ABSTRACT
To understand the effects of the eIF-5A gene in response to abiotic stress, the 909 bp full-length cDNA of eIF-5A, including a 480 bp open reading frame encoding 159 amino-acid (aa) residues, was isolated from the leaf of herbal plant (Apocynum venetum) by rapid-amplification of cDNA ends. The deduced molecular weight of the encoding protein was 17.48 kDa with a theoretical pI of 5.61 and predicted no signal peptide. Real-time polymerase chain reaction analysis revealed that AveIF-5A gene expression was induced by cold, salt and drought stress. To determine the biological function of this gene, recombinant plasmids expressing AveIF-5A and AveIF-5A1 (86–156 aa truncated polypeptide) were used to transform Escherichia coli. The analysis of the growth curves of recombinant E. coli revealed that AveIF-5A and AveIF-5A1 improved the resistance of transformed E. coli to low-temperature, drought and salt stress. These results could provide experimental evidence of the function of the AveIF-5A gene as a valuable gene resource in plant resistance-breeding.
Introduction
The eukaryotic translation initiation factor 5A (eIF-5A), which is found in all eukaryotic organisms, is the only known cellular protein containing the unusual amino-acid hypusine, which is essential for the function of eIF-5A. Hypusine eIF-5A is known to interact with components of the 80S ribosome and translation elongation factors 2 in a hypusine-dependent manner.[Citation1,Citation2] eIF-5A was originally isolated from immature red blood cells and was identified as a translation initiation factor,[Citation3] but recent research suggests that the activity of eIF-5A is not absolutely essential for general protein biosynthesis. The role of eIF-5A in translation is to act as a nucleocytoplasmic shuttle protein that selectively translocates specific subsets of mRNAs from the nucleus to the cytoplasm.[Citation4] eIF-5A plays important roles in many cellular and molecular activities, such as cell proliferation, protein translation, mRNA degradation, cell cycle transition, cell senescence,[Citation5] apoptosis [Citation6,Citation7] and development.[Citation8] The previous reports about eIF-5A are largely focused on human [Citation9] and yeast cells,[Citation10] but the precise cellular function of eIF-5A remains largely unknown, especially in plants. Although the full-length cDNA encoding eIF-5A has been isolated from several plant species such as Arabidopsis thaliana,[Citation11–13] pumpkin,[Citation14] maize,[Citation15] tomato,[Citation16] Tamarix androssowii [Citation17] and so on, the function and mechanism of this gene in higher plants are not clear, especially its biological function under abiotic stress.
Compared to glycophytes, halophytes are well adapted to salinity, and can solve the problem of salinity adaptation more efficiently. The unique genetic makeup of halophytes also allows them to survive and grow under salt stress conditions.[Citation18] As a result, halophytes are useful organisms in research on salt tolerance mechanisms. Our studies have concentrated on an extreme halophyte, Apocynum venetum. It is a perennial herb or half-shrub and is widely distributed in the temperate zones of Eurasia and North America, especially in central and northwestern China. A. venetum belongs to the Apocynaceae family and grows widely in saline-alkali soil, river banks, alluvial plains and sandy wastelands.[Citation19] Because of its resistance to cold, drought, saline-alkaline and sandy stress, A. venetum could provide an advantage for studying the salt tolerance mechanisms and could serve as a potential resource in plant-breeding for salt tolerance.
In this study, the eIF-5A gene was cloned from A. venetum and its expression pattern was characterized under cold, drought and saline-alkaline stress conditions by real-time polymerase chain reaction (PCR) and was functionally assayed by prokaryotic expression. This research provides data for the better understanding of the biological function of the eIF-5A gene as a valuable gene resource for plant-breeding applications.
Materials and methods
Plant growth and stress treatment
Seeds of A. venetum were harvested from the botanical garden of Northeast Forestry University of China. The seeds were disinfected in 10% NaClO solution for 5 min, rinsed in sterile water 4–5 times and sowed on Murashige and Skoog medium.[Citation20] Culture flasks were incubated in a growth chamber at 26 °C/22 °C (day/night) and a 16 h photoperiod. When the plants formed six leaves, the seedlings were transferred to plastic pots containing potting mix (nutrient soil:vermiculite:perlite,1:1:1, v/v/v). One week after transplantation, the seedlings were divided into four groups. One group was grown under normal conditions (as described above) as a control and the other three groups were subjected to stress by 200 mmol/L NaHCO3, 20% polyethylene glycol (PEG) 6000 or low-temperature (5 °C), respectively. The leaf tissues were harvested from three seedlings of these four groups at 0, 12, 24, 48 and 72 h and frozen immediately in liquid nitrogen. The tissues were then stored at −80 °C until use.
Cloning of the AveIF-5A gene from A. venetum and sequence analysis
Total RNA of A. ventum was extracted from frozen leaf tissues by using Trizol reagent (Invitrogen), according to the manufacturer's instructions. To obtain a partial cDNA sequence of AveIF-5A, degenerate primers were designed: sense primer 5'-CNCARCARGCNGGNACNA-3' and anti-sense primer 5'-ATYTGYTCYTCNCCCAT-3' (R = A/G, Y = C/T, N = A/C/G/T). The full-length cDNA sequence of AveIF-5A was cloned using 5'/3' rapid-amplification of cDNA ends (RACE) (5'/3' RACE Kit, 2nd Generation, Roche, Basel, Switzerland). For 5'-RACE, a gene-specific primer, SP1 (5'-CTTTCCCTCAGCAAATC CATC-3'), was required to transcribe the mRNA into first-strand cDNA. The second nested primer, SP2 (5'-TGGTAATCAACACGGTTCACAT-3') located upstream of SP1, was used for the first PCR amplification. A further nested primer SP3 (5'-CTTGCCAGTCTTGGAAGTTGA-3') was used for the second round PCR. For 3'-RACE, the Oligo dT-anchor primer was used to transcribe the mRNA into first-strand cDNA. A gene-specific forward primer, SP5 (5'-CTGAGGCTCCCAACAGATGA-3'), was required. PCR anchor primer SP4 (5'-AGATATTGTTCCCTCTTCCCAC-3') was applied to obtain a specific band in agarose gel electrophoresis. DNA sequencing was done by Sangon Biotech company (Shanghai, China).
The open reading frame (ORF) of AveIF-5A was assembled by ORF finder (http://www.ncbi.nlm.nih.gov/gorf/gorf.html). The molecular weight (MW) and the theoretical isoelectric point (pI) were calculated by ProtParam software (http://www.expasyorg/tools/s-protaram.html). The signal peptide was predicted by SignalP 3.0 (http://www.cbs.dtu.dk/services/SignalP/). Conserved domain was predicted by conserved domain database (CDD) from national center of biotechnology information (NCBI) (http://www.ncbi.nlm.nih.gov/cdd/). The deduced amino-acid sequences of AveIF-5A, along with other reported plant eIF-5As, were aligned using Clustal W2. A phylogenetic tree was constructed using the neighbour-joining method provided by the MEGA5 software. Bootstrapping (1000 replicates) was performed to quantify the relative support for branches of the inferred phylogenetic tree.
AveIF-5A gene expression profiles in response to abiotic stress
Total RNA was extracted from leaves of A. venetum in the three stress treatments and the control, using Trizol reagent. Total RNA (1 µg) was reverse transcribed with reverse transcriptase M-MLV (Takara) in a volume of 10 µL. The synthesized cDNA was then diluted to a final volume of 100 µL. Real-time PCR was performed in a volume of 20 µL, containing 2 µL of diluted template cDNA, 10 µL of 2 × SYBR premix Ex Taq (Takara) and 0.5 µL of each forward and reverse primer (10 µmol/L). The amplification was performed using the following cycle parameters: 94 °C for 30 s, followed by 44 cycles of 94 °C for 12 s, 58 °C for 30 s, 72 °C for 40 s and 1 s at 81 °C for plate reading. Specific primers, 5'-TTCTCTCCCTTTTCTCAGTCCTT-3' and 5'-CACAGACAGTTTACTTGGGACC-3', were used for real-time PCR of AveIF-5A. The actin gene was used as an internal control in order to normalize the amount of total RNA in each reaction, by using the following primers: 5'-TGTGAGTCACACTGTGCCAATCTA-3' and 5'-ATATCCACATCACACTTCATGATGG-3'.
There were three independent biological replications for each treatment. Three experimental technical replications were performed for each sample to assess the reproducibility. The mean of the nine replications was used for relative expression quantitation. Gene expression levels were calculated by the 2−△△Ct method. The data were analysed by Duncan's test using SPSS16.0.
AveIF-5A gene stress tolerance assays
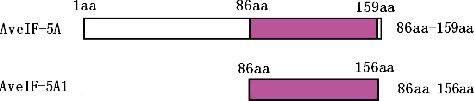
The full-length ORF of AveIF-5A and a truncated cDNA of AveIF-5A1 (AveIF-5A86–156 aa) (), with a Hind III restriction site at the 5' end and a XhoIsite at the 3' end was obtained by PCR using the following primers: 5'-CCCAAGCTTCGCGAAGAAGACGATCATGT-3'/5'-CCGCTCGAGGACACA GACAGTTTACTTGG-3' and 5'-CCCAAGCTTATGGTGAACCGTGTTGATTACCA-3'/5'-CCGCTC GAGTTAAATATCCTTGAGGGCGCAGA-3'. The fragments were inserted into the Hind III/XhoIsite of prokaryotic expression vector pET32a in order to allow expression as a Trx fusion protein in Escherichia coli. These recombinant plasmids were transformed into E. coli BL21 along with the pET32a empty vector as a control. E. coli BL21 with pET32a–AveIF-5A, pET32a–AveIF-5A1 and pET32a was grown in Luria Bertani (LB) medium to optical density (OD) of 0.6 at 600 nm (OD600) and then 1 mmol/L of IPTG (isopropyl β-D-1-thiogalactopyranoside) was added to the medium. After a 12-h incubation at 25 °C, cultured cells were added to LB basal medium for cultivation at 16 °C and LB medium containing 0.5 mol/L NaCl and 20% PEG for cultivation at 37 °C, respectively.
The original OD600 values of all E. coli groups were adjusted to the same value at 0 h. The OD values were measured every 2 h. Three independent biological replications were performed for each treatment. Also three technical replicates were performed for each biological sample.
Results and discussion
Cloning and sequence analysis of the eIF-5A gene
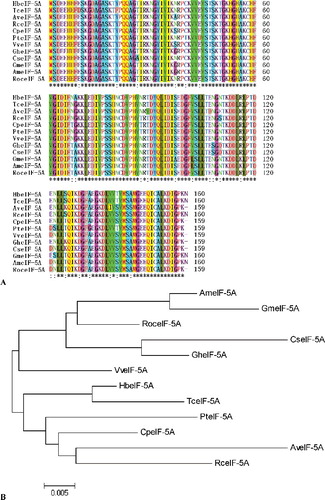
According to homology sequences from Medicago sativa, Hevea brasiliensis, Rosa chinensis, Vitis vinifera, Solanum tuberosum, Manihot esculenta, Populus deltoids and Populus tremula × Populus alba, by means of designing degenerate primers, a partial eIF-5A cDNA of 388 bp was obtained from A. venetum. Based on the sequences mentioned above, 5'/3'-RACE gene-specific primers (SP1–SP5) were designed. A 283 bp fragment was obtained from 5'-RACE and a 500 bp fragment from 3'-RACE to assemble the full-length gene.
The 909 bp full-length cDNA of the eIF-5A gene was isolated from the leaves of A. venetum by RACE, including an 89 bp 5' untranslated region, a 340 bp 3' untranslated region and a 480 bp ORF encoding 159 amino-acid residues. The putative protein sequence of AveIF-5A was aligned with other reported plant eIF-5A sequences and showed significant sequence homology among them ((A)). The identity of AveIF-5A with eIF-5A from other plants was above 90% at the amino-acid level. Moreover, the constructed phylogenetic tree showed that AveIF-5A was most similar to RceIF-5A from R. communis and had a longer evolutional distance from AmeIF-5A of A. mongolicus and GmeIF-5A of G. max ((B)). The conserved domain of AveIF-5A was between 86 and 156 aa that belonged to S1_eIF-5A super family (). The MW of the deduced protein was 17.48 kDa with a theoretical pI of 5.61. There was no predicted signal peptide in the eIF-5A amino-acid sequence.
Expression analysis of the eIF-5A gene from A. venetum under abiotic stress conditions
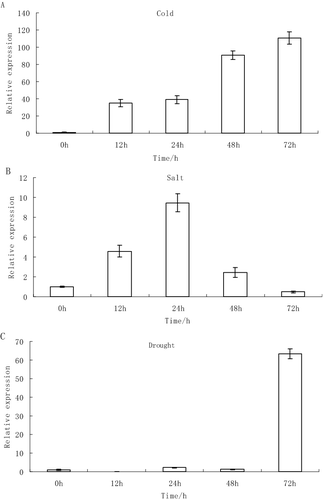
The real-time PCR expression analysis of the eIF-5A gene in leaves of A. venetum in response to cold, salt and drought stress is shown in . In conditions of low-temperature treatment, the expression of the AveIF-5A gene was significantly higher than that in the control group from 12 to 72 h (P < 0.01) and the highest expression value was observed at 72 h ((A)). Under NaHCO3 stress, there was an early increase in the expression of the AveIF-5A gene, but it was later down-regulated and the highest gene expression value was at 24 h, which was significantly different (P < 0.01) from that at the other time points ((B)). Under drought stress, the relative expression of AveIF-5A was lower between 0 and 48 h, but then increased sharply (P < 0.01) at 72 h ((C)). These results indicated that the expression profiles may be different in cold, salt and drought stress. Several studies have demonstrated that eIF-5A expression greatly changes in plants under various stress conditions. For example, RceIF-5A expression was up-regulated in Rosa chinensis under high temperature, oxidative and osmotic stress conditions but no significant differences were observed under NaCl, LiCl and CdCl2 stress.[Citation21] This evidence suggests that eIF-5A in plants plays an important role in response to some types of abiotic stress.
Function analysis of the eIF-5A gene in transformed E. coli under abiotic stresses
Over-expression of plant stress-related genes in E. coli has been reported to attribute stress tolerance enhancement.[Citation22] Although eIF-5A does not exist in bacteria, its expression from wheat,[Citation23] Chinese rose [Citation24] and Tamarix androssowii [Citation25] increased the tolerance levels of the bacterial cells to stress. In this study, to determine the function of the eIF-5A gene under abiotic stress conditions, the growth of two transformed E. coli strains containing AveIF-5A and AveIF-5A1, and the wild E. coli strain were compared. Under normal conditions (in LB liquid medium at 37 °C), the pET32a-AveIF-5A (complete ORF) recombinant E. coli, pET32a-AveIF-5A1 (truncated polypeptide) recombinant E. coli and vector alone (pET32a) E. coli showed similar exponential growth curves ((A)). However, at low-temperature or in the presence of 0.5 mol/L NaCl and 20% PEG6000, there were differences in the growth of the recombinant E. coli and the control. Under low-temperature and NaCl stress, the pET32a-AveIF-5A and pET32a-AveIF-5A1 recombinant cells showed higher growth rates compared to the strain with the vector alone, whose growth was inhibited ((B,C)). In the presence of 20% PEG, the vector-only strain grew more slowly than the recombinant cells up to 10 h. After that, the bacterial growth was similar in the control and recombinant E. coli ((D)). These results indicated that AveIF-5A could enhance the abiotic stress tolerance of recombinant bacterial cells. Also, the specific conserved region of eIF-5A might play important roles with complete eIF-5A under stress.
Figure 4. Growth kinetics of recombinant E. coli cells expressing AveIF-5A and AveIF-5A1 grown in liquid nutrient medium in: normal conditions (A), low-temperature (16 °C) (B), 0.5 mol/L NaCl (C) and 20% PEG stress (D).
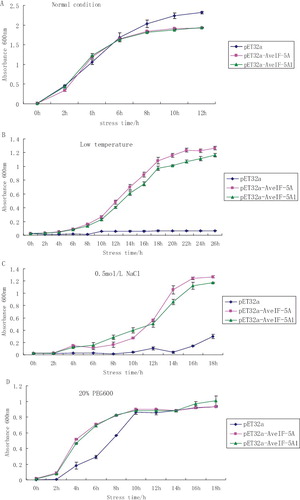
Based on the observed different response to different stress factors in recombinant E. coli and control, it could be suggested that the expression of AveIF-5A and AveIF-5A1 in E. coli could significantly enhance the ability of host bacteria to resist low-temperatures and salt stress. Our results that increasing the duration of exposure to drought stress did not lead to significant changes in the drought resistance of recombinant E. coli compared to control cells, further suggest that the potential of the AveIF-5A and AveIF-5A1 expression product to improve the resistance to drought in host bacteria was restricted within certain limits, except at the initial stage.
AveIF-5A and AveIF-5A1 could be involved in different protective pathways in response to different stress factors. However, further research is needed to elucidate which pathway AveIF-5A is related to. Expression analysis of the eIF-5A gene in A. venetum in this study provides insights for further study of eIF-5A functions in plants and important information to improve plant tolerance to abiotic stresses via gene transformation.
Conclusions
In this work, we successfully cloned the full-length cDNA of eIF-5A from the leaf of A. venetum. AveIF-5A gene expression could be induced under cold, salt and drought stress. The full-length AveIF-5A and the truncated AveIF-5A1 could improve the resistance of transformed E. coli to low-temperature, drought and salt stress. These results provide theoretical basis for eIF-5A functional research and suggest that the gene could potentially find application as a gene resource in genetic engineering-breeding.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Park MH. The post-translational synthesis of a polyamine-derived amino acid, hypusine, in the eukaryotic translation initiation factor 5A (eIF5A). JPN Biochem Soc. 2006;139:161–169.
- Lee SB, Park JH, Kaevel J, et al. The effect of hypusine modification on the intracellular localization of eIF5A. Biochem Biophys Res Commun. 2009;383:497–502.
- Kemper WM, Berry KW, Merrick WC. Purification and properties of rabbit reticulocyte protein synthesis initiation factor M2Bα and M2Bβ. J Biol Chem. 1976;251:5551–5557.
- Jao DL, Yu CK. Subcellular localization of the hypusine-containing eukaryotic initiation factor 5A by immunofluorescent staining and green fluorescent protein tagging. J Cell Biochem. 2002;86:590–600.
- Hopkins M, Taylor C, Liu ZD, et al. Regulation and execution of molecular disassembly and catabolism during senescence. New Phytol. 2007;175:201–214.
- Feng HZ, Chen QG, Feng J, et al. Functional characterization of the Arabidopsis eukaryotic translation initiation factor 5A-2 that plays a crucial role in plant growth and development by regulating cell division, cell growth, and cell death. Plant Physiol. 2007;144:1531–1545.
- Seko Y, Fujimura T, Yao T, et al. Secreted tyrosine sulfated-eIF5A mediates oxidative stress-induced apoptosis. Sci Rep. 2015;5:13737.
- Belda-Palazón B, Almendáriz C, Martí E, et al. Relevance of the axis spermidine/eIF5A for plant growth and development. Front Plant Sci [Internet]. 2016 [cited 2016 Mar 25];7:245. Available from: http://dx.doi.org/10.3389/fpls.2016.00245
- Lee YB, Joe YA, Wolff EC, et al. Complex formation between deoxyhypusine synthase and its protein substrate, the eukaryotic translation initiation factor 5A (eIF5A) precursor. Biochem J. 1999;340:273–281.
- Valentini SR, Casolar JM, Oliveira CC, et al. Genetic interactions of yeast eukaryotic translation initiation factor 5A (eIF5A) reveal connections to poly(A)-binding protein and protein kinase C signaling. Genetics. 2002;160:393–405.
- Liu ZD, Duguay J, Ma FS, et al. Modulation of eIF5A1 expression alters xylem abundance in Arabidopsis thaliana. J Exp Bot. 2008;59:939–950.
- Ma FS, Liu ZD, Wang TW, et al. Arabidopsis eIF5A3 influences growth and the response to osmotic and nutrient stress. Plant Cell Environ. 2010;33:1682–1696.
- Xu XY, Ding ZJ, Chen L, et al. An eukaryotic translation initiation factor, AteIF5A-2, affects cadmium accumulation and sensitivity in Arabidopsis. J Integr Plant Biol. 2015;57:848–858.
- Ma Y, Miura E, Ham BK, et al. Pumpkin eIF5A isoforms interact with components of the translational machinery in the cucurbit sieve tube system. Plant J. 2010;64:536–550.
- Łebska M, Ciesielski A, Szymona L, et al. Phosphorylation of maize eukaryotic translation initiation factor 5A (eIF5A) by casein kinase 2. J Biol Chem. 2010;285:6217–6226.
- Wang TW, Lu L, Wang D, et al. Isolation and characterization of senescence-induced cDNAs encoding deoxyhypusine synthase and eucaryotic translation initiation factor 5A from tomato. J Biol Chem. 2001;276:17541–17549.
- Wang LQ, Xu CX, Wang C, et al. A eukaryotic translation initiation factor 5A from Tamarix androssowii (Tamarisk), TaeIF5A1, can form a homodimer and interact with other proteins. Plant Omics J. 2014;7:468–473.
- Yadav NS, Singh VK, Singh D, et al. A novel gene SbSI-2 encoding nuclear protein from a halophyte confers abiotic stress tolerance in E. coli and tobacco. Plos One [Internet]. 2014 [cited 2015 Sep 15];9:e1019261–19. Available from: http://dx.doi.org/10.1371/journal.pone.0101926
- Zhang WM, Xiao ZC, Gu GP, et al. On the resources utilization of Apocynum and its classification. Chinese Wild Plant Resour. 2006;25:15–19.
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Phisiol Plant. 1962;15:473–497.
- Xu JY. Function analysis of eIF-5A in Rosa chinensis [M.Sc. thesis]. Shanghai: College of Life, Fudan University; 2010. Chinese.
- Liu Y, Zheng YZ. PM2, a group 3 LEA protein from soybean, and its 22-mer repeating region confer salt tolerance in Escherichia coli. Biochem Biophys Res Commun. 2005;331:325–332.
- Zhou JP, Yang ZJ, Bai L, et al. Construction of wheat translation initiation factor 5A(eIF5A) expression vector and its prokaryotic expression. Chin J Appl Environ Biol. 2007;13:301–303.
- Shi JL, Xu JY, Jiang CH, et al. Increasing the thermotolerance of E.coli with expression of eIF-5A from Rosa chinensis. China Biotechnol. 2008;28:18–24.
- Yang CP, Jiang J, Tian G, et al. Cloning and expression of translation initiation factor 5A (eIF-5A) gene from Tamarix androssowii. Plant Physiol Commun. 2005;41:433–438.