ABSTRACT
Curcumin is a polyphenolic substance with attractive pharmacological activities (e.g. antioxidant, anti-inflammatory, anticancer). Incorporation of curcumin in polymeric micelles could overcome the problems associated with its instability and low aqueous solubility. The aim of this study was to load curcumin in polymeric micelles based on Pluronic® P 123 or Pluronic® F 127 triblock copolymers and evaluate the antioxidant and neuroprotective effects after micellization. The micelles were prepared and loaded with curcumin by applying the dissolution method. Higher encapsulation efficiency was observed in the micelles formulated with Pluronic® P 123. These micelles were characterized with small size and narrow size distribution. The effects of micellar curcumin were investigated in two in vitro models. First, the capacity of micellar curcumin to inhibit iron/ascorbic acid-induced lipid peroxidation in rat liver microsomes was evaluated. Micellar curcumin and free drug showed similar inhibition of lipid peroxidation. Second, micellar curcumin and free curcumin showed protective potential in a model of 6-hydroxydopamine induced neurotoxicity in rat brain synaptosomes. The results from both methods indicated preservation of antioxidant and neuroprotective activity of curcumin in micelles. The small micellar size, high loading capacity and preservation of antioxidant activity of curcumin into Pluronic micelles, suggested their further evaluation as a curcumin delivery system.
Introduction
Polymeric micelles are attractive drug carriers because of their numerous advantages, among them the capacity to provide sustained release of incorporated drug, improvement of the bioavailability of drugs with poor absorption and drug protection from different factors.[Citation1] For example, the study of Yoncheva et al. [Citation2] has reported an improved oral absorption of paclitaxel loaded in stabilized Pluronic micelles. An improvement of oral bioavailability has also been demonstrated by its incorporation in other types of polymeric micelles.[Citation3] Insulin has been incorporated in arginine polymeric micelles, which provided protective effect against enzymatic degradation of insulin and hypoglycemic effect in diabetic rats.[Citation4]
Curcumin is a polyphenolic substance that is attractive due to various activities like antioxidant, anti-inflammatory, anticancer ones, etc. However, the administration of curcumin is associated with very low bioavailability.[Citation5,Citation6] For example, oral administration in humans at a dose of 2 g/kg resulted in undetectable or very low serum levels (0.006 µg/mL).[Citation7] Different reasons for the low bioavailability were reported, in particular chemical instability, poor absorption, rapid metabolism and a very short biological half-life in circulation and visceral organs.[Citation5,Citation8] Another problem of curcumin is associated with its instability in certain conditions (i.e. pH, light exposure). In recent years, many research groups have attempted to overcome these drawbacks.[Citation9] Suppression of alkaline hydrolysis of curcumin has been shown by its encapsulation in cationic micelles composed of cetyltrimethylammonium bromide and dodecyltrimethylammonium bromide.[Citation10] Other studies have reported an improved solubility and higher cytotoxic effect of curcumin loaded in various types of micelles.[Citation11,Citation12] Loading of curcumin in poly(2-(dimethylamino)ethyl methacrylate)-poly(ϵ-caprolactone)-poly(2-(dimethylamino)ethyl methacrylate) micelles improved its antioxidant activity in vitro and enabled intracellular transport.[Citation13]
This study evaluated the possibility of incorporating curcumin in polymeric micelles and preserving its antioxidant and neuroprotective properties. These activities were assayed using two in vitro methods: (1) determination of the capacity of micellar curcumin to inhibit lipid peroxidation in isolated rat liver microsomes and (2) capacity to protect rat brain synaptosomes in a model of 6-hydroxydopamine-induced neurotoxicity. The micelles were based on the triblock copolymers poly(ethylene oxide)-b-poly(propylene oxide)-b-poly(ethylene oxide) (PEO–PPO–PEO) (Pluronic). These copolymers were chosen as carriers because of their biocompatibility and safety of use. Two types of copolymers were selected, taking into account their different properties, in particular Pluronic® P 123 (PEO20PPO70PEO20) and Pluronic® F 127 (PEO101PPO56PEO101).
Materials and methods
Chemicals and reagents
Pluronic® P 123 (PEO20PPO70PEO20) and Pluronic® F 127 (PEO101PPO56PEO101) were supplied by (BASF, Ludwigshafen, Germany). Curcumin, 2-thiobarbituric acid (TBA), trichloroacetic acid (TCA), nicotineamideadenine dinucleotide phosphate (reduced) (NADPH), HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), Percoll reagent, MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide), ascorbic acid and bovine serum albumin (fraction V) were purchased from Sigma Chemical Co. (Germany); 2,2′-dinitro-5,5′-dithiodibenzoic acid (DTNB) and 6-hydroxydopamine (6-OHDA) were obtained from Merck (Germany). All other reagents were of analytical grade.
Preparation of micelles
The micelles were prepared by the dissolution method adapted from the study of Kabanov et al.[Citation14] In the present study, the copolymer (Pluronic® P 123 or F 127) was dissolved in purified water (10 mg/mL). The temperature was raised and kept at 37 °C and curcumin was added to the solution. Two ratios between curcumin and copolymers were applied, in particular 1:25 and 1:50 (wt/wt). The dispersions were incubated for 1 h under gentle stirring (700 r/min). After incubation, the micellar dispersions were filtered through a 0.22 nm filter and kept for further studies. The filter was rinsed with ethanol and the collected fractions were measured for determination of non-encapsulated curcumin.
Characterization of curcumin-loaded mixed micelles
The size, polydispersity and zeta-potential were determined by Zetamaster analyzer (Malvern Zetasizer Nano ZS, Worcestershire, UK). Samples were evaluated at 25 °C with a scattering angle of 90°.
Curcumin encapsulation was found as a difference between its initial concentration and the concentration determined in the rinsing fractions collected after washing of the filter. The concentration of curcumin was determined by ultraviolet (UV)-spectrophotometry at 428 nm (Hewlett Packard 8452A). The standard curve was prepared in the concentration range of 2–10 mg/mL (r > 0.9994). The encapsulation efficiency was calculated as follows: Encapsulation efficiency = (Total amount of drug – Free drug)/Total amount of drug.
In vitro curcumin release
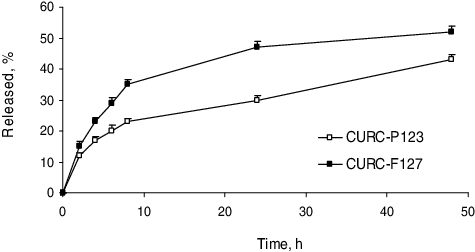
In vitro release of curcumin from the micelles was examined in a phosphate buffer (pH = 7.0). The freshly prepared micellar dispersion was introduced into a dialysis membrane bag (MW = 6000–8000 g/mol, Spectrum labs), which was further placed into 100 mL of phosphate buffer containing 10% ethanol. The sample was agitated (50 r/min) and the temperature was constantly maintained at 37 °C throughout the study. At predetermined time intervals (2, 4, 6, 8, 24, 48 h), samples were withdrawn from the medium outside the dialysis bag and the concentration of the released curcumin was determined by UV-Vis spectrophotometry as described above.
Animals
Male Wistar rats were purchased from the National Breeding Center, Sofia, Bulgaria. Food and water were provided ad libitum. At least seven days of acclimatization were allowed before the study. The animal's health was regularly monitored by a veterinary physician. All performed procedures were approved by the Institutional Animal Care Committee and were carried out according to Ordinance No. 15/2006 for humane treatment of experimental animals (vivarium certificate of registration of farm No. 0072/01.08.2007).
Isolation of rat liver microsomes
Male Wistar rats (200.0–220.0 g) were fasted overnight and were sacrificed by cervical decapitation. Livers were thoroughly perfused with 1.15% KCl and homogenized with four volumes of ice-cold 0.1 mol/L potassium phosphate buffer, pH = 7.4. The liver homogenate was centrifuged at 9000 × g for 30 min at 4 °C and the resulting postmitochondrial fraction (S9) was centrifuged at 105,000 × g for 60 min at 4 °C. The microsomal pellets were resuspended in 0.1 mol/L potassium phosphate buffer, pH = 7.4, containing 20% glycerol. Aliquots of liver microsomes were stored at −70 °C. Microsomal protein content was determined according to the method of Lowry et al.[Citation15]
Non-enzymatic iron/ascorbic acid (Fe2+/AA) induced lipid peroxidation assay (LPO)
The microsomes were preincubated with the empty micelles (10 mg/mL), curcumin-loaded micelles and free curcumin in equal concentrations (8.7 μg/mL) at 37 °C for 15 min. The LPO was started with a 20 μmol/L solution of iron sulphate and 0.5 mmol/L of ascorbic acid.[Citation16] The reaction of LPO was stopped 20 min after initiation with a mixture of TCA 25% and TBA 0.67%. The quantity of malondialdehyde (MDA), the main lipid peroxidation product, was assessed as described by Deby and Goutier.[Citation17] The absorbance was measured at 535 nm, using a Spectro UV-VIS Split spectrophotometer. The amount of MDA was calculated using a molar extinction coefficient of 1.56 × 105 mol−1 cm−1. The final results were presented as nmol/mg protein.
Isolation and incubation of synaptosomes
Synaptosomes were prepared from the brains of adult male Wistar rats, as previously described by Taupin et al.[Citation18] The brains were homogenized in 10 vol. of cold Buffer 1, containing: 5 mmol/L HEPES and 0.32 mol/L of sucrose (pH = 7.4). The brain homogenate was centrifuged twice at 1000 × g for 5 min at 4 °C. The supernatant was collected and centrifuged three times at 10,000 × g for 20 min at 4 °C. The pellet was resuspended in ice-cold Buffer 1. The synaptosomes were isolated by using Percoll reagent to prepare the gradient. Synaptosomes were resuspended and incubated in Buffer 2, containing: 290 mmol/L NaCl, 0.95 mmol/L MgCl2.6H2O, 10 mmol/L KCl, 2.4 mmol/L CaCl2.H2O, 2.1 mmol/L NaH2PO4, 44 mmol/L HEPES and 13 mmol/L D-glucose. Incubations were performed in a 5% CO2 + 95% O2 atmosphere. The content of synaptosomal protein was determined according to the method of Lowry et al. [Citation15], using serum albumin as a standard. Isolated rat synaptosomes were exposed to 6-OHDA (150 µmol/L), as a model of dopamine-induced oxidative neurodegeneration; to free curcumin (8.7 µg/mL); or curcumin-loaded polymeric micelles (8.7 curcumin µg/mL).
Glutathione (GSH) content and MTT-dye reduction assay
The levels of glutathione (GSH) were determined in rat brain synaptosomes with the Ellman reagent (DTNB), which forms coloured complexes with –SH groups at pH = 8, with maximum absorbance at 412 nm, as described by Robyt et al.[Citation19] The viability of synaptosomes was measured by MTT-test as previously described.[Citation20] After incubation with the tested compounds, synaptosomes were treated with MTT solution (0.5 mg/mL) for 1 h at 37 °C and were centrifuged at 15,000 × g for 1 min. The formed formazan crystals were dissolved in dimethyl sulfoxide. The absorbance was measured spectrophotometrically at 580 nm.
Data analysis
Statistical analysis was performed by analysis of variance, followed by Student's t-test. Data are presented as mean values with standard errors of the mean (±SEM) from three independent experiments. The values of P < 0.05, P < 0.01 and P < 0.001 were considered statistically significant.
Results and discussion
Encapsulation efficiency and physicochemical properties of curcumin-loaded micelles
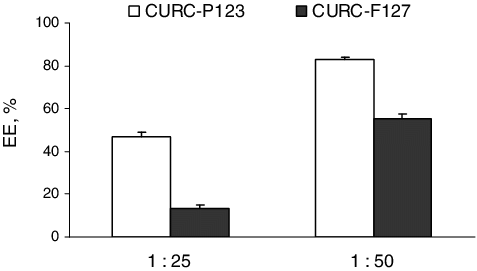
This study dealt with the loading of curcumin in polymeric micelles based on Pluronic® P 123 or Pluronic® F 127 triblock copolymers and evaluation of its antioxidant and neuroprotective effect after micellization. These two types were selected taking into account their different hydrophilicity and molecular weight. Various methods have been reported for incorporation of drugs in Pluronic micelles.[Citation21,Citation22] In this study, the loading of curcumin was conducted under heating of the aqueous copolymer solution at 37 °C.[Citation14] The temperature was applied aiming to ensure curcumin loading into the PPO-core of the micelles. The loading of curcumin into both types of the micelles was studied by varying the ratio between curcumin and the amount of copolymers. Our results showed that the ratio significantly influenced the encapsulation efficiency of curcumin (). At both ratios, the efficiency was observed to be higher in the micelles formulated with Pluronic® P 123. At a 1:50 ratio, the encapsulation efficiency reached 83% in the case of Pluronic® P 123 micelles, whereas for Pluronic® F 127, it was about 55%. A recent study reported higher loading of curcumin in Pluronic® F 127 micelles, which is probably due to the different method of loading.[Citation23] In our study, the different encapsulation efficiency of curcumin in both copolymeric micelles correlated with the values of the ratio between the total weight of hydrophobic (PO) and hydrophilic (EO) units for both copolymers (). This ratio was higher for Pluronic® P 123, which would contribute for the higher loading of the hydrophobic drug into these micelles.
Figure 1. Size and polydispersity index of curcumin-loaded micelles based on Pluronic® P123 (CURC-P123) and Pluronic® F127 (CURC-F127). Mean values ± SEM.
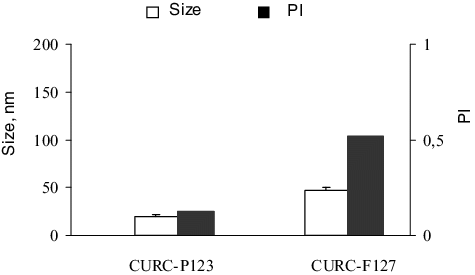
Table 1. Total weights of propylene oxide (PO) and ethylene oxide (EO) units and their ratio for Pluronic® P 123 and Pluronic® F 127 copolymers.
The physicochemical properties of curcumin-loaded micelles are shown in and . The micelles formulated with Pluronic® P 123 were of a small diameter and had narrow size distributions. The micelles based on Pluronic® F 127 consisted of more than one size fraction, which has also been observed by other authors.[Citation24] There are many reports that the presence of drug molecules could increase the size of the micelles.[Citation25,Citation26] In this study, it is very probable that the presence of the hydrophobic drug increased the size and the polydispersity of the micelles formulated with the more hydrophilic Pluronic® F 127. Regarding the zeta-potential, both types of micelles had slightly negative zeta-potential values (), which correlated with the non-ionic properties of Pluronic copolymers.
Figure 2. Zeta-potential values of Pluronic® P123 and Pluronic® F127 micelles loaded with curcumin. Mean values ± SEM.
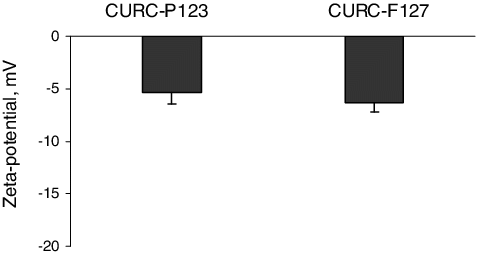
Figure 3. Encapsulation efficiency of curcumin into micelles depending on the initial ratio between curcumin and both copolymers. Mean values ± SEM.
The in vitro release study performed in phosphate buffer (pH = 7.0) revealed that curcumin was released faster from Pluronic® F 127 micelles than from Pluronic® P 123 micelles (). The slower release from the micelles formulated with the more hydrophobic Pluronic® P 123 could be explained by the higher affinity of curcumin to these micelles. The higher affinity was suggested regarding the higher encapsulation efficiency in these micelles (). In general, the release rate was similar to that observed in another study with curcumin-loaded micelles.[Citation11]
Inhibition of iron/ascorbic acid (Fe2+/AA) induced lipid peroxidation in rat liver microsomes
Oxidative stress is the underlying mechanism and the main reason for different pathological processes. The increased generation of reactive oxygen species (ROS) leads to lipid peroxidation and, consequently, to functional and structural impairments in the cell membranes and cytotoxicity. As a natural polyphenol, curcumin can act as a free radical scavenger and protector against tissue damage caused by excessive ROS production.[Citation27,Citation28] Reddy and Lokesh [Citation29] have studied the activity of curcumin as an antioxidant in the inhibition of lipid peroxidation of rat liver microsomes.
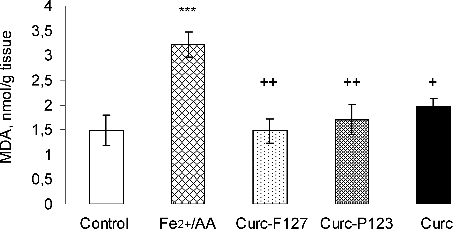
In our study, the in vitro antioxidant activity of curcumin loaded into micelles was investigated in a Fe2+/AA-induced lipid peroxidation model. Rat liver microsomes were used for induction of non-enzyme lipid peroxidation.[Citation30] In particular, a significant three-fold increase in the amount of MDA vs. untreated controls was observed after Fe2+/AA treatment (P ≤ 0.001). The effects of equal concentrations of free and micellar curcumin on Fe2+/AA-induced lipid peroxidation in rat liver microsomes are presented in . Free curcumin statistically significantly decreased the Fe2+/AA-induced lipid damage by 39% (P ≤ 0.05). Curcumin loaded in Pluronic® P 123 micelles showed similar protection against Fe2+/AA-induced lipid damage; in particular, it decreased the MDA formation by 47% (P ≤ 0.01). Similar results were observed in the case of curcumin loaded in Pluronic® F 127 micelles, where MDA formation was reduced by 54% (P ≤ 0.01). Consequently, the antioxidant activity of curcumin was preserved after incorporation into the micelles. These results indicate that incorporation of curcumin into the micelles could be considered advantageous, since the micelles could provide further protection of curcumin during storage.
Rat brain synaptosomal protection by curcumin-loaded polymeric micelles
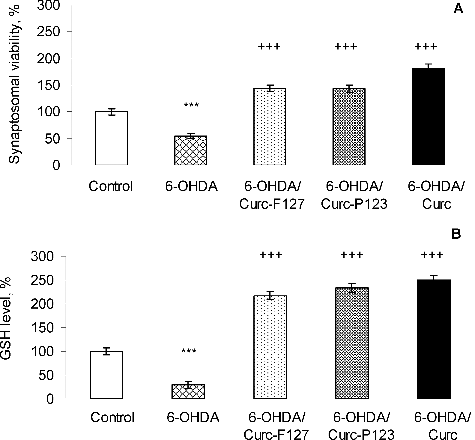
It is known that curcumin exerts its antioxidant properties acting as a scavenger for reactive oxygen and nitrogen species.[Citation31,Citation32] In our experiments, we evaluated the protective potential of curcumin loaded in polymeric micelles against 6-hydroxydopamine-induced neurotoxicity in rat brain synaptosomes (). 6-OHDA is a neurotoxin which structurally resembles dopamine. It shows high affinity for the dopamine transporter and, once inside the neuron, it accumulates and undergoes non-enzymatic auto-oxidation, thus promoting ROS formation.[Citation33] Thus, it was interesting to evaluate the protective effects of curcumin-loaded polymeric micelles in a model of 6-OHDA-induced oxidative stress and neurotoxicity in rat brain synaptosomes. As shown in , the incubation of rat brain synaptosomes with 150 µmol/L 6-OHDA significantly reduced the synaptosomal viability and the GSH levels by 46% and 71%, respectively vs. the untreated control synaptosomes (P ≤ 0.001). This result was in agreement with a previous study.[Citation34] Our results showed that the treatment with the micellar and free curcumin diminished the lesions caused by 6-OHDA in the synaptosomes. Curcumin loading in Pluronic® P 123 and F 127 polymeric micelles significantly attenuated the decrease in synaptosomal viability and GSH depletion induced by 6-OHDA and these effects were similar to those of free curcumin. Our recent study evaluated the protective effect of curcumin loaded in microparticles in the same model and it was considered that curcumin counteracted the in vitro toxicity of 6-hydroxydopamine through its antioxidant properties.[Citation35] The present study indicated that the neuroprotective effect of curcumin is preserved after loading into Pluronic® micelles in a model of 6-OHDA-induced oxidative stress in rat brain synaptosomes.
Conclusions
The study revealed an opportunity to develop polymeric micelles based on well-known copolymers as a platform for curcumin delivery. The micelles formulated with Pluronic® P 123 were characterized with smaller size and higher curcumin loading. Curcumin loaded in both types of micelles showed comparable antioxidant properties to those of free curcumin in a model of iron/ascorbic-acid-induced lipid peroxidation in vitro. Similar results were obtained regarding the neuroprotective activity in a model of 6-OHDA-induced oxidative stress in rat brain synaptosomes. Thus, the preservation of the antioxidant and neuroprotective activity, the smaller size and higher loading indicated the advantages of Pluronic® P 123 micelles for further evaluation as a curcumin delivery system.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Kataoka K, Harada A, Nagasaki Y. Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv Drug Delivery Rev. 2001;47:113–131.
- Yoncheva K, Calleja P, Agueros M, et al. Stabilized micelles as delivery vehicles for paclitaxel. Int J Pharm. 2012;436:258–264.
- Zhao Y, Li Y, Ge J, et al. Pluronic-poly (acrylic acid)-cysteine/Pluronic L121 mixed micelles improve the oral bioavailability of paclitaxel. Drug Dev Ind Pharm. 2014;40:1483–1493.
- Zhang ZH, Abbad S, Pan RR, et al. N-octyl-N-arginine chitosan micelles as an oral delivery system of insulin. J Biomed Nanotechnol. 2013;9:601–609.
- Anand P, Kunnumakkara AB, Newman RA, et al. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–818.
- Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as “Curecumin”: from kitchen to clinic. Biochem Pharmacol. 2008;75:787–809.
- Shoba G, Joy D, Joseph T, et al. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64:353–356.
- Bisht S, Mizuma M, Feldmann G, et al. Systemic administration of polymeric nanoparticle-encapsulated curcumin (NanoCurc™) blocks tumor growth and metastases in preclinical models of pancreatic cancer. Mol Cancer Ther. 2010;9:2255–2264.
- Naksuriya O, Okonogi S, Schiffelers R, et al. Curcumin nanoformulations: a review of pharmaceutical properties and preclinical studies and clinical data related to cancer treatment. Biomaterials. 2014;35:3365–3383.
- Leung MH, Colangelo H, Kee TW. Encapsulation of curcumin in cationic micelles suppresses alkaline hydrolysis. Langmuir. 2008;24:5672–5675.
- Zhao L, Du J, Duan Y, et al. Curcumin loaded mixed micelles composed of Pluronic P123 and F68: preparation, optimization and in vitro characterization. Coll Surf B: Biointerfaces. 2012;97:101–108.
- Liang J, Wu W, Lai D, et al. Enhanced solubility and targeted delivery of curcumin by lipopeptide micelles. J Biomater Sci Polymer Ed. 2015;26:369–383.
- Yoncheva K, Kamenova K, Perperieva T, et al. Cationic triblock copolymer micelles enhance antioxidant activity, intracellular uptake and cytotoxicity of curcumin. Int J Pharm. 2015;490:298–307.
- Kabanov A, Batrakova EV, Melik-Nubarov NS, et al. A new class of drug carriers: micelles of poly(oxyethylene)-poly(oxypropylene) block copolymers as microcontainers for drug targeting from blood in brain. J Contr Rel. 1992;22:141–157.
- Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275.
- Mansuy D, Sassi A, Dansette PM, et al. A new potent inhibitor of lipid peroxidation in vitro and in vivo, the hepatoprotective drug anisyldithiol thione. Biochem Biophys. 1986;135:1021–1051.
- Deby C, Goutier R. New perspectives on the biochemistry of superoxide anion and the efficiency of superoxide dismutases. Biochem Pharmacol. 1990;39:399–405.3
- Taupin P, Zini S, Cesselin F, et al. Subcellular fractionation on Percoll gradient of mossy fiber synaptosomes: morphological and biochemical characterization in control and degranulated rat hippocampus. J Neurochem. 1994;62:1586–1595.
- Robyt JF, Ackerman RJ, Chittenden CG. Reaction of protein disulfide groups with Ellman's reagent: a case study of the number of sulfhydryl and disulfide groups in Aspergillusoryzae – amylase, papain, and lysosome. Arch Biochem Biophys. 1971;147:262–269.
- Mungarro-Menchaca X, Ferrera P, Moran J, et al. Beta-amyloid peptide induces ultrastructural changes in synaptosomes and potentiates mitochondrial dysfunction in the presence of ryanodine. J Neurosci Res. 2002;68:89–96.
- Chen F, Rice KC, Liu XM, et al. Triclosan-loaded tooth-binding micelles for prevention and treatment of dental biofilm. Pharm Res. 2010;27:2356–2364.
- Pepic I, Lovric J, Hafner A, et al. Powder form and stability of Pluronic mixed micelle dispersions for drug delivery applications. Drug Dev Ind Pharm. 2014;40:944–951.
- Sahu A, Kasoju N, Goswami P, et al. Encapsulation of curcumin in pluronic block copolymer micelles for drug delivery applications. J Biomater Appl. 2011;25:619–639.
- Chiappetta DA, Hocht C, Taira C, et al. Efavirenz-loaded polymeric micelles for pediatric anti-HIV pharmacotherapy with significantly higher oral bioavailaibility. Nanomed. 2010;5:11–23.
- Allen C, Maysinger D, Eisenberg A. Nano-engineering block copolymer aggregates for drug delivery. Colloids Surf B. 1999;16:3–27.
- Basak R, Bandyopadhyay R. Encapsulation of hydrophobic drugs in Pluronic F127 micelles: effects of drug hydrophobicity, solution temperature, and pH. Langmuir. 2013;29:4350–4356.
- Ishrat T, Hoda MN, Khan MB, et al. Amelioration of cognitive deficits and neurodegeneration by curcumin in rat model of sporadic dementia of Alzheimer's type (SDAT). Eur Neuropsychopharmacol. 2009;19:636–647.
- Bisht K, Wagner KH, Bulmer AC. Curcumin, resveratrol and flavonoids as anti-inflammatory, cyto- and DNA-protective dietary compounds. Toxicology. 2010;278:88–100.
- Reddy AC, Lokesh BR. Studies on spice principles as antioxidants in the inhibition of lipid peroxidation of rat liver microsomes. Mol Chem Biochem. 1992;111:117–124.
- Zaidi SI, Agarwal R, Eichler G, et al. Photodynamic effects of new silicon phthalocyanines: in vitro studies utilizing rat hepatic microsomes and human erythrocyte ghosts as model membrane sources. Photochem Photobiol. 1993;58:204–210.
- Barzegar AA, Moosavi-Movahedi A. Intracellular ROS protection efficiency and free radical-scavenging activity of curcumin. PLoS ONE [Internet]. 2011 [cited 2016 Apr 14];6:e26012. Available from: http://dx.doi.org/10.1371/journal.pone.0026012
- Trujillo J, Chirino YI, Molina-Jijon E, et al. Renoprotective effect of the antioxidant curcumin: recent findings. Redox Biol. 2013;1:1–9.
- Blandini F, Armentero MT, Martignoni E. The 6-hydroxydopamine model: news from the past. Parkisonism Relat Disord. 2008;14:S124–S129.
- Stokes AH, Freeman WM, Mitchell SG, et al. Induction of GADD45 and GADD153 in neuroblastoma cells by dopamine-induced toxicity. Neurotoxicology. 2002;23:675–684.
- Yoncheva K, Kondeva-Burdina M, Tzankova V, et al. Curcumin delivery from poly(acrylic acid-co-methylmethacrylate) hollow microparticles prevents dopamine-induced toxicity in rat brain synaptosomes. Int J Pharm. 2015;486:259–267.