ABSTRACT
Hypocrellins is regarded as the new generation of potential photosensitizer in the photodynamic therapy. However, the current production of HA in large quantity is limited, whether extracted from the fruit bodies of Hypcrella bambusae and Shiraia bambusicola, or its fermented mycelia. To break through the bottleneck, we screened over 350 fungal strains from the uncommon moso bamboo (Phyllostachys edulis) seeds, and acquired 14 isolates of Shiraia sp. Hypocrellins were generally extracted from the stroma of S. bambusicola, and however the hypocrellin-producing strains in our study were identified as endophytic fungi, where several of them exhibited significantly high content of hypocrellins. One of them, a newly isolated Shiraia sp. strain ZZZ816 was selected for further research. The hypocrellin production conditions of the strain ZZZ816 under submerged fermentation were optimized by one-factor-at-a-time and orthogonal experiment design. The optimum parameters for the quid fermentation were determined as follows: incubation time (144 h), initial pH (6.0), volume of medium (100/250 mL), rotary speed (130 rpm), mycelial age (60 h) and inoculation level (10%). The optimum culture medium constituents were determined as follows: yeast extract 20g/L, malt sugar 40g/L, FeSO4·H2O 0.5g/L, urea 4.0g/L, MgS04·7H20 0.5g/L and initial pH 5.0. Using submerged fermentation of the optimum production conditions, the maximum content of hypocrellin A from the strain ZZZ816 was 921.6 mg/L, more than the other industrial strains published in the previous reports. The results demonstrated that Shiraia sp. strain ZZZ816 could enhance the hypocrellin production tremendously with optimizing the fermentation progress.
Introduction
Perylenequinones are regarded as a group of reactive oxygen species (ROS)-generating photosensitizers with a basic 3,10-dihydroxy-4,9-perylenequinone chromophore, but differing mainly in side chains. Hypocrellins, mainly composing of hypocrellin A (HA) and hypocrellin B (HB), is a type of naturally occurring perylenequinonoid pigments from filamentous fungi and a mixture of rapidly interconverting diastereomers. It has been proved that HA and HB had similar photosensitizing characteristics, generating active oxygen at the chief target of the cellular membrane and the photosensitized damage were dependent on the structure of different hypocrellins. Furthermore, hypocrellin could vary from form A to form B under the change of pH, and hypocrellin A usually has higher production level and plays more important role than hypocrellin B.[Citation1–Citation3] Because of their unique architectures and desirable photoactivated biological profiles, hypocrellins have also attained a great deal of attentions as a new generation of potential photosensitizers in the photodynamic therapy and with excellent light-induced anticancer and antiviral activities, especially of its antiviral activity against the human immunodeficiency virus.[Citation4–6] Hypocrellins have more advantages than the present photosensitizer Photofrin II and are regarded as potentially better photosensitizing agents.[Citation7]
In addition to pharmaceutical industry, hypocrellins also have a wide range of potential application fields like agricultural, cosmetic, food and feed industries. Hypocrellins inhibited the growth of phytopathogens (Arthrinium sacchari, A. Phaeospermum, B. fuckeliana and T. cucumeris) considerably and displayed low toxicity, low aggregation and rapid metabolism in vivo, as a promising green pesticide.[Citation8] Similarly, owing to anti-inflammatory activity, analgesic activity and low toxicity, hypocrellins also take a vital part in the skin care product “Jianfushuang”, which has been granted as the earliest patent in the cosmetic industries in China (Publication Number: CN85103693). The recent study has been carried out that the extent of encapsulation from hypocrellin liposome could be achieved by the best way macroporous resin column chromatography.[Citation9] For expanding the extent of hypocrellin application in the food and feed industries, the inclusion complex of HA with hydroxypropyl-β-cyclodextrin was prepared to improve the water solubility and it is very likely that hypocrellins could enhance the appearance, quality and health care value of food or feed as herbal/natural supplements.[Citation10,Citation11]
Hypocrellins were first isolated from stromata of Hypcrella bambusae in the 1980s, and then discovered in Shiraia bambusicola. Unfortunately, because of the fruit bodies gathered from the wild, the corresponding production and quality were greatly influenced by climate, distribution and collecting time, so the natural resource of hypocrellins is now in short supply and cannot meet its extensive potential applications. To overcome the limit of resource, there is an alternative way extraction of hypocrellins from anamorphic mycelium of the fungi S. bambusicola by solid-state fermentation or submerged fermentation methods.[Citation12,Citation13] Many researches aimed to enhance the production of hypocrellins from mycelium, including screening of industrial strains,[Citation12,Citation14,Citation15] optimization of extraction technology,[Citation16,Citation17] control of fermentation process [Citation13,Citation18] and search of microbial elicitors.[Citation19,Citation20]
The fermentation process takes a vital part in the yield of the ultimate products, and generally requires a statistical method to analyse the effects of multiple variables simultaneously, for minimizing the assay time, cutting the cost of experiments, optimizing the procedure of mycelium cultivation and saving manual labour. Orthogonal array design (OAD) is a type of fractional factorial design, where the series of trials were assigned by an orthogonal array. The results obtained from experimental procedures can be managed by analysis of variables and direct comparative observation. This method has been applied in many different fields, which contained mass production of bioactive compounds from filamentous fungi.[Citation21]
The present work attempts to investigate hypocrellin-producing strains of Shiraia sp. from new plantation – moso bamboo, and achieve the higher yield of hypocrellins by optimization of cultivation conditions.
Materials and methods
Chemicals and reagents
Standard hypocrellin was purchased from Yunnan Institute of Microbiology (Yunnan, China). The methanol, acetonitrile and isopropanol used for chemical purification were purchased from Fisher Scientific Co (Fair Lawn, USA) and high-performance liquid chromatography (HPLC) grade level. Other chemicals used in the study were of analytical grade.
Endophytic fungi and culture conditions
Fresh and healthy seeds were collected from moso bamboo in one plantation (110° 17’ ∼110° 47’ E,25° 04’ ∼25° 48’ N) in Guilin City in the Guangxi Zhuang Autonomous Region in China. More than 100 seeds were randomly selected for fungal isolations. They were surface-sterilized by 75% ethanol for 30 sec, 5% NaOCl for 10 min and rinsed with sterile water. After sterilization, each seed was cut into three fragments and these samples were individually planted onto 2% potato dextrose agar media (PDA, containing (g/L): potato 200, dextrose 20 and agar 20; pH 6.0.), at 20 °C without light. The fungal cultures isolated from seeds were recorded and deposited in the China Forestry Culture Collection Center (CFCC).
The fresh mycelia of different endophytic fungi were grown on plates at 25 ˚C for more than seven days. Five plugs (6 mm in diameter) of growing culture plus the adhering mycelium were subsequently added to 250 mL Erlenmeyer flasks containing 100 mL of Potato Dextrose Broth media (PDB, containing (g/L): potato 200 and dextrose 20; pH 6.0. All liquid cultures were kept at 25 ˚C for 7–10 d with shaking (150 rpm). The fermentation of each fungus was filtered to separate the filtrates from the mycelia.
DNA extraction, amplification, sequencing and molecular identification
Fungal mycelia from subcultured colonies were scraped from the surface of the agar and frozen at −20 °C for one night in order to extract DNA. Extractions were performed using E.Z.N.A.™ Fungal DNA Mini Kit (Omega Biotech Inc., Norcross, United States) and the target regions of ITS rDNA were amplified by ITS1-F/ITS4 (ITS, internal transcribed spacer).[Citation22] The polymerase chain reaction (PCR) mixture (25 μL, total volume) contained 0.5 μL template, 0.5 μL of each primer (25 μM each), 12.5 μL 2× MasterMix (including 10× buffer, deoxyribonucleoside triphosphates (dNTP) and Taq polymerase) and ddH2O (Tiangen Biotech Co. Ltd., Beijing, China). Thirty-five cycles consisting of denaturation at 94 °C (30 s), annealing at 50 °C (45 s) and extension at 72 °C (60 s) were run and the final extension step at 72 °C for 7 min was performed using Techne TC512 (Keison Products Co. Ltd., Beijing, China). Finally, the purified amplicons were sequenced by Invitrogen Biotechnology Co. Ltd. (Beijing, China). To identify the isolates, sequences were subjected to a BLAST search with the NCBI database (http://www.ncbi.nlm.nih.gov/). Only matches of sequences published in journals were used. Priority was given to sequences derived from authoritative material, such as ex-type or ex-epitype cultures. The sequences of the present study were also deposited at GenBank.
Extraction of cell-associated hypocrellin from Shiraia sp.
After cultivation for several days, the fermented mycelia were harvested by centrifugation at 12 000 rpm for 10 min at 4 °C after being rinsed three times with distilled water. The mycelia was then dried out through vacuum freeze drying and grounded into powder. To extract with chemicals, the mycelium pellets (0.1 g) were accurately weighed and extracted with 4 mL acetone by Soxhlet extraction for 12 h at 70–80°C. The acetone was dried under vacuum condition at rotavapor at 45 °C. At last, the residues were redissolved in 10 mL acetone and used to determine the hypocrellin content.
UV–Vis spectrophotometer and high-performance liquid chromatography (HPLC) analysis
The samples were diluted according to a certain percentage and were identified by the Lambda-35 UV–Vis Spectrophotometer (PerkinElmer Co., Norwalk, USA) in the wavelength of 300–600 nm. The standard hypocrellin was used as a control.
The Agilent 1200 HPLC system was used to analyse the content of hypocrellin from extracts, and had been equipped with a Zorbax Extend – C18 column (5 μm × 4.6 mm × 150 mm). The operating condition was at a flow rate of 1.0 mL/min and with the acetonitrile/water (40/60 v/v) as mobile phase. For each sample, the injection volume was 10 μL and external standard method applied in the quantitative analysis with standard hypocrellin as standards by the detection wavelength of 465 nm.
Cultivation condition optimization for hypocrellin production
To optimize the course of hypocrellin production, the process of submerged fermentation was conducted on the basal cultivation condition (fermentation time 144 h, culture medium loading volume 100 mL, revolution 150 rpm, mycelial age 60 h and inoculation level 10%) from the basal medium of initial pH 7.0. The influence of fermentation time (72, 96, 120, 144, 168 and 192 h), culture medium loading volume (40, 60, 80, 100, 120 and 140 mL), revolution (90, 110, 130, 150, 170 and 210 rpm), mycelial age (36, 40, 44, 48, 52, 60, 64 and 70 h), inoculation level (2.5, 5, 7.5, 10, 15 and 20%) and pH (3.0, 4.0, 4.5, 5.0, 5.5, 6.0, 6.5, 7.0, 7.5 and 8.0) were assessed by analysing the hypocrellin yield.
Culture medium optimization for hypocrellin production
To test the effect of medium composition on mycelial growth and hypocrellin yield in shake flask cultures, the carbon sources (maltose, sorbitol, glucose, sucrose, galactose, xylose, starch, mannitol, lactose and rhamnose), nitrogen sources (organic nitrogen sources: polypeptone, yeast extract, tryptone, beef extract, peptone, casein and potato juice; inorganic nitrogen sources: NH4Cl, NaNO3, (NH4)2SO4, NH4NO3, KNO3 and urea), inorganic salt (KH2PO4, K2HPO4, MgSO4, VB1, VB2, ZnSO4 and FeSO4·7H2O) were selected respectively for the influence of changes in hypocrellin production.
An OAD was employed to optimize the culture medium composition for hypocrellin yield. The processing parameters, yeast extract (A), maltose (B), urea (C), pH (D) and FeSO4·7H2O (E) were studied and optimized by a four-factor, five-level OAD test, L16(4)5 ().
Table 1. Taxon designation of Shiraia species from Moso bamboo seeds based on sequence data from the internal transcribed spacer regions of nuclear ribosomal DNA (ITS rDNA).
Statistical analysis
All the experiments of fermentation treatments were set in a complete randomized design with three replicates, and the mean results of three sets of observations were expressed as mean ± SD values. Statistical analysis of the data was conducted by the one-way analysis of variance (ANOVA) using SPSS 19.0 for Windows (SPSS Inc., Chicago, USA). The means were compared by Student–Newman–Keuls test method and p < 0.05 was considered to indicate statistical significance.
Results and discussion
Over 350 distinct strains were isolated from moso bamboo seeds, and the nuclear ribosomal ITS (nr ITS) DNA sequence analyses were conducted to confirm the identification of these endophytic fungi. Among them, the representatives of Shiraia sp. were selected from each morphotype and the ITS region was sequenced for these isolates (14 in total). ITS sequences were compared with those deposited in GenBank using a BLAST search (http://www.ncbi.nlm.nih.gov/), and all of the 14 isolates represented high similarity with Shiraia spp. ().
Table 2. Orthogonal design of culture medium optimization.
It is necessary to confirm whether hypocrellins were synthesized by fungal endophytes from moso bamboo. Rapid identification of perylenequinonoid derivatives was carried out by chemistry colour response. As evident from (a), the pigments from Shiraia sp. were red in acid condition, turned dark purple when FeCl3 was added and became green under alkaline solution. (b) displayed that the same full-wave spectra appeared in the samples (isolate ZZZ816) and HA standard, which demonstrated that the bright red bioactive agents were produced by Shiraia spp. under submerged fermentation. The accurate quantitative results of HA were analysed by HPLC, and the retention time of authentic HA standard was 19.115 min ((c)). At the same retention time, we screened the production of HA from all cultural Shiraia species (), and highest yield strain ZZZ816 was used for optimization.
Figure 1. (a) Colour reaction of the pigments produced by Shiraia sp.: (1) pigment acetone extract with FeCl3 solution (1 mol/L, 0.1 mL); (2) pigment acetone extract with hydrochloric acid solution (1 mol/L, 0.1 mL); (3) pigment acetone extract with sodium hydroxide solution (1 mol/L, 0.1 mL). (b) UV–vis absorbing spectrum of the main pigment components: (1) standard hypocrellin A; (2) hypocrellins produced by Shiraia sp. 816. (c) HPLC chromatograms of authentic compound hypocrellin A: (1) standard hypocrellins (HA, hypocrellin A; HB, hypocrellin B); (2) cell extracts of Shiraria sp. 816.
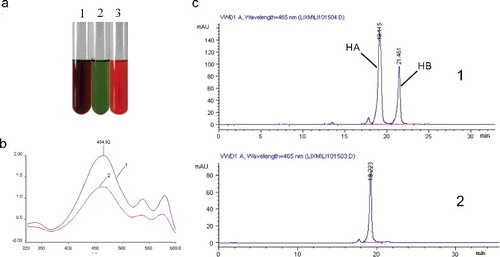
Table 3. Hypocrellin A produced by Shiraia species from Moso bamboo seeds under submerged fermentation.
The effects of cultivation conditions on HA and DCW (dry cell weight) were investigated for the first time and the results presented in indicated that HA production was simultaneously affected by incubation time, initial pH, volume of medium, rotary speed, mycelial age and inoculation level.
Figure 2. Effects of various parameters from cultivation condition on hypocrellin A production within the single-factor experiments. DCW (♦), hypocrellin A (▪). Data are shown as mean ± SD (n = 3).
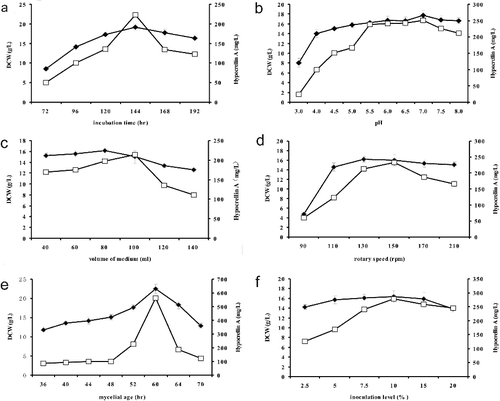
The curves from (a) demonstrated that accumulation of HA increased quickly after 120 h of culturing, and the maximum yield (222.79 mg/L) appeared on the 144 h. When the cultivation time was extended after 144 h, the production of HA decreased remarkably. The growth curve profile of DCW was similar to that of HA, but the growth rate was slower than that of HA.
The effect of different initial culture pH (3.0, 4.0, 4.5, 5.0, 5.5, 6.0, 6.5, 7.0, 7.5 and 8.0) was examined in parallel experiments, and both the content of DCW and HA reached their maximum at initial pH 5.5–7.0 ((b)). However, when strain ZZZ816 has grown at initial pH 6.5–8.0, there would be no crucial by-product of mannitol extracted from filtrates.
The volume of liquid medium and rotary speed also had significant influence on the DCW and HA content, and the results are illustrated in (c–d). The optimal yield of HA was obtained in the 250-mL Erlenmeyer flask containing 100 mL liquid medium, but the DCW decreased gradually with 80–140 mL ((c)). The results from (d) indicated that higher HA production appeared at the rotary speed of 180 rpm, and greater biomass anticipated the rotary speed of 130 rpm.
In order to monitor the effect of inoculation procedures on fungal biomass and the yield of HA, mycelial age and inoculation level were optimized respectively by one-factor-at-a time. The results from (e,f) presented that the most DCW and HA were harvested at mycelial age of 60 h and inoculation level of 10%.
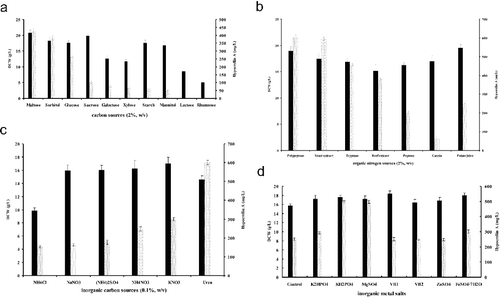
In the present work, one-factor-at-a-time and orthogonal matrix methods were applied to optimize the medium for HA production. To investigate the effects of the substrates on the mycelial growth and HA production, a basal medium included changing one independent variable (carbon, nitrogen or mineral source) while fixing the others at contain levels. Ten carbon sources (maltose, sorbitol, glucose, sucrose, galactose, xylose, starch, mannitol, lactose and rhamnose) were tested in the shake flask cultures by the defined medium, and the highest HA yield of ZZZ816 and mycelial biomass was obtained on the defined media with 5% maltose ((a)). In this experiment, the influence of several organic (polypeptone, yeast extract, tryptone, beef extract, peptone, casein, potato juice) ((b)) and inorganic nitrogen (NH4Cl, NaNO3, (NH4)2SO4, NH4NO3, KNO3 and urea) ((c)) sources were assessed, and the addition of yeast extract or urea produced comparably higher amounts of HA. Considering that different minerals also have a great effect on mycelial growth and HA, various inorganic metal salts and trace elements were tested, and KH2PO4 (0.1%), MgSO4·7H20 (0.05%) and FeSO4·H2O (0.05%) were chosen to optimize the medium composition ((d)).
Figure 3. Effect of different carbon sources (a), organic nitrogen sources (b), inorganic nitrogen sources (c) and inorganic salts (d) on DCW (■) and hypocrellin A content (░). Data are shown as mean ± SD (n = 3). Statistical analysis of the data was performed with SSPS 18.0 using Student–Newman–Keuls test for determining significant difference (p < 0.05).
To further improve the HA production, an orthogonal L16(4)5 test design was applied to get the best concentration of culture medium composition. Maltose, yeast extracts, urea, KH2PO4 and pH are regarded as the most important factors that affect the main ingredients and the field of HA by submerged fermentation. Total evaluation index of OAD was analysed by statistical software SSPS 13.0 (). The results from displayed that there are great yield differences among each set in the extraction process. As shown in , the yield of HA depended largely on maltose (R = 20.83) and pH (R = 20.824). Urea also had a significant impact on the yield. For influence of extraction factors and levels on yield of HA, decreased order of effort of all factors was A > E > C > D > B. The optimal medium composition (A2B3C3D1E2) for generating highest HA yield was obtained, as follows: yeast extract 20 g/L, maltose 40 g/L, urea 4.0 g/L and initial pH 5.0.
Table 4. Results of orthogonal for culture medium optimization.
The optimum culture medium constituents for the production of HA by the strain ZZZ816 were determined as follows: yeast extract 20 g/L, malt sugar 40 g/L, FeSO4·H2O 0.5 g/L, urea 4.0 g/L, MgS04·7H20 0.5 g/L and initial pH 5.0. The optimum parameters for the submerged fermentation were as follows: incubation times (144 h), initial pH (6.0), volume of medium (100 mL/250 mL), rotary speed (130 rpm), mycelial age (60 h) and inoculation level (10%).
Using the optimum production conditions under submerged fermentation, the maximum concentration of HA from the strain ZZZ816 was 921.63 mg/L (DCW 9.98 g/L), 3.55-fold on the preliminary fermentation, as the highest yield among all recorded in the published reports.
In our previous work, over 500 fungal strains were isolated from the uncommon moso bamboo (Phyllostachys edulis) seeds and branches, and it is noticeable that many strains of Shiraia sp. were identified as fungal endophytes.[Citation8] In contrast to hypocrellin biosynthesis strains generally originating from the fruiting body of S. bambusicola Henn., we screened the hypocrellin-producing strains based on these endophytic fungi, and some of them exhibited significantly high content of hypocrellins, compared to the unmodified strains from the traditional resource of the fruiting body. As far as we know, it is the first time that the industrial strains of Shiraia sp. were isolated from the novel plantations, and in the present study, we demonstrated that it could improve tremendously the production efficiency of the active agent in further research.
In the previous reports, the different fermentation parameters proved to be effective on the production of HA of Shiraia sp. The various carbon (glucose, lactose, maltose, sucrose, mannitol, xylose and fructose) and nitrogen (yeast extract, peptone, urea, (NH4)2SO4, NaNO3, casein and NH4NO3) sources played significant roles in the enhancement of hypocrellin on the laboratorial scales, and higher hypocrellin-yield was obtained with glucose and (NH4)2SO4, as only one carbon and nitrogen source respectively.[Citation13] The different levels of metal ions (Cu2+, Fe3+, Cr3+ and Ca2+) added to the submerged fermentation medium culture, have also been screened for the highest hypocrellin yield, and 0.005% of Cr3+ content, 0.003%–0.005% of Fe3+ content, 0.03% of Ca2+ content and 0.03% of Cu2+ content were favourable to the hypocrellin production.[Citation23] Except the optimization of medium culture composition, several surfactants (sodium dodecyl sulfate [SDS], Tween 40, Tween 80, Triton X-114 and Triton X-100) have been applied to improve hypocrellin production under submerged fermentation, and the experiments have finally reached the anticipated goal by Triton X-100.[Citation20] Furthermore, it has been proved that addition of bacterial and fungal elicitor into a Czapek medium is effective for advancing the efficiency of fermentation process.[Citation19] In the present study, we also optimized the cultivation condition and culture medium composition, and the results displayed that HA increased remarkably by the appropriate parameters for the liquid fermentation.
In recent reports, a mutant strain of S. bambusicola by UV-62 mutation was used to produce the hypocrellin, and the final yield was about 196.9 mg/L.[Citation13] Cai et al.[Citation20] used Shiraia sp. SUPER-H168 to produce hypocrellin with a Czapek medium containing Triton X-100, and the yield of overall hypocrellin was about 780.6 mg/L. Microbial elicitors had taken effect on the production of hypocrellin by the wild isolates of S. bambusicola, and the experimental value of hypocrellin production was 102.60 mg/L.[Citation19] Compared with the published achievements, our optimum content of hypocrellin is significantly high among them, which raised the prospect that a new S. bambusicola strain ZZZ816 is of great potential for reducing the cost of hypocrellin production and expanding the application range.
Conclusion
Hypocrellins, one type of naturally occurring perylenequinonoid pigments with a basic 3,10-dihydroxy-4,9-perylenequinone chromophore, play the important role in the photodynamic therapy. To break through the yield bottleneck, a newly isolated Shiraia sp. strain ZZZ816 was selected for further research. By the optimization of submerged fermentation parameters and medium constituents, the maximum content of HA reached 921.6 mg/L, significantly different from the other industrial strains. At the base of high-efficiency production, the mutagenesis of spores and the addition of elicitors are anticipated to further increase the final yield.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Zhang MH, Chen S, An JY, et al. Separation and identification of hypocrellin-B and fatty-acids as ingredients in Hypocrella bambusae (b et br) sacc. Chin Sci Bull. 1989;34:1008–1014.
- Zhao K, Jiang L. Conversion of hypocrellin A in alkaline and neutral media. Youji Huaxue. 1989;9:252–254.
- Nenghui W, Zhiyi Z. Relationship between photosensitizing activities and chemical structure of hypocrellin A and B. J Photoch Photobio B. 1992;14:207–217.
- Shigematsu H, Suzuki M, Takahashi T, et al. Aberrant methylation of HIN-1 (high in normal-1) is a frequent event in many human malignancies. Int J Cancer. 2005;113:600–604.
- Mulrooney CA, O'Brien EM, Morgan BJ, et al. Perylenequinones: isolation, synthesis, and biological activity. Eur J Org Chem. 2012;21:3887–3904.
- Olivo M, Chin W. Perylenequinones in photodynamic therapy: cellular versus vascular response. J Environ Pathol Toxicol Oncol. 2006;25:223–237.
- Zhenjun D, Lown JW. Hypocrellins and their use in photosensitization. Photochem Photobiol. 1990;52:609–616.
- Shen X, Zheng D, Gao J, et al. Isolation and evaluation of endophytic fungi with antimicrobial ability from Phyllostachys edulis. Bangl J Pharmacol. 2012;7:249–257.
- Qiu Y, Lu Z, Sun P. Preparation of hypocrellin liposome and determination of its extent of encapsulation. China Surfact Det Cosmet. 2011;41:422–425.
- Su YJ, Rao SQ, Cai YJ, et al. Preparation and characterization of the inclusion complex of hypocrellin A with hydroxypropyl-beta-cyclodextrin. Eur Food Res Technol. 2010;231:781–788.
- Su YJ, Si SH, Qiao LW, et al. The effect of a hypocrellin A enriched diet on egg yolk quality and hypocrellin A distributions in the meat of laying hens. Eur Food Res Technol. 2011;232:935–940.
- Liang XH, Cai YJ, Liao XR, et al. Isolation and identification of a new hypocrellin A-producing strain Shiraia sp. SUPER-H168. Microbiol Res. 2009;164:9–17.
- Yang HL, Xiao CX, Ma WX, et al. The production of hypocrellin colorants by submerged cultivation of the medicinal fungus Shiraia bambusicola. Dyes Pigments. 2009;82:142–146.
- Meng L, Sun P, Tang H, et al. Endophytic fungus Penicillium chrysogenum, a new source of hypocrellins. Biochem Syst Ecol. 2011;39:163–165.
- Dong T, Pan W, Zhao Y, et al. Screening of higher hypocrellin A with strains of Shiraia bambusicola by genome-shuffling. Shengwu Jiagong Guocheng. 2012;10:25–29.
- Fang WU, Jiayou LI. Extraction of hypocrellin from the solid fermentation substrate with microwave-assisting. Acta Agr Zhejiang. 2011;23:604–606.
- Su YJ, Yin XY, Rao SQ, et al. Natural colourant from Shiraia bambusicola: stability and antimicrobial activity of hypocrellin extract. Int J Food Sci Tech. 2009;44:2531–2537.
- Cai YJ, Liang XH, Liao XR, et al. High-Yield hypocrellin A production in solid-state fermentation by Shiraia sp. SUPER-H168. Appl Biochem Biotech. 2010;160:2275–2286.
- Du W, Liang Z, Zou X, et al. Effects of microbial elicitor on production of hypocrellin by Shiraia bambusicola. Folia Microbiol. 2013;58:283–289.
- Cai Y, Liao X, Liang X, et al. Induction of hypocrellin production by Triton X-100 under submerged fermentation with Shiraia sp. SUPER-H168. N Biotechnol. 2011;28:588–592.
- Strobel R, Sullivan G. Experimental design for improvement of fermentations. Manual Ind Microbiol Biot. 1999;2:80–93.
- Gardes M, Bruns TD. ITS primers with enhanced specificity for basidiomycetes‐application to the identification of mycorrhizae and rusts. Mol Ecol. 1993;2:113–118.
- Xiang X, Zheng A, Xie L. Effect of different metal ions for submerged culture of Shiraia bambusicola. Chin Tradit Herbal Drugs. 2011;42:164–166.
