ABSTRACT
Hashimoto's thyroiditis (HT) is an organ-specific, mainly Th1 autoimmune disorder. New evidence has accumulated for involvement of Treg-produced cytokines. Interleukin (IL)-12 and IL-10 are immunoregulatory cytokines with antagonistic effect on Th differentiation. This study was designed to investigate the correlation of circulating IL-10 and IL-12p40 with their genotypes in 124 HT patients in different stages of disease. The IL-10 and IL-12p40 circulating level in the serum of HT patients and healthy controls was determined by enzyme-linked immunosorbent assay. Genotyping for the 3’UTR A/C IL12B polymorphism was performed using restriction fragment length polymorphism–polymerase chain reaction and genotyping for −1082 A/G by amplification refractory mutation system (ARMS)-PCR. The results showed significantly enhanced IL-12p40 serum quantity both in euthyroid and hypothyroid stages compared to controls and insignificantly decreased level after levothyroxine treatment. Regarding the 3’UTR A/C IL12B polymorphism, a significantly higher frequency of the AA genotype was observed in HT patients than in healthy individuals. The serum IL-10 level was significantly increased in hypothyroid HT patients compared to controls and euthyroid patients. Stratification based on the −1082 A/G polymorphism revealed a significantly enhanced level of circulating IL-10 in hypothyroid patients with a GG genotype compared to AA and AG genotypes. There were also significant differences between the circulating IL-10 quantity in hypo- and euthyroid patients with GG genotypes. In conclusion, the IL-12p40 and IL-10 serum level in HT patients was dependent on the genotype and HT stages, suggesting that enhanced IL-10 serum level in combination with enhanced IL-12p40 is associated with hypothyroidism in HT development.
Introduction
Hashimoto's thyroiditis (HT) is the most frequent organ-specific autoimmune disease of the thyroid. The dysfunction of the gland may be clinically evident or subclinical depending on the degree of thyroid parenchyma destruction. Hypothyroidism may, in some cases, be developed early in life, whereas in others, a euthyroid state may be maintained in old age. HT is a multifactorial disease that develops as a result of a complex interaction between genetic and environmental factors that influence thyroid autoimmunity.[Citation1]
The role of cytokines in the pathogenesis of HT has been extensively investigated.[Citation2–4] Coordinated cytokine production after activation controls proliferation, differentiation and functions of immune cells and is a key factor that regulates the type and magnitude of the immune response. The proper balance between pro-inflammatory cytokines (tumour necrosis factor alpha (TNF-α), interleukin (IL)-6 and IL-12p40) and the anti-inflammatory IL-10 controls the appearance of normal or pathological inflammatory response [Citation5] and is therefore likely to play a critical role in the development of autoimmune diseases. IL-12 and IL-10 are immunoregulatory cytokines with antagonistic effect on Th differentiation.
IL-12p70 is a pro-inflammatory cytokine composed of two subunits, p40 and p35, encoded by the IL12B and IL12A genes, respectively. IL-12 induces the differentiation of CD4+ T lymphocytes from a Th0 to a Th1 phenotype and promotes cell-mediated immunity.[Citation6,Citation7] The role of IL-12 in the pathogenesis of autoimmune thyroid diseases has been widely investigated and it is well established that they have a pivotal role in the pathogenesis of disease.[Citation8,Citation9]
IL-10 has important immunoregulatory functions. Its protective effect in autoimmunity has been demonstrated. IL-10 suppresses Th1-mediated immune response through down-regulation of pro-inflammatory cytokine secretion from both Th1 and activated macrophages.[Citation10,Citation11] It also inhibits antigen-presenting cells by down-regulating major histocompatibility complex class II (MHC-II).[Citation12] In addition to these inhibitory actions, IL-10 promotes B-cell-mediated functions, enhancing proliferation, differentiation and antibody production.[Citation13] In recent studies, the involvement of IL-10 in the pathogenesis of HT has been reported.[Citation14–16]
It remains unclear whether the production of IL-10 and IL-12p40 controls autoimmune inflammation or it promotes pathogenic processes in HT. Studies on cytokine gene polymorphism in autoimmune diseases have revealed a correlation between cytokine levels and various cytokine gene variants, which may explain the differences in the clinical presentation and course of diseases. Functional polymorphisms in IL-12p40 (IL12B) have been reported to be associated with susceptibility to some autoimmune diseases. Several intronic polymorphisms and a single nucleotide polymorphism (SNP) at position +16974 (+16974 A/C) in the 3'-untranslated region (UTR) of IL12B have been found to correlate with IL-12 secretion.[Citation17–20] IL-10 promoter polymorphisms possibly contributing to predisposition to autoimmune disorders have also been identified.[Citation21] Approximately, 75% of the variation in IL-10 production appears to be genetically determined.[Citation22]
As cytokine gene polymorphisms may affect the cytokine levels, the aim of this study was to investigate the correlation of circulating serum IL-10 and IL-12p40 with their genotypes in the development of HT in a cohort of Bulgarian patients.
Subjects and methods
Subjects
We investigated 124 out-patients (from the Department of Endocrinology, Stara Zagora University Hospital (Bulgaria), with autoimmune thyroiditis. The group of the TH patients consisted of 9 (7.3%) males and 115 (92.7%) females from 18 to 74 years of age, with a mean age of 48.0 years and standard deviation (±SD) of 13.3 years. Seventy-two healthy subjects were included as controls. The sex and age distribution of the healthy controls was: 6 (8.3%) males and 66 (91.7%) females; mean (±SD) age of 42.2 ± 14.5 years and a range of 19–74 years. In all patients, diagnosis had been made by clinical examination of the thyroid gland, elevated anti-thyroid peroxydase antibodies and/or typical hypoechogenicity of the thyroid in high-resolution sonography. In negative thyroid peroxidase antibodies (TPO) Abs patients, fine needle aspiration biopsy was performed and typical cytological features of autoimmune thyroiditis were found. Serum levels of thyroid-stimulating hormone (TSH) and free thyroxin (fT4) were determined. Fasting venous blood samples were collected in the morning between 8.00 and 10.00. Serum samples were routinely collected and stored frozen at −20 °C until assayed. At the time of sampling, none of the patients and control subjects had clinical signs or symptoms of intercurrent illness. Patients were divided into three sub-groups according to the thyroid function: Group I (n = 41) included subjects with normal thyroid function (TSH and fT4 within the normal range); Group II (n = 17) included patients with hypothyroidism (high levels of TSH and low or normal serum levels of fT4); Group III (n = 66) included subjects with hypothyroidism treated with levothyroxine (LT4) in a dosage to maintain TSH and fT4 within the normal range. The medication of levothyroxine (range: 50–200 µg) was given in the fasting state.
Informed consent was obtained from all participants in the study, according to the ethical guidelines of the Helsinki Declaration.
Cytokine determination
The circulating level of IL-10 and IL-12p40 in serum of HT patients and healthy controls was determined by enzyme-linked immunosorbent assay (ELISA) using commercially available kits (Quantakine® HS and Quantakine®, R&D systems, Abingdon, UK, for IL-10 and IL-12p40, respectively). The developed colour reaction was measured as optical density (OD490 or OD450) units for IL-10 and IL-12p40, respectively, on an ELISA reader (Rosys Anthos 2010, Salzburg, Austria). The concentration of cytokines was determined by using a standard curve constructed with the kit's standards. The minimum detectable dose of the IL-12p40 ELISA kit is less than 15 pg/mL and that of the IL-10 ELISA kit is less than 0.5 pg/mL.
DNA extraction
Genomic DNA was extracted using a genomic blood DNA purification kit (NucleoSpin Blood L, Macherey-Nagel, Duren, Germany) and stored at −80n °C until use. The concentration of resulting DNA was measured spectrophotometrically at 260 nm by using GeneQuant 1300 spectrophotometer (GE Healthcare Life Sciences, Switzerland). The ratio of absorptions at 260 nm vs. 280 nm was used to assess the purity of DNA samples.
Genotyping of SNP in 3’UTR of IL12B
Genotyping for the IL12B A/C polymorphism in 3’UTR was performed by restriction fragment length polymorphism–polymerase chain reaction (PCR) assay after amplification of a 1046 bp fragment with forward primer: 5’-ATT TGG AGG AAA AGT GGA AGA-3’ and reverse primer: 5’-AAT TTC ATG TCC TTA GCC ATA-3’. PCR amplification was carried out in 20 µL volumes containing 10 × PCR buffer, 0.5 U Taq polymerase (Thermo Scientific, Waltham, MA, USA), 3.0 mmol/L MgCl2, 100 µmol/L of each deoxyribonucleoside triphosphate and 0.4 µmol/L of each primer. The cycling parameters for IL12B A/C SNP in 3’UTR were as follows: initial incubation step of 5 min at 95 °C; 30 cycles: 1 min at 94 °C, 1 min at 54.3 °C and 2 min at 72 °C; and a final extension step of 7 min at 72 °C completed the reaction. PCR amplification was performed in a GeneAmp PCR System 9700 (Applied Biosystems, Foster City, CA, USA). Amplified products (10 µL) were digested using 10 U TaqI (Thermo Scientific, Waltham, MA, USA) per reaction for 4 h at 65 °C. The +16974C allele yields two fragments, 906 bp and 140 bp, respectively.
Genotyping of SNP in IL-10
Genotyping for the −1082 A/G SNP in IL10 was performed by amplification refractory mutation system (ARMS)–PCR. The following primers were used: 5’ primer specific for the IL10-1082G allele (5’-CCTATCCCTACTTCCCCC-3’), 5’ primer specific for the IL10-1082A allele (5’-CCTATCCCTACTTCCCCT-3’) and IL10 generic 3’ primer (5’-AGCAACCACTCCTCGTCGCAAC-3’); аnd the amplification mixture used was as described above. The cycling parameters were as follows: initial incubation step of 5 min at 95 °C; 30 cycles: 30 s at 94 °C, 1 min at 60 °C and 1 min at 72 °C; and a final extension step of 7 min at 72 °C completed the reaction. PCR amplification was performed in a GeneAmp PCR System 9700 (Applied Biosystems, Foster City, CA, USA). Primers and reagents for PCR reactions were supplied by Metabion GmbH (Munich, Germany) and Thermo Scientific (Waltham, MA, USA), respectively. PCR products were visualized in a 2% agarose gel stained with ethidium bromide (0.5 mg/mL). In each PCR run, a heterozygous control template was used to ensure accuracy. For quality control, 10% of random selected samples containing both cases and controls were analysed a second time without finding any discrepancies.
Statistical analysis
All data were analysed using SPSS V.16.0. Differences in IL-10 and IL-12p40 serum levels in patients with different stages of HT and healthy controls were analysed by the Mann–Whitney U-test. Differences were considered significant when the p-value was less than 0.05. The differences in genotype distribution and allele frequency among cases and controls were analysed using the χ2 test. When 2 × 2 tables contain small observed frequencies, Yates' corrected p-values (denoted as pc) were taken into account. StatPages.net (http://statpages.org/index.html) was used to estimate the odds ratios (OR), expressed with their 95% confidence intervals for disease susceptibility and severity in relation to IL12B polymorphisms. The goodness of fit to the Hardy–Weinberg equilibrium and the expected frequencies of each genotype in comparison with the observed values for patients and healthy controls were determined using a χ2 test. In all cases, two-tailed p-values less than 0.05 were considered significant.
Results and discussion
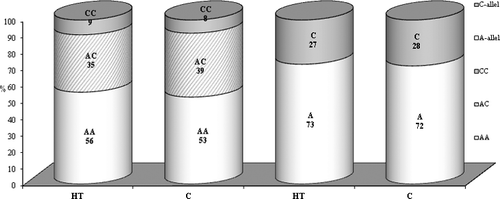
To investigate the influence of the studied SNPs on the circulating serum level of IL-10 and IL-12p40 in TH development, we performed genotyping and cytokine quantitative assessment in patients and control groups. There were no significant differences in the genotype and allele frequencies of the IL12B polymorphism between HT patients and controls (χ = 0.387, p = 0.82) (). However, when the HT patients were stratified according to the thyroid function into euthyroid and hypothyroid patients, we observed that the individuals who are carriers of the CC genotype have an elevated risk of developing euthyroid HT (OR = 1.68) than hypothyroidism (OR = 0.84) compared to controls, but this difference was not statistically significant.
Figure 1. Distribution of allele and genotype frequencies of 3’UTR A/C in IL12B among Hashimoto's thyroiditis (HT) patients and healthy subjects (C).
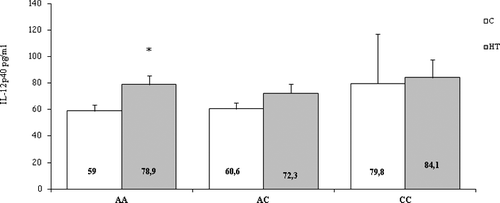
All patients and healthy controls had detectable values of serum IL-12p40. The concentrations of IL-12p40 in the study groups are shown in . The quantity of IL-12p40 in serum was elevated in both euthyroid and hypothyroid stages compared to controls (82.3 ± 10.1 pg/mL and 83.4 ± 14.1 pg/mL, respectively, vs. 62.3 ± 3.6; p = 0.025) and non-significantly decreased after levothyroxine treatment (mean ± SEM, 72.3 ± 5.1) (). Regarding the 3’UTR A/C IL12B polymorphism, it was shown that the serum level of IL-12p40 was significantly higher in HT patients with the AA genotype (78.9 ± 6.9 pg/mL) in comparison with AA healthy individuals (58.97 ± 4.7 pg/mL, p = 0.019) as presented in
Table 1. Serum concentrations of IL-12p40 and IL-10 in Hashimoto's thyroiditis (HT) patients and healthy controls.
Figure 2. Serum levels of IL-12p40 in Hashimoto's thyroiditis (HT) patients and controls (C). EU – patients in euthyroid stage; HYPO – patients with hypothyroidism; TREATMENT – patients treated with levothyroxine. Results are presented as mean values with standard errors of the means (±SEМ).
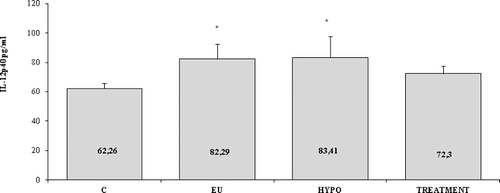
Figure 3. Serum levels of IL-12p40 in Hashimoto's thyroiditis (HT) patients and controls (C) according to the genotypes of the 3’UTR A/C IL12B polymorphism. Results are presented as means ±SEМ.
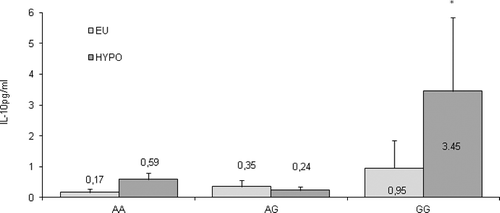
The serum concentrations of IL-10 in healthy controls and patient groups () showed that, although quite a few controls and patients had undetectable serum levels of the cytokine, a significant increase in the serum level of IL-10 was found in hypothyroid HT patients compared both to controls and to euthyroid patient group (mean ± SEM, 1.98 ± 1.59 vs. 0.74 ± 0.22 and 0.48 ± 0.3, respectively; p = 0.038). Moreover, the quantity of circulating IL-10 decreased after treatment with levothyroxine (mean ± SEM, 1.31 ± 0.94) as seen from . The stratification of the patients based on the −1082 A/G SNP genotype () revealed a significantly enhanced level of circulating IL-10 in hypothyroid patients with a GG genotype compared to AA and AG genotypes (3.45 ± 2.4 pg/mL vs. 0.59 ± 0.2 pg/mL and 0.24 ± 0.1 pg/mL, respectively; p = 0.048). Also, significant differences between the circulating IL-10 quantity in hypo- and euthyroid patients with GG genotypes were detected (3.45 ± 2.4 pg/mL vs. 0.95 ± 0.9 pg/mL; p = 0.028). In contrast, the AA and AG genotypes did not show differences in the circulating IL-10 among patients at different stages.
Figure 4. Serum levels of IL-10 in Hashimoto's thyroiditis (HT) patients and controls (C). EU – patients in euthyroid stage; HYPO – patients with hypothyroidism; TREATMENT – patients treated with levothyroxine. Results are presented as means ±SEМ.
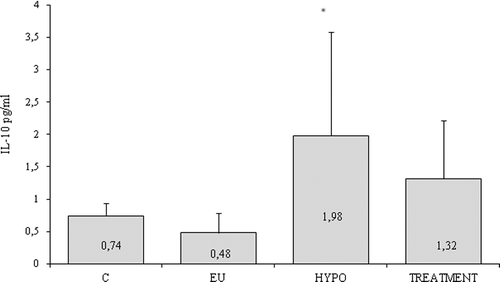
Figure 5. Serum levels of IL-10 in Hashimoto's thyroiditis (HT) patients (EU – patients in euthyroid stage; HYPO – patients with hypothyroidism) according to the −1082 A/G IL10 SNP genotypes. Results are presented as means values ±SEМ.
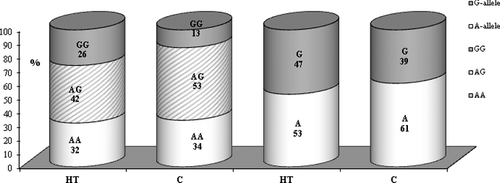
The results for the distribution of the allele and genotype frequencies of the −1082 A/G polymorphism of IL-10 are presented in . Regarding the polymorphism rs1800896, a significantly higher frequency of the homozygous genotype GG (26% vs. 13%; OR = 2.19, p = 0.024) was shown in the studied cases than in the controls.
Figure 6. Distribution of allele and genotype frequencies of −1082 A/G in IL-10 among Hashimoto's thyroiditis (HT) patients and healthy subjects (C).
In this study, it was observed that the IL-12 and IL-10 levels in the serum of hypothyroid HT patients were significantly higher when compared to the levels in the control group and gene polymorphisms were related to these cytokines. Due to its ability to stimulate Th1 responses, IL-12 is considered as a key cytokine in the pathogenesis of organ-specific autoimmune diseases, which are often mediated by cellular immunity.[Citation23]
Our results, as well as data from our previous work demonstrated that serum IL-12 levels were increased in all sub-groups in HT patients regardless of the functional state of the disease,[Citation8] which suggests that IL-12 might affect the immune response. However, the results of other investigators concerning the IL-12 serum levels in different stages of HT are contradictory. Phenekos et al. [Citation9] have shown that IL-12 serum concentrations were significantly higher than those in normal controls in HT patients. Zhang et al. [Citation24] found that the mean levels of IL-12 in euthyroid HT patients were significantly higher than those in normal controls; however, they observed no differences in IL-12 serum levels between hypothyroid HT patients and normal controls. The study of Guclu et al. [Citation25] showed a statistically significant decrease in the IL-12 serum levels in HT hypothyroid patients after treatment with levothyroxine for about 12 weeks.
During iodine-induced autoimmune (lymphocytic) thyroiditis in non-obese diabetic mice, IL-12 is produced in the thyroid gland early and throughout the course of the disease.[Citation26] Kimura et al. [Citation27] have shown that the local production of IL-12 in the thyroid enhances the expression of sodium-iodide symporter and inhibits thyroid hormonogenesis, thus inducing primary hypothyroidism; also the disease-promoting effect of IL-12 was independent of interferon-γ. On the basis of our results and data from experimental models and clinical studies, it may be suggested that the effect of IL-12 on the initiation and regulation of immune responses and also on thyroid function is crucial in HT.
The results from the analysis of the 3’UTR A/C polymorphisms in the promoter region of the IL-12 cytokine gene in our study cohort showed that, when the allele frequencies of the patients and the control group were examined alone, there were no significant differences in the genotype and allele frequencies of the IL12B polymorphism between the two groups. In a work in which Hunt et al. [Citation28] investigated the relationship between autoimmune hypothyroidism and 15 polymorphisms in nine cytokine gene, it was shown that were no significant differences between the study groups for IL-12 and IL-10 polymorphisms and sub-group analysis revealed no association with clinical parameters of disease. Similarly, the IL-12p40 polymorphism did not seem to play a major role on genetic susceptibility to autoimmune thyroid diseases in a Japanese population.[Citation29,Citation30]
However, when we analysed the serum levels of IL-12p40 in HT patients and controls according to the genotype of 3’UTR A/C IL12B polymorphisms, we found significantly higher serum level of IL-12p40 for the AA genotype in HT in comparison with AA healthy individuals. On the basis of this finding, it could be supposed that the carriers of the AA genotype of IL12B, with their higher production if Il-12, may more frequently develop HT.
Another cytokine that was investigated in our study was IL-10. This cytokine has dual anti-inflammatory and immunoregulatory roles. We observed a significant increase in the serum levels of IL-10 in hypothyroid HT patients compared to euthyroid HT and controls. In addition, we detected lower serum levels of IL-10 in the group of HT patients treated with levothyroxine as compared to those in the group of hypothyroid patients. At the same time, the role of IL-10 in HT development is still unclear and the data are scarce. Takeoka et al. [Citation31] examined the serum concentrations of IL-10 in patients with autoimmune thyroid diseases and concluded that IL-10 was not related to the severity of HT. Phenekos et al. [Citation9] did not detect IL-10 in the sera of HT patients. In a recent study, Kristensen et al. [Citation15] examined the differentiation of B cells into IL-10-producing cells and found that autoimmune thyroiditis is not associated with reduced frequency of IL-10+ B cells. Moreover, Yu et al. [Citation16] demonstrated that IL-10 influences the susceptibility to experimental autoimmune thyroiditis. In general, our results support the hypothesis that IL-10 plays a critical role in the development and progression of autoimmune processes in HT. Considering the increased circulating levels of IL-10 in the sera of hypothyroid HT patients, it is possible that the cytokine production may influence the clinical presentation of the disease.
The rs1800896 polymorphisms of the promoter region that belongs to the IL-10 cytokine gene were also examined in our study. The comparative analysis of the IL-10 −1082 A/G allele frequencies of the patients and the control group revealed a significantly higher frequency of the homozygous GG genotype in HT patients. Baki et al. [Citation32] as well as Hunt et al. [Citation28] did not report differences in the G allele frequency in HT patients compared to controls. Inoue et al. [Citation33] studied the functional −592 A/C polymorphism in the gene encoding IL-10 and reported that the CC genotype, which is associated with higher production of IL-10, was more frequent in patients with severe HT than in those with mild HT. These collective data demonstrate that the −1082 A/G polymorphism in the IL-10 gene alone has a contradictory impact on the susceptibility to HT. However, in our study, the stratification of patients based on the −1082 A/G SNP genotype revealed a significantly enhanced level of circulating IL-10 in hypothyroid patients with a GG genotype compared to those with AA and AG genotypes. In addition, significant differences between circulating IL-10 quantity in hypo- and euthyroid patients with GG genotypes were detected. In contrast, the AA and AG genotypes did not reveal differences in the circulating IL-10 among patients with HT in different stages of disease.
Our data demonstrate that the production of IL-10 and IL-12 in HT patients depends on the combined effect of the 1082 A/G and 3’UTR A/C polymorphisms of the cytokine genes. This interaction may determine the magnitude of the immune reaction and the severity of the clinical presentation of the disease and development of hypothyroidism. However, we believe that the interactions of IL-12 and IL-10 and their role in the clinical course of autoimmune thyroiditis might also be influenced by the serum levels of other regulatory cytokines and cytokines from the Th17 cascade. Future studies are needed to show whether the polymorphisms in the genes of other regulatory and Th17 cytokines are also involved in the clinical expression of the severity of autoimmune HT in Bulgarian patients.
Conclusions
On the basis of our results, it was shown that the IL-12p40 and IL-10 serum level in patients with HT demonstrated significant differences depending on the genotype and HT stages. Our data provide evidence that enhanced serum levels of IL-10 in combination with enhanced IL-12p40 can be considered to be associated with hypothyroidism in HT development.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Weetman AP. Immunopathogenesis of autoimmune thyroiditis. Eur Thyroid J. 2012;1:243–250.
- Ajjan RA, Weetman AP. Cytokines in thyroid autoimmunity. Autoimmunity. 2003;36:351–359.
- Ganesh BB, Bhattacharya P, Gopisetty A, et al. Role of cytokines in the pathogenesis and suppression of thyroid autoimmunity. J Interferon Cytokine Res. 2011;31:721–731.
- Cogni G, Chiovato L. An overview of the pathogenesis of thyroid autoimmunity. Hormones. 2013;12:19–29.
- Hackett TL, Holloway R, Holgate ST, et al. Dynamics of pro-inflammatory and anti-inflammatory cytokine release during acute inflammation in chronic obstructive pulmonary disease: an ex vivo study. Respiratory Res [Internet]. 2008 [cited 2016 Jan 13];9:47. Available from: http://dx.doi.org/10.1186/1465-9921-9-47
- Trinchieri G, Pflanz S, Kastelein RA. The IL-12 family of heterodimeric cytokines: new players in the regulation of T cell responses. Immunity. 2003;19:641–644.
- Gately MK, Desai BB, Wolitzky AG, et al. Regulation of human lymphocyte proliferation by a heterodimeric cytokine, IL-12 (cytotoxic lymphocyte maturation factor). J Immunol. 1991;147:874–882.
- Gerenova J, Manolova I, Gadjeva V. Hashimoto's disease – involvement of cytokine network and role of oxidative stress in the severity of Hashimoto's thyroiditis. In: Springer D, editor. A new look at hypothyroidism. Rijeka: InTech; 2012. p. 91–124.
- Phenekos C, Vryonidou A, Gritzapis AD, et al. Th1 and Th2 serum cytokine profile characterize patients with Hashimoto's thyroiditis (Th1) and Graves’ disease (Th2). Neuroimmunomodulation. 2004;11:209–213.
- de Waal Malefyt R, Yssel H, de Vries JE. Direct effects of IL-10 on subsets of human CD4+ T cell clones and resting T cells. Specific inhibition of IL-2 production and proliferation. J Immunol. 1993;150:4754–4765.
- Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–2095.
- Ding L, Linsley PS, Huang LY, et al. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J Immunol. 1993;51:1224–1234.
- Rousset F, Garcia E, Defrance T, et al. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc Natl Acad Sci USA. 1992;89:1890–1893.
- Nielsen CH, Hegedüs L, Rieneck K, et al. Production of interleukin (IL)-5 and IL-10 accompanies T helper cell type 1 (Th1) cytokine responses to a major thyroid self-antigen, thyroglobulin, in health and autoimmune thyroid disease. Clin Exp Immunol. 2007;147:287–295.
- Kristensen B, Hegedüs L, Lundy SK, et al. Characterization of regulatory B cells in Graves' disease and Hashimoto's thyroiditis. PLoS One [Internet]. 2015 [cited 2016 Jan 13];10(5):e0127949. Available from: http://dx.doi.org/10.1371/journal.pone.0127949
- Yu Z, Liu T, Liu S, et al. Interleukin 10 influences susceptibility to experimental autoimmune thyroiditis independently of the H-2 gene. Int J Mol Med. 2015;35:413–424.
- Hall MA, McGlinn E, Coakley G, et al. Genetic polymorphism of IL-12 p40 gene in immune-mediated disease. Genes Immun. 2000;1:219–224.
- Morahan G, Huang D, Ymer SI, et al. Linkage disequilibrium of a type 1 diabetes susceptibility locus with a regulatory IL12B allele. Nat Genet. 2001;27:218–221.
- Seegers D, Zwiers A, Strober W, et al. A TaqI polymorphism in the 3’UTR of the IL-12 p40 gene correlates with increased IL-12 secretion. Genes Immun. 2002;3:419–423.
- Stanilova S, Miteva L. Taq-I polymorphism in 3’UTR of the IL-12B and association with IL-12p40 production from human PBMC. Genes Immun. 2005;6:364–366.
- Karimabad MN, Arababadi MK, Hakimizadeh E, et al. Is the IL-10 promoter polymorphism at position-592 associated with immune system-related diseases? Inflammation. 2013;36:35–41.
- Westendorp RG, Langermans JA, Huizinga TW, et al. Genetic influence on cytokine production and fatal meningococcal disease. Lancet. 1997;349:170–173.
- Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146.
- Zhang JA, Zhang J, Xu L, et al. Measurement of IL-12 and IL-18 in sera of patients with autoimmune thyroid disease. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2006;22:630–632.
- Guclu F, Ozmen B, Kirmaz C, et al. Down-regulation of the auto-aggressive processes in patients with hypothyroid Hashimoto's thyroiditis following substitutive treatment with L-thyroxine. Eur Cytokine Netw. 2009;20:27–32.
- Bonita RE, Rose NR, Rasooly L, et al. Kinetics of mononuclear cell infiltration and cytokine expression in iodine-induced thyroiditis in the NOD-H2h4 mouse. Exp Mol Pathol. 2003;74:1–12.
- Kimura H, Tzou SC, Rocchi R, et al. Interleukin (IL)-12-driven primary hypothyroidism: the contrasting roles of two Th1 cytokines (IL-12 and interferon –γ). Endocrinology. 2005;146:3642–3651.
- Hunt PJ, Marshall SE, Weetman AP, et al. Cytokine gene polymorphisms in autoimmune thyroid disease. J Clin Endocrinol Metab. 2000;85:1984–1988.
- Ikeda Y, Yoshida W, Noguchi T, et al. Lack of association between IL-12B gene polymorphism and autoimmune thyroid disease in Japanese patients. Endocr J. 2004;51:609–613.
- Yang JM, Nagasaka S, Yatagai T, et al. Interleukin-12p40 gene (IL-12B) polymorphism and Type 1 diabetes mellitus in Japanese: possible role in subjects without having high-risk HLA haplotypes. Diabetes Res Clin Pract. 2006;71:164–169.
- Takeoka K, Watanabe M, Matsuzuka F, et al. Increase of serum interleukin-10 in intractable Graves' disease. Thyroid. 2004;14:201–205.
- Baki M, Akman FE, Vural P, et al. The combination of interleukin-10 −1082 and tumor necrosis factor α −308 or interleukin-6 −174 genes polymorphisms suggests an association with susceptibility to Hashimoto's thyroiditis. Int Immunopharmacol. 2012;4:543–546.
- Inoue N, Watanabe M, Wada M, et al. IL-10 −592 A/C polymorphism is associated with severity of Hashimoto's disease. Cytokine. 2013;64:370–374.
