?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In this study, the antimicrobial and antioxidant activities of root methanolic extracts of Glycyrrhiza glabra var. glandulifera (Waldst. & Kit.) Boiss. (Fabaceae) were investigated. Plant samples were collected from different habitats in the East Mediterranean part of Turkey. The plant extracts were evaluated for antimicrobial activities against nine bacterial and two yeast strains using disc-diffusion and minimum inhibitory concentration methods. The antioxidant activity was determined by using the DPPH (1,1-diphenyl-2-picrylhydrazyl) method. The antimicrobial assays indicated that the plant root extracts were more effective against Gram-positive bacteria than against Gram-negative ones. In addition, the extracts had higher antimicrobial effect against Candida species than against bacteria. The extracts showed good antioxidant activity, with a median inhibitory concentration (IC50) in the range of 588 ± 0.86 µg/mL to 2190 ± 1.73 µg/mL. Results indicated that different environmental conditions in each habitat might affect the contents of chemical compounds and biological activity in the natural licorice populations of. This study also supported the traditional use of licorice and as well as suggested that it may also be its beneficial role in the treatment of other infections. The obtained results indicated that different environmental conditions in each habitat might affect the contents of chemical compounds and the biological activity in the natural licorice populations.
Introduction
The genus Glycyrrhiza includes well-known traditional medicinal plants growing in various parts of the world. The roots of the plants have been medically used by human beings over 4000 years. The Glycyrrhiza genus consists of about 30 species, of which 15 species have been studied so far.[Citation1–3] The roots of Glycyrrhiza glabra (licorice), which is one of the most common species of this genus, contain glycyrrhizin, or glycyrrhizinic acid,[Citation4] glabridin, glabrene, glabrol, licoflavonol, glycerol, licoricone, formononetin, phaseollinisoflavan, hispaglabridin A and B, 3-hydroxy glabrol, 3-methoxy glabridin,[Citation5–11] glabranin isomer, narigenin and lupiwightenone.[Citation12,Citation13] Licorice root extracts have been shown to be beneficial in the treatment of eye diseases, throat infections, peptic ulcers, liver diseases, joint diseases, arthritic conditions, immunodeficiency,[Citation14] cough, tuberculosis, respiratory diseases, cancer, diabetes, endocrine disorders,[Citation15] kidney diseases,[Citation16] bronchitis, asthma, psoriasis, eczema, haemorrhoids,[Citation17] epilepsy, chronic hepatitis, heart diseases [Citation3] and orodental diseases.[Citation18] It has also been shown that extracts help regulate the estrogen–progesterone ratio [Citation19–21] and the gastrointestinal system.[Citation15] However, the effects of environmental variables on the antimicrobial and antioxidant activities have not been sufficiently studied in licorice. The objective of this work was to study the relationship between the ecological characteristics and the biological activities of methanolic extracts of licorice plants growing in different habitats.
Materials and methods
Plant materials
Specimens (roots and rhizomes) of Glycyrrhiza glabra var. glandulifera (Waldst. & Kit.) Boiss. were collected from 15 different localities of the East Mediterranean part of Turkey ( and ). The botanical identification was carried out by Dr V. Altay from the Department of Biology, Faculty of Sciences and Arts, Mustafa Kemal University, based on Davis.[Citation22]
Table 1. List of the localities, habitat types and altitudes of G. glabra var. glandulifera samples.
Preparation of plant extracts
The plant roots were shade-dried at room temperature and then ground into powder. About 20 g of each powdered sample was extracted with 250 mL of methanol for 24 h. The extracts were filtered and concentrated under vacuum to obtain crude extracts.
Micro-organisms
The test organisms included six Gram-positive bacteria [Staphylococcus aureus ATCC 6538, Enterococcus faecalis ATCC 51299, Micrococcus luteus, Bacillus cereus 7064, vancomycin-resistant Enterococcus (VRE) and methycilline resistant Staphylococcus aureus (MRSA)]; three Gram-negative bacteria (Escherichia coli ATCC 11293, Pseudomonas aeruginos and Klebsiella pneumoniae) and two yeast species (Candida krusei ATCC 6258 and Candida parapsilosis ATCC 22019). They were all obtained as pure cultures from the Molecular Biology and Microbiology Laboratory, Department of Biology, Faculty of Arts and Science, Sinop University, Turkey.
Evaluation of antimicrobial activity
The antimicrobial activity of each methanol extract sample was evaluated by using the disc-diffusion method.[Citation23] All bacteria were maintained at −20 °C in nutrient agar (NA) and all yeast, in Sabouraud dextrose agar (SDA, Difco) containing 20% (v/v) glycerol. Before testing, the bacteria were transferred to nutrient broth (NB) and the fungi, to Sabouraud dextrose broth (SDB, Difco), and were cultured overnight at 37 °C. Then, the turbidity was adjusted to equivalent to 0.5 McFarland standards (approximately 108 CFU/mL for bacteria and 105 or 106 CFU/mL for fungi). Aliquots of 100 μL of micro-organisms were spread over the surface of NA plates. Sterile Whatmann No. 1 filter paper discs (6 mm) were soaked with 25 μL of extract residue diluted in the corresponding extract solvent (1000 μg/1 mL of 12.5% dimethyl sulfoxide (DMSO)) and placed on the surface of the freshly inoculated medium. The media were incubated for 24 h at 37 °C. Antibiotic susceptibility discs including bacitracin (0.04 U), ceftazidime (30 μg), imipenem (10 μg), novobiocin (5 μg), polymyxin B (300 U), tetracycline (30 μg), ampicillin (10 μg) and cycloheximide were used as control, and negative controls were 12.5% DMSO, methanol and deionized water. The antimicrobial activity was evaluated by measuring the diameter of the inhibition zones. The experiment was performed in triplicate. The values were expressed as means with standard deviations (±SD).
The minimum inhibitory concentration (MIC) was determined by the serial tube dilution method. Methanol extracts of plants (1000 mg) were dissolved in 12.5% DMSO to obtain initial stock solutions. The stock solutions were stirred into 0.9 mL of NB (for bacteria) and SDB (for fungi) in glass tubes in order to adjust to concentrations of 1000–0.001 mg/mL. All tubes were inoculated with 100 μL of standardized inoculums of each organism and incubated for 24 h at 37 °C (for bacteria) and 48–72 h at 25 ± 1 ˚C (for fungi). The MIC of the extracts was taken as the lowest concentration that showed no growth. The experiment was performed in duplicate.
Determination of free-radical-scavenging activity
The free-radical-scavenging activity assay of the plant extracts was determined by the DPPH (1,1-diphenyl-2-picrylhydrazyl) method.[Citation24,Citation25] The powdered root samples were dissolved to a concentration of 1000 µg/mL in ethanol. Solutions of different concentrations (1000, 500, 250, 125 and 62.5 µg/mL) were prepared by the serial dilution technique. One millilitre of ethanol extract solution of each concentration was mixed with 4 mL of a DPPH-ethanol solution (0.1 mmol/L). Thirty minutes later, the absorbance was measured at 517 nm (Helios Alpha, Thermo Scientific). The DPPH-radical-scavenging activity was calculated by using the following equation:where AB is the absorbance of the positive control and AS is the absorbance of the sample containing the tested extract. Ascorbic acid was used as a standard or positive control. Reaction mixture without a sample was used as the negative control.
All experiments were run in triplicate. The concentration providing 50% inhibition (IC50) was calculated from the resulting graph plot by interpolation.
Data analysis
All experiments were carried out in triplicates and values are expressed as means with standard deviations (±SD). Graphics were drawn using MS Office Excel 2007.
Results and discussion
Ethnopharmacological studies suggest that natural chemical compounds isolated from medicinal plants have been used to treat the adverse effects of several bacterial, fungal and viral infectious by all civilizations from ancient times to the present.[Citation26–31] Previous investigations have reported that G. glabra is a valuable medicinal plant due to its antimicrobial and antioxidant properties.[Citation32–34] In this study, we compared the antioxidant and antimicrobial potential of root extracts from G. glabra collected from different localities in Turkey.
Antioxidant activity
The results for the DPPH-radical-scavenging activity of the methanolic extracts of licorice roots are shown in . The IC50 values of the extracts are given in . The IC50 values of the extracts were found to be between 588 ± 0.86 µg/mL and 2190 ± 1.73 µg/mL and that of the control (ascorbic acid) was determined to be 745 ± 0.05 µg/mL.
Figure 2. DPPH free-radical-scavenging activity of methanolic extracts (samples 1–15) as compared to ascorbic acid (AA) as a standard.
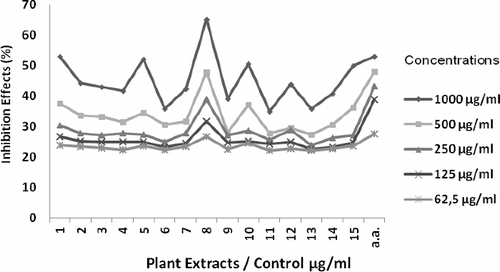
Figure 3. IC50 values of the plants methanol extracts collected from 15 sampling points (samples 1–15) as compared to ascorbic acid (AA).
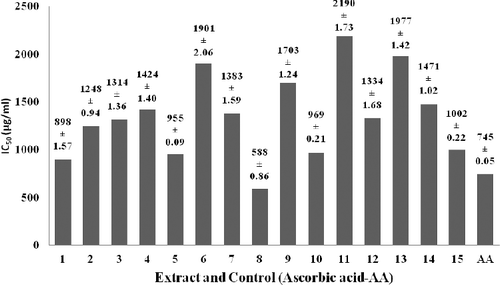
According to observed experimental data in terms of IC50, the plant designated as number 8 showed higher antioxidant activity (IC50 of 588 ± 0.86 µg/mL) compared to the control and the other studied plant extracts. In addition, the antioxidant activity of the plant extracts numbers 1, 5, 10 and 15 was close to that of the control (). These results support the data reported by Chopra et al.,[Citation3] who observed that methanolic extracts of licorice roots displayed good antioxidant activity (IC50 = 359.45 µg/mL), which is in a similar range to the IC50 determined in our study. Al-Bachir and Al-Adawi [Citation34] also demonstrated that licorice roots powder showed antioxidant activity with an IC50 value of 87.152 µg/mL versus 22.78 µg/mL for ascorbic acid.
Antimicrobial activity
The antimicrobial activities of the plant extracts collected from different habitats against nine bacteria and two yeasts using the disc-diffusion and broth microdilution (MIC) procedures are presented in and . According to the results of the disc-diffusion assays, all the plant methanolic extracts inhibited the growth of B. cereus, E. faecalis, K. pneumonia, MRSA, S. aureus, VRE, C. krusei and C. parapsilosis. However, there was no activity against E. coli, K. pneumoniae and M. luteus. Our results that the three tested Gram-negative bacteria were more resistant to the extracts than the tested Gram-positive bacteria and yeasts could most likely be explained by the differences in the cell wall structure, since the lipopolysaccharide layer in the outer membrane of Gram-negative bacteria is known to serve as a strong permeability barrier to many environmental substances.
Table 2. Inhibition zones (mm) of the methanolic plant extracts collected from 15 different localities against tested micro–organisms using disc–diffusion method.
Table 3. MIC values (μg/mL) of the methanolic plant extracts collected from 15 different localities against different micro–organisms, using the broth microdilution method.
Interestingly, in our study, the plant extract designated as number 7 was the only extract that showed activity against P. aeruginosa (9 mm, ). Our results indicated that the extracts had higher antimicrobial activity against Candida species than against the tested bacterial strains. Moreover, the antimicrobial activity of some plant extracts was found to be higher than that of some standard commercial antibiotics. Notably, extract number 10 showed more potent activity against B. cereus, MRSA, S. aureus and VRE than novobiocin, tetracycline, polymyxin B and ceftazidime ().
In addition, our data demonstrated a good correlation between the disc-diffusion results and the MIC test. The MIC values of different extracts against Gram-positive bacteria were found to be in the range of 100 to ≥1000 µg/mL. The most effective extract was No. 10 (MIC value of 100 µg/mL or less) against all the tested micro-organisms. In addition, the MIC values of the extracts against the yeasts used in the test were found to be in the range of 10–1000 µg/mL. All the extracts showed high anti-yeast activity against C. parapsilosis, ranging from 10 to 100 µg/mL ().
These results are in agreement with previous reports that G. glabra methanolic extracts have antibacterial and antioxidant activity. For example, Chopra et al. [Citation3] reported that methanolic extracts of licorice roots showed moderate antibacterial activity. In another study, Sultana et al. [Citation2] determined that G. glabra methanolic extracts exhibited antibacterial activity against Gram-positive (S. aureus, Bacillus megaterium and B. subtilis) and Gram-negative (E. coli, P. aeruginosa and Salmonella paratyphi) bacteria. Al-Bachir and Al-Adawi [Citation34] reported relatively high total aerobic plate counts, including coliform, E. coli and Klebsiella spp., after treatment with licorice roots powder. Gupta et al. [Citation14] also found that the root extracts of G. glabra showed activity against Mycobacterium tuberculosis H37Ra and H37Rv at a concentration of 500 µg/mL.
Imakiire et al. [Citation35]. and Fukai et al. [Citation9] demonstrated that compounds extracted from G. glabra and G. uralensis have good antibacterial activity against Helicobacter pylori. Fukai et al. [Citation10] determined that 19 flavonoids isolated from G. glabra, G. inflata and G. uralensis were active against methycilline sensitive Staphylococcus aureus (MSSA), MRSA, M. luteus, B. subtilis, E. coli, K. pneumoniae and P. aeruginosa. In our study, however, we could not confirm any effect against E. coli, K. pneumonia, M. luteus and P. aeruginosa (except for one extract). We hypothesize that these different results might be due to differences in the ecological conditions under which the collected plants have grown.
The effects of environmental conditions on plant growth and development, reproduction and distribution are well known in plant ecology.[Citation36–39] Many studies have been carried out about the relationships among plant chemical contents, biological activity and environmental variables in natural and cultivated plant species.[Citation40–42] This information has been used to determine the medicinal value and economic importance of plant products.[Citation43]
Based on the results from our study on the antimicrobial and the antioxidant activities of methanolic extracts obtained from Glycyrrhiza growing in different habitats, it could be summarized that the plant genetic and environmental factors have important effects on the production and the quality of medicinal plants.[Citation44] Previous reports have demonstrated that geographic variations result in changes in the content of some metabolites, e.g. glycyrrhizin.[Citation45] For example, Hosseini et al. [Citation46] studied the effects of soil and climatic conditions on the content of glycyrrhizic acid in various Iranian populations and reported that the glycyrrhizin content was significantly correlated with temperature, suggesting that temperature is an important factor affecting the amount of the glycyrrhizin acid in licorice. The same authors [Citation46] also reported that some physicochemical soil properties are important regarding the accumulation of active compounds in natural populations of G. glabra. Similarly, Zhang et al. [Citation38] and Zhou,[Citation39] reported that soil variables had a significant effect on the content of glycyrrhizic acid in Glycyrrhiza spp. Glycyrrhizin has also been reported to vary in the range of 1.96%–2.24% in the roots of G. glabra from Italian populations.[Citation47] In addition, many previous studies reported that light intensity influences the biosynthesis of secondary metabolites.[Citation48,Citation49] In this context, our results showed that the three extracts whose antioxidant properties were the highest were obtained from populations (designated as population 1, 8 and 10) that are similar in terms of climate type (rainy Mediterranean climate and low-rain Mediterranean climate), cardinal direction, habitat properties and geographical coordinates (). Also, the extracts from populations 6, 8 and 10 had the highest antimicrobial effects and their habitats (water channel, field and roadside) were under the anthropogenic pressure. Among our extracts, those that showed the highest antimicrobial and antioxidant effects originated from populations in the south-east direction. Therefore, our results indicate that there is a correlation between light intensity and the biological effects of the studied plants. In addition, it could be speculated that the observed differences in the antioxidant and antimicrobial properties of G. glabra populations could possibly be attributed to different environmental conditions in each habitat, which might affect the contents of active chemical compounds in natural licorice populations differently.
Conclusions
Significant differences were observed in the antimicrobial and antioxidant activities of root extracts of G. glabra var. glandulifera from different habitats. The extracts with most potent antioxidant properties were obtained from populations originating from regions with similar types of climate (rainy Mediterranean climate and low-rain Mediterranean climate), whereas the extracts that showed the strongest antimicrobial effect originated from habitats under anthropogenic pressure. Thus, it could be suggested that the differences in the biological activities might be attributed, at least in part, to the influence of environmental factors.
Acknowledgments
The authors are grateful to Dr V. Altay, Department of Biology, Faculty of Science and Art, Mustafa Kemal University for providing identifications of plant taxa.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Blumenthal M, Goldberg A, Brinckmann J., et al. Herbal medicine: expanded commission E monographs. American Botanical Council; 2000.
- Sultana S, Haque A, Hamid K, et al. Antimicrobial, cytotoxic and antioxidant activity of methanolic extract of Glycyrrhiza glabra. Agr Bio J N Am. 2010;1:957–960.
- Chopra PKPG, Saraf BD, Inam F, et al. Antimicrobial and antioxidant activities of methanol extract roots of Glycyrrhiza glabra and HPLC analysis. Int J Pharm Pharmacol Sci. 2013;5:157–160.
- Tang W, Eisenbrand G. Chinese drugs of plant origin. Berlin: Springer-Verlag; 1992. p. 567–588.
- Kinoshita T, Saitoh T, Shibata S. Flavonols of licorice root. Chem Pharm Bull. 1976;24:991–994.
- Mitscher LA, Park S, Omoto S, et al. Antimicrobial agents from higher plants, Glycyrrhiza glabra L. Some antimicrobial isoflavans, isoflavenes, flavanones and isoflavones. Heterocycles. 1978;9:1533–1537.
- Mitscher LA, Park YH, Clark D, et al. Antimicrobial agents from higher plants, antimicrobial, isoflavanoids and related substances from Glycyrrhiza glabra L. var. typica. J Nat Prod. 1980;43:259–269.
- Saitoh T, Noguchi H, Shibata S. A new isoflavone and the corresponding isoflavanone of licorice root. Chem Pharma Bull. 1978;26:144–147.
- Fukai T, Marumo A, Kaitou K, et al. Anti-Helicobacter pylori flavonoids from licorice extract. Life Sci. 2002;71:1449–1463.
- Fukai T, Marumo A, Kaitou K, et al. Antimicrobial activity of licorice flavonoids against methicillin-resistant Staphylococcus aureus. Fitoterapia. 2002;73:536–539.
- Fukai T, Satoh K, Nomura T, et al. Preliminary evaluation of antinephritis and radical scavenging activities of glabridin from Glycyrrhiza glabra. Fitoterapia. 2003;74:624–629.
- Biondi DM, Rocco C, Ruberto G. New dihydrostibene derivatives from the leaves of Glycyrrhiza glabra and evaluation of their antioxidant activity. J Nat Prod. 2003;66:477–480.
- Biondi DM, Rocco C, Ruberto G. Dihydrostilbene derivatives from Glycyrrhiza glabra leaves. J Nat Prod. 2005;68:1099–1102.
- Gupta VK, Fatima A, Faridi U, et al. Antimicrobial potential of Glycyrrhiza glabra roots. Elseiver J Ethnopharmacol. 2008;116:377–380.
- Asl NM, Hosseinzadeh H. Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phytother Res. 2008;22:709–724.
- Vivekanand JHA. Herbal medicines and chronic kidney disease. Nephro. 2010;15:10–17.
- Sofia H, Walter TM. Review of Glycyrrhiza glabra L. siddha papers. Med J. 2009;2:1–7.
- Messier C, Epifano F, Genovese S, et al. Licorice and its potential beneficial effects in common orodental diseases. Oral Dis. 2011;18:32–39.
- Kumagai A, Nishino K, Shimomura A, et al. Effect of glycyrrhizin on estrogen action. Endocrinol Japon. 1967;14:34–38.
- Nomura T, Fukai T, Akiyama T. Chemistry of phenolic compounds of licorice (Glycyrrhiza species) and their estrogenic and cytotoxic activities. Pure Appl Chem. 2002;74:1199–1206.
- Simmler C, Pauli GF, Chen SN. Phytochemistry and biological properties of glabridin. Fitoterapia. 2013;90:160–184.
- Davis PH. Flora of Turkey and the East Aegean Islands. Edinburgh: Edinburgh University Press; 1970.
- Bauer AW, Kirby WMM, Sherris JC, et al. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45:493–496.
- Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–1200.
- Kumar LS, Prasad KS, Revanasiddappa HB. Synthesis, characterization, antioxidant, antimicrobial, DNA binding and cleavage studies of mononuclear Cu (II) and Co (II) complexes of 3-hydroxy-N'-(2-hydroxybenzylidene)-2-naphthohydrazide. Eur J Clin Chem. 2011;2:394–403.
- Chapatwala KD, De la Cruz AA, Miles DH. Antimicrobial activity of juncusol, a novel 9-10-dihydrophenanthrene from the marsh plant Juncus roemerianus. Life Sci. 1981;29:1997–2001.
- Khan MR, Omoloso AD. Antibacterial, antifungal activity of Harpullia petiolaris. Fitoterapia. 2002;73:331–335.
- Voravuthikunchai S, Lortheeranuwat A, Jeeju W, et al. Effective medicinal plants against enterohaemorrhagic Escherichia coli O157:H7. J Ethno. 2004;94:49–54.
- Fiore C, Eisenhut M, Krausse R, et al. Antiviral effects of Glycyrrhiza species. Phytother Res. 2008;22:141–148.
- Mekseepralard C, Kamkaen N, Wilkinson JM. Antimicrobial and antioxidant activities of traditional Thai herbal remedies for aphthous ulcers. Phytother Res. 2010;24:1514–1519.
- Shen X, Sun X, Xie Q, et al. Antimicrobial effect of blueberry (Vaccinium corymbosum L.) extracts against the growth of Listeria monocytogenes and Salmonella Enteritidis. Food Control. 2014;35:159–165.
- Visavadiya NP, Soni B, Dalwadi N. Evaluation of antioxidant and anti-atherogenic properties of Glycyrrhiza glabra root using in vitro models. Int J Food Sci Nutr. 2009;60:135–149.
- Ali EM. Phytochemical composition, antifungal, antiaflatoxigenic, antioxidant, and anticancer activities of Glycyrrhiza glabra L. and Matricaria chamomilla L. essential oils. J Med Plants Res. 2013;7:2197–2207.
- Al-Bachir M, Al-Adawi M. Comparative effect of irradiation and heating on the microbiological properties of licorice (Glycyrrhiza glabra L.) root powders. Int J Radiat Bio. 2014;91:112–116.
- Imakiire M. Legumes component studies, flavonoid in licorice oil extract with anti-Helicobacter pylori action. Paper presented at: The 14th Kyushu Branch meeting of the Pharmaceutical Society of Japan. 14th Annual Meeting; 1997 May 3–6; Tokyo, Japan.
- Harper J. Biology of plant populations. London: Blackwell Scientific Publications; 1977.
- Begon M, Harper L, Townsend CR. Ecology. Boston (MA): Blackwell Scientific Publications; 1990. p. 267–350.
- Zhang JT, Xu B, Li M. Relationships between the bioactive compound content and environmental variables in Glycyrrhiza uralensis populations in different habitats of North China. Int J Exp Bot. 2011;80:161–166.
- Zhou CM. Cultivation techniques for Glycyrrhiza uralensis. Xinjiang Farmland Reclamation Sci Technol. 2006;15:14–15.
- Hayashi H, Sudo H. Economic importance of licorice. Plant Biotechnol. 2009;26:101–104.
- Alimuddin S, Hemlata R, Patel NM. Evaluation of antimicrobial activity of stem bark of Ficus bengalensis Linn, collected from different geographical regions. Phcog J. 2010;2:178–180.
- Zhang JT. Applied ecology. Beijing: Science Press; 2003.
- Gende L, Maggi M, Van Baren C, et al. Antimicrobial and miticide activities of Eucalyptus globules essential oils obtained from different Argentine regions. Span J Agric Res. 2010;8:642–650.
- Bowes KM, Zheljazkov VD. Essential oil yields and quality of fennel grown in Nova Scotia. Hortic Sci. 2005;39:1640–1643.
- Oloumi H, Hassibi N. Study the correlation between some climate parameters and the content of phenolic compounds in roots of Glycyrrhiza glabra. J Med Plant Res. 2011;5:6011–6016.
- Hosseini SMA, Souri MK, Farhadi N, et al. Changes in glycyrrhizin content of Iranian licorice (Glycyrrhiza glabra L.) affected by different root diameter and ecological conditions. Agri Commun. 2014;2:27–33.
- Usai M, Vincenzo P, Domenico A. Glycyrrhizin variability in subterranean organs of Sardinian Glycyrrhiza glabra subspecies glabra var. glabra. Nat Prod Commun. 1995;58:1727–1729.
- Zavala JA, Ravetta DA. Allocation of photoassimilates to biomass, resin and carbohydrates in Grindelia chiloensis as affected by light intensity. Field Crop Res. 2001;69:143–149.
- Coelho GC, Rachwal MFG, Dedecek RA, et al. Effect of light intensity on methylxanthine contents of Ilex paraguariensis A. St. Hil. Biochem Syst Ecol. 2007;35:75–80.