ABSTRACT
A high-frequency and reproducible protocol for induction of adventitious shoot buds and plant regeneration from cotyledonary petiole explants of Jatropha curcas L. has been developed. The cotyledonary petiole explants of J. curcas cultured directly on medium supplemented with thidiazuron (TDZ) induce regeneration of poor quality shoot buds that have a low regeneration frequency. However, treating the explants with high concentrations (10–60 mg/L) of TDZ solution for certain time periods (5–80 min) significantly increased the regeneration frequency and improved the quality of the regenerated shoot buds. The best shoot buds induction (88.42%) and number of shoot buds (12.67) per explant were observed when in vitro explants were treated with 20 mg/L TDZ solution for 20 min before being transferred on hormone-free medium after 30 days. Regeneration was also influenced by the orientation (horizontal or vertical) of the explants on the medium, and by the origin of the cotyledonary petioles (in vitro or in vivo) used for the preparation of explants. We performed subsequent experiments for elongation and rooting of the regenerated shoot buds. Addition of L-arginine to the medium was conducive to the elongation of the shoot buds. A concentration of 7.5 mg/L L-arginine yielded the best results. The elongated shoots could initiate roots to become intact plantlets in half-strength Murashige and Skoog medium containing 0.1 mg/L indole-3-butyric acid. After acclimatization, these plantlets could be transplanted to the soil and the growth was normal. Therefore, application of the methods described here helped to increase plant regeneration efficiency.
Introduction
Jatropha curcas L. is a woody plant with wide distribution in tropical and sub-tropical areas. These plants have several benefits such as extraction of pharmaceutical compounds and insecticides.[Citation1–3] However, the most important economic aspect of this tree is its high seed oil content (up to 60%), which can be processed to make ‘biodiesel’ for automobile and airplane use.[Citation4,Citation5] Although the oil content in the seeds of J. curcas is generally high, the yield of seeds is low in most cases. Furthermore, some traits of this tree are undesirable for field management. Therefore, genetic transformation has been suggested as an ideal solution for breeding of advanced J. curcas varieties for large-scale cultivation and for enhancing the seed yield.[Citation6–10]
Most conventional genetic transformation systems require an essential step of shoot bud regeneration from the genetically transformed tissues (explants). However, since most such techniques reported for J. curcas are very time-consuming, needing about 90–140 days for regeneration of intact plants,[Citation11] more efficient tissue culture methods are needed for species improvement through genetic engineering and large-scale production of quality planting material. There have been several reports on the regeneration of plants in J. curcas tissue cultures.[Citation12–19] However, the regeneration efficiency was still not satisfactory. With the innovations in culture methods, here we have established an efficient protocol for the recovery of plantlets from cotyledonary petiole explants of J. curcas.
Materials and methods
Plant sources
Mature seeds were collected from cloned J. curcas trees coded M-19 at a farm in Haikou, Hainan Island, China.[Citation11]
Preparation of explants
For preparation of in vitro cotyledonary petiole explants, the shells of seeds were removed and the embryos were surface-sterilized by 2% sodium hypochlorite (NaClO) for 5 min and rinsed five times in sterile distilled water. The sterilized embryos were placed on hormone-free Murashige and Skoog (MS) basal medium.[Citation20] Explants (0.5 cm in length) were isolated from the cotyledonary petioles of 20-day-old seedlings. For in vivo explants, the seeds were planted in moist soil; cotyledonary petiole explants (0.5 cm in length) were collected from 20-day-old seedlings, and sterilized with 2% sodium hypochlorite (NaClO) for 20 min and rinsed five times in sterile distilled water.
Preparation of culture medium and maintenance of the cultures
Uniform culture conditions were applied in all experiments. Basal MS formula was used for all tissue culture experiments. The pH of the medium was adjusted to 5.8–6.0 using 1 mol/L NaOH, prior to autoclaving at 1.4 kg/cm2 for 20 min. All culture treatments were kept at 25 ± 1 °C under a 12-h photoperiod of 60–80 μmol/(m2 s) intensity (cool white fluorescent tubes).
Treatment of cotyledonary petiole explants with thidiazuron (TDZ) solution
TDZ (Sigma-Aldrich Co., St Louis, MO, USA) was dissolved in 1 mol/L NaOH solution. The solution was diluted with purified water to prepare concentrations of 0, 10, 20, 30 and 60 mg/L, adjusted with 1 mol/L HCl to reach a pH value range of 5.8–6.0, and filter-sterilized before using it for treatment of the cotyledonary petiole explants.
The cotyledonary petiole explants were soaked in a glass bottle containing TDZ solution for different time periods (0, 5, 20, 40 and 80 min). The explants were blotted on sterilized filter paper post treatment.
Regeneration culture
For inducing shoot buds regeneration, in vitro cotyledonary petiole explants were placed horizontally on hormone-free MS medium after treatment with TDZ solution for different time periods. For comparison, in vitro cotyledonary petiole explants were also treated by conventional methods and placed horizontally on MS medium containing different concentrations of TDZ (0, 0.1, 0.3 and 0.6 mg/L) as reported previously.[Citation18,Citation21] The in vitro and in vivo explants were also placed vertically with their morphological up-side up for comparison. The percentage of induction of shoot buds and the number of shoot buds per explant were determined after 30 days of culture.
Shoot buds elongation culture
For shoot buds elongation, the regenerated shoot buds were transferred along with the mother tissues (explants) to MS medium supplemented with 0.5 mg/L 6-benzyladenine (6-BA; Sigma-Aldrich Co., St Louis, MO, USA), 0.2 mg/L kinetin (KT; Sigma-Aldrich Co., St Louis, MO, USA), 0.25 mg/L indole-3-acetic acid (IAA; Sigma-Aldrich Co., St Louis, MO, USA) and 0.25 mg/L gibberellic acid (GA3; Sigma-Aldrich Co., St Louis, MO, USA).[Citation14] Various L-arginine (Sigma-Aldrich Co., St Louis, MO, USA) concentrations (0, 7.5, 15 and 30 mg/L) were used to supplement the elongation medium. The length of the elongated shoots was recorded after 15 days of culture.
Rooting culture
For rooting of the shoots, shoots at least 10 mm in length were isolated from the mother tissues and transferred onto fresh half-strength MS medium containing indole-3-butyric acid (IBA; Sigma-Aldrich Co., St Louis, MO, USA) of different concentrations (0, 0.1, 0.3 and 0.6 mg/L) and the results were assessed on days 10, 15, 20, 30 and 40.
Acclimatization and transplantation of the regenerated plantlets
Plantlets were taken from culture bottles, washed for removing residuals of medium, transplanted to pots with sterilized sand and soil in a 1:1 ratio and covered with transparent plastic sheets for about 15 days. The established plants were transferred to a greenhouse (temperature 25 ± 3 °C and relative humidity 70%–80%) for further growth.
Evaluation of the results and data analysis
All the experiments were set up in a completely randomized factorial design and repeated three times with 30 replicates per treatment. Statistical analysis of the data was carried out using SPSS 17.0 software and the significance of differences among means was determined by Duncan's multiple range tests at p ≤ 5%. The results were expressed as means with ±standard deviation (SD) from three independent experiments.
Results and discussion
Regeneration of adventitious buds from cotyledonary petiole explants with conventional culture methods
It is well known that Agrobacterium-mediated transformation contributes to improving one or a plurality of features of plant species. Furthermore, the regeneration of adventitious buds from the genetically transformed tissues (explants) is, in most cases, a pre-condition. Cotyledonary petiole explants were inoculated directly onto media supplemented with 0.05–1 mg/L of TDZ to induce adventitious buds regeneration via conventional tissue culture methods in J. curcas.[Citation18,Citation21] However, the regeneration effect of adventitious buds was unsatisfactory, and the highest rate of shoot buds regeneration (41.66%–59.11%) and the maximum quantity of induced adventitious buds per explant (4.59–9.75) were yielded subsequently, when 0.5 mg/L TDZ was added to the medium.[Citation18,Citation21]
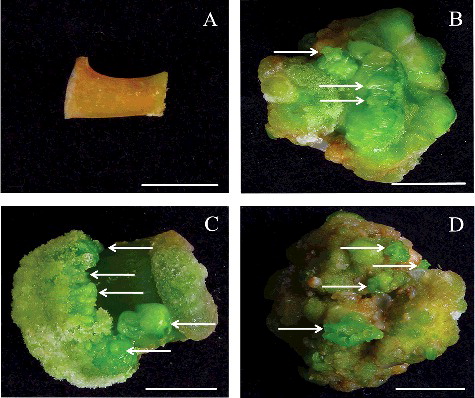
In our study, in vitro cotyledonary petiole explants were treated by conventional methods and placed horizontally on MS medium containing different concentrations of TDZ (0, 0.1, 0.3 and 0.6 mg/L). TDZ effectively induced adventitious buds regeneration ( and (A–D)). The concentration of 0.3 mg/L was found to be most effective, and the highest percentage of shoot buds induction (41.68%) and highest number of induced shoot buds (4.31) per explant were observed ( and (C)). However, when TDZ was supplemented with concentration of 0.6 mg/L, the regeneration percentage of adventitious buds decreased significantly, then the percentage of shoot buds induction was 28.69% and the number of induced shoot buds per explant was 3.38, whilst the regenerated buds were of poorer quality ( and (D)). These results were in line with our previous report [Citation11] that 0.3 mg/L TDZ was the optimum concentration for inducing adventitious buds regeneration and further cultivation via conventional methods. ( and (C)).
Table 1. Effects of various concentrations of TDZ on the conventional regeneration of adventitious buds from in vitro cotyledonary petiole explants of J. curcas after 30 days.
Regeneration of shoot buds from cotyledonary petiole explants treated with TDZ before culture
In the present study, a low regeneration rate (below 42%), similar to previous reports, was observed via conventional methods.[Citation18,Citation21] Furthermore, our previous results showed that when hypocotyl explants were treated with high concentrations of cytokinin (BA) solution before placing onto hormone-free MS medium, the bud regeneration effect was markedly enhanced in soybean.[Citation22] In order to verify whether this treatment method could be applied in J. curcas, cotyledonary petiole explants were incubated with different concentrations of TDZ solution for 20 min before being transferred onto MS medium without any hormones. As shown in , the obtained results confirmed our previous observation [Citation11] that the response of adventitious buds induction was influenced significantly by TDZ concentration. In vitro petiole explants were treated with various concentrations (0, 10, 20, 30 and 60 mg/L) of TDZ solution for 20 min before transfer on hormone-free MS medium. As a result, the percentage of induction of adventitious buds and the number of induced shoot buds per explant were also influenced by the concentration of TDZ solution ( and (A–D)). When 20 mg/L of TDZ solution was applied in treatment, the highest rate of adventitious buds regeneration (88.42%) and the highest quantity of regenerated shoot buds per explant (12.67) were obtained ( and (B)); nevertheless, when the application of TDZ concentrations was higher than 20 mg/L, the shoot bud regeneration effect was prominently inhibited (). Comparative analysis of the results shown in and (A–D) suggests that the best and most effective results were obtained when 20 mg/L TDZ was applied.
Table 2. Effect of treating explants with TDZ solution of various concentrations on the regeneration of adventitious buds from in vitro cotyledonary petiole explants of J. curcas after 30 days.*
Figure 2. Direct induction of adventitious buds from cotyledonary petiole explants of J. curcas. Treatment of in vitro petiole explants with 10 mg/L (A), 20 mg/L (B), 30 mg/L (C) and 60 mg/L (D) TDZ solution for 20 min before transfer and horizontal placement on hormone-free MS medium after 30 days in culture (bar = 0.5 cm). Treatment of explants with 20 mg/L TDZ solution for 20 min before inoculation: in vitro petiole explants in vertical position (E), in vivo petiole in horizontal position (F), in vivo petiole in vertical position (G) on hormone-free MS medium after 30 days in culture (bars = 0.5 cm).
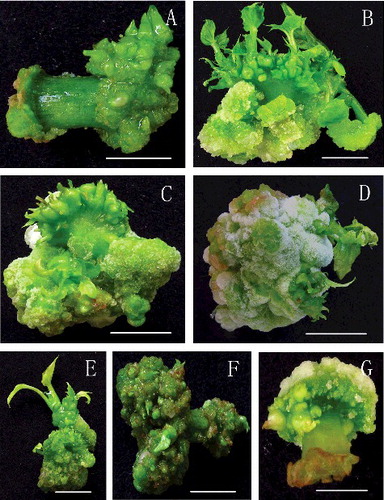
For the purpose of studying the effect of TDZ process-time on the induction of shoot buds regeneration, in this study, in vitro cotyledonary petiole explants were soaked in 20 mg/L of TDZ solution for different lengths of time before inoculation onto MS medium without any hormones. The experimental results suggested that the regeneration effect of adventitious buds was markedly affected by process-time (). When the explants were treated with TDZ solution for 5 min before culture, the lowest percentage of shoot buds induction (50.53%) and least number of regenerated buds (6.62) per explant were observed (). After incubation of the explants in 20 mg/L TDZ solution for 20 min, the best regeneration rate (88.42%) and the highest quantity of regenerated buds per explant (12.67) were obtained ( and (B)). Comparing to our experiments using the conventional approach in the present study, these results were increased by 47%. However, when the explants were incubated in TDZ solution for 40 and 80 min, the effect on adventitious buds regeneration was impeded dramatically compared to that achieved by treatment for 20 min; with the rate of adventitious buds regeneration changing from 54.13% to 67.88% and the quantity of induced adventitious buds per explant, from 6.74 to 8.35 ().
Table 3. Effect of the duration of treatment of explants with TDZ solution on the regeneration of adventitious buds from in vitro cotyledonary petiole explants of J. curcas after 30 days.*
In the present study, the conventional methods for the induction of shoot buds regeneration by direct placement of the explants onto a medium containing TDZ resulted in relatively lower regeneration rates (less than 42%) of shoot buds and the regenerated shoot buds were of poor quality ( and ). However, the regeneration frequency can be increased and the quality of the regenerated buds can be improved effectively by treating the explants with high concentrations of TDZ solution before culture ( and ), and caused the formation of bigger buds simultaneously ((A–D)) as compared with the conventional methods ((A–D)). Therefore, the concentration of TDZ solution and the time periods of treatment significantly influenced the regeneration response ( and ). What is more, further culture of the regenerated buds showed that the regenerated buds were easily elongated (data not shown).
TDZ is a potent cytokinin for woody plant tissue culture.[Citation23] In plant tissue cultures, cytokinin is an essential factor for the induction of shoot bud formation in most cases.[Citation15,Citation18,Citation24–29] The present study showed that cytokinin is only required for a short duration during induction of shoot buds formation. The higher quality of the regenerated buds (bigger buds) was obtained by the new method, which is closely consistent with our previous work in soybeans and J. curcas.[Citation11,Citation22]
The success of the experimental procedure presented here suggested that the induction of adventitious buds formation might not require cytokinin during the whole culture period; when the process of cell division for the formation of adventitious buds was triggered, the presence of exogenous cytokinin might be no longer necessary, and on the contrary, long presence in a form incorporated in the medium might have only negative effects. Furthermore, it has been well documented in many text books and academic journals that cytokinin has the effects of apical suppression and hinders the growth of roots.[Citation11,Citation22]
Effects of origin and orientation of the explants on shoot buds regeneration
To investigate the effect of origin (in vivo or in vitro) and placement orientation (vertical or horizontal) on adventitious buds induction, in vivo or in vitro cotyledonary petioles were treated with 20 mg/L TDZ solution for 20 min before transfer on hormone-free MS medium vertically or horizontally. Our findings suggested that origin and placement orientation of explants both significantly influenced plant regeneration at the concentrations of TDZ solution tested. In vitro explants responded more efficiently than in vivo explants. The percentage of induction of shoot buds varied from 50.92% to 88.42% for in vitro explants ( and (B and E)) and 39.11%–68.46% for in vivo explants ( and (F and G)), whilst the number of shoot buds induced per explant varied from 6.45 to 12.67 for in vitro explants and 4.76 to 9.37 for in vivo explants (). Furthermore, we also investigated the effect of the orientation of the explants on regeneration. The orientation of explants significantly influenced the response of shoot buds induction, with no exception for explants of both different origin sources. Horizontal placement of the explants on the medium was confirmed to be much more conducive to regeneration than the vertical one ( and (B and E–G)), as previously observed by us.[Citation11] The rate of adventitious buds regeneration altered from 68.46% to 88.42% with horizontal placement on the medium ( and (B and F)) but from 39.11% to 50.92% in the case of vertical positioning on the medium ( and (E and G)), whilst the number of induced shoot buds per explant varied from 9.37 to 12.67 in the horizontal position and 4.76 to 6.45 in the vertical position (). The percentage of induction of shoot buds and the number of induced shoot buds differed significantly depending upon the orientation and the source of explants (), which is in agreement with similar observations reported previously by other authors as well.[Citation18,Citation21]
Table 4. Effect of orientation (horizontal or vertical) and source of the explants (in vitro or in vivo) on the regeneration of adventitious buds from cotyledonary petiole explants of J. curcas after 30 days.*
Effects of L-arginine on the elongation of the regenerated shoot buds
Poor quality of the regenerated shoot buds has been a major problem that impedes further development of the regenerated buds in J. curcas tissue cultures. This difficulty has been partially overcome by our innovative method of ‘high TDZ concentration–short treatment duration’. Although the quality of the regenerated shoot buds was greatly improved in our study, new methods for stimulating the elongation of the shoot buds are still desired. L-arginine, an amino acid that has been scarcely used in plant tissue cultures was reported to have beneficial effects in tissue culture of apple.[Citation30]
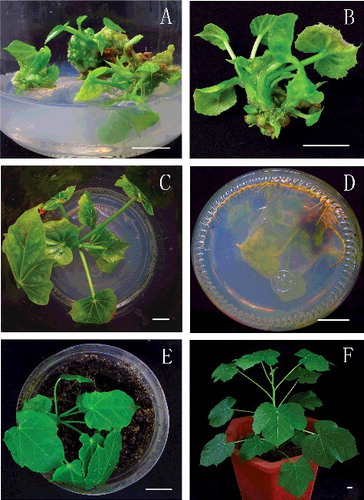
To investigate the effect of L-arginine on the elongation of regenerated buds, the explants with regenerated shoot buds were transferred and placed on fresh MS medium supplemented with 6-BA, KT, IAA, GA3 and various concentrations (7.5–30 mg/L) of L-arginine for 15 days. As shown in , the concentration of L-arginine significantly influenced the elongation of the shoot buds. The most suitable concentration for promoting the elongation of the shoot buds was 7.5 mg/L, which resulted in vigorous and healthy shoot buds, and the best elongation (1.81 cm) ( and (A and B)). When L-arginine was used at concentrations higher than 7.5 mg/L, the elongation of adventitious buds was markedly inhibited (). Thus, it could be concluded that L-arginine stimulated the elongation of the regenerated shoot buds in our study when it was additionally supplemented to the shoot buds elongation medium.
Table 5. Effects of various concentrations of L-arginine on elongation of the regenerated shoot buds from cotyledonary petiole explants of J. curcas after 15 days.
Figure 3. Elongation of adventitious buds, rooting of elongated shoot buds and transplantation of the regenerated plants to the soil. (A) Regenerated adventitious buds were inoculated into elongation medium supplemented with 7.5 mg/L L-arginine for 15 days of culture; (B) close-up of an elongated shoot bud; (C) rooting of elongated shoot buds on half-strength MS medium containing 0.1 mg/L IBA after 30 days in culture; (D) a view from the bottom of the same culture; (E) 20-day-old acclimatized plant; (F) a regenerated plant growing in a pot after acclimatization (bars = 1 cm).
In the present study, 0.75 mg/L L-arginine was considered the optimum concentration for the elongation of shoot buds. Our results indicated that L-arginine, like GA3,[Citation11] may have a positive effect on facilitating the elongation of regenerated buds. Furthermore, shoots multiplication is an essential procedure usually needed in plantlet regeneration of J. curcas.[Citation12,Citation16,Citation17,Citation31] Nevertheless, shoots multiplication and buds elongation were carried out synchronously in the present study, which vastly shortened the culture period for gaining complete plants without losing the quality and quantity of regenerated buds per explant, confirming the results from our previous report.[Citation11]
Effects of IBA on shoots rooting cultures
With the purpose of obtaining intact plants, auxin is frequently supplemented into the rooting medium.[Citation32–34] IBA belongs to the auxins family and has been shown to be efficient in the rooting of a great variety of plants.[Citation14,Citation35–39] In order to test whether IBA could facilitate the rooting of elongated shoot buds in J. curcas, shoots at least 10 mm in length were isolated from the mother tissues and cultured vertically in half-strength MS medium containing various concentrations of IBA (0.1–0.6 mg/L). The results were scored from day 10 to day 40 of culture at 10-day intervals. In our study, the results showed that the concentrations of IBA and the time period of culture significantly influenced the response of shoot rooting. As shown in , supplementing IBA to the medium could effectively stimulate the initiation and growth of roots, with 0.1 mg/L IBA giving the best effect and the highest rooting rate (43.39%) ( and (C and D)). The rooting of shoots could indeed be seen to begin before 10 days of culture, but it was necessary to extend the time to obtain satisfying rooting efficiency. The comparison of the data obtained on day 30 and day 40 indicated that culture periods longer than 30 days had only a little positive effect on rooting, suggesting that a culture period of 30 days was suitable for rooting, as previously observed.[Citation11] The rooting rate in this study was 42.83%, the number of induced roots per shoot was 8.29, and the average length of roots was 4.20 cm (). (C) and (D) shows a healthy plantlet that developed from a shoot cultured for 30 days on half-strength MS medium containing 0.1 mg/L IBA ( and (C and D)). Regenerated plantlets were successfully acclimatized and transplanted to soil with the methods provided above ((E and F)) and no abnormal phenomena were observed up to this point.
Table 6. Effects of various concentrations of IBA on the rooting of regenerated shoots from cotyledonary petiole explants of J. curcas.
The induction of rooting by auxins is usually utilized to obtain regenerative plants from shoots.[Citation32–34] However, higher levels of IBA applied to plants might prevent the development of roots.[Citation40] In our experiments, at 0.3 mg/L IBA and higher concentrations, induction of roots was inhibited. Moreover, significant differences in the response of shoots rooting were observed among the concentrations of IBA and the period of culture in this study ().
In the present study, with application of the described procedure for inducing adventitious buds regeneration from cotyledon petiole explants, which were acquired from 20-day-old aseptic seedlings of J. curcas, the regeneration effect was markedly promoted compared to that previously reported the conventional approach [Citation11,Citation18,Citation21]: the regeneration rate was 29%–47% increased. Moreover, because there was such an abundant quantity and good quality of regenerated buds in our study extra shoots multiplication was not necessary, the culture period could be shortened to less than 28 days.[Citation18,Citation21] In addition, using cotyledon petiole explants in our present study gave a 23% higher regeneration rate of adventitious buds as compared to our previous approach based on plant regeneration from another type and source of explants, petiole explants isolated from physiologically mature J. curcas trees.[Citation11]
To the best of our knowledge, this is the first investigation of the effect of L-arginine on shoot elongation in J. curcas, and good results were obtained by supplementing a proper concentration of L-arginine into the elongation medium. In addition, it might take 90–140 days to obtain intact regenerated plantlets via conventional methods,[Citation12,Citation16–18,Citation21,Citation31,Citation41] whilst by applying the optimized culture technique in this study, the cultivation period was reduced by 30–80 days. Since an efficient method for plant regeneration from cotyledonary petiole explants of J. curcas was established in full, it might be introduced, for example, in Agrobacterium or biolistic bombardment-mediated transformation. The proposed protocol could prove particularly useful because the transformed explants in both these genetic transformation methods need induced regeneration of adventitious buds by means of a well-established plant tissue culture protocol to produce transgenetic seedlings.[Citation8–10,Citation41]
Conclusions
A high-efficiency in vitro culture protocol for obtaining regenerated plantlets from cotyledonary petiole explants of J. curcas was presented. With the help of improved adventitious shoot regeneration, ameliorative shoot elongation and efficient root induction, an intact plantlet could be obtained in 60–75 days of culture by using the described culture protocol, rather than at 90–140 days of culture by conventional methods. This high-efficiency and reproducible method might be applied to the production of transgenic plants through Agrobacterium/biolistic bombardment-mediated transformation.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Gupta RC. Pharmacognostic studies on ‘Dravanti’. Part I. Jatropha curcas Linn. Indian Acad Sci. 1985;1:65–81.
- Openshaw K. A review of Jatropha curcas: an oil plant unfulfilled promise. Biomass Bioenergy. 2000;19:1–15.
- Adebowale KO, Adedire CO. Chemical composition and insecticidal properties of the underutilized Jatropha curcas seed oil. Afr J Biotechnol. 2006;10:901–906.
- Liberalino AAA, Bambirra EA, Moraes-Santos T, et al. Jatropha curcas L. seeds: chemical analysis and toxicity. Arq Biol Technol. 1988;31:539–550.
- Ghosh A, Chaudhary DR, Reddy MP, et al. Prospects for Jatropha methyl ester (biodiesel) in India. Int J Environ Stud. 2007;64:659–674.
- Li MR, Li HQ, Jiang HW, et al. Establishment of an Agrobacterium-mediated cotyledon disc transformation method for Jatropha curcas. Plant Cell Tissue Organ Cult. 2008;92:173–181.
- He Y, Pasapula V, Li X, et al. Agrobacterium tumefaciens-mediated transformation of Jatropha curcas: factors affecting transient transformation efficiency and morphology analysis of transgenic calli. Silvae Genet. 2009;58:123–128.
- Kumar N, Vijayanand KG, Sudheer Pamidimarri DVN, et al. Stable genetic transformation of Jatropha curcas via Agrobacterium tumefaciens-mediated gene transfer using leaf explants. Ind Crops Prod. 2010;32:41–47.
- Qu J, Mao HZ, Chen W, et al. Development of marker-free transgenic Jatropha plants with increased levels of seed oleic acid. Biotechnol Biofuels. 2012;5:1–11.
- Kajikawa M, Morikawa K, Inoue M, et al. Establishment of bispyribac selection protocols for Agrobacterium tumefaciens- and Agrobacterium rhizogenes-mediated transformation of the oil seed plant Jatropha curcas L. Plant Biotechnol. 2012;29:145–153.
- Liu Y, Tong X, Hui WK, et al. Efficient culture protocol for plant regeneration from petiole explants of physiologically mature trees of Jatropha curcas L. Biotechnol Biotech Equip. 2015;29:479–488.
- Sujatha M, Mukta N. Morphogenesis and plant regeneration from tissue cultures of Jatropha curcas. Plant Cell Tissue Organ Cult. 1996;44:135–141.
- Wei Q, Lu WD, Liao Y, et al. Plant regeneration from epicotyl explants of Jatropha curcas. J Plant Physiol Mol Biol. 2004;30:475–478.
- Deore AC, Johnson TS. High-frequency plant regeneration from leaf-disc cultures of Jatropha curcas L. an important biodiesel plant. Plant Biotech Rep. 2008;2:10–15.
- Khurana-Kaul V, Kachhwaha S, Kothari SL. Direct shoot regeneration from leaf explants of Jatropha curcas in response to thidiazuron and high copper contents in the medium. Biol Plant. 2010;54:369–372.
- Kumar N, Reddy MP. Plant regeneration through the direct induction of shoot buds from petiole explants of Jatropha curcas: a biofuel plant. Ann Appl Biol. 2010;156:367–375.
- Kumar N, Vijayanand KG, Reddy MP. In vitro regeneration from petiole explants of non-toxic Jatropha curcas. Ind Crops Prod. 2011;33:146–151.
- Kumar N, Reddy MP. Thidiazuron (TDZ) induced plant regeneration from cotyledonary petiole explants of elite genotypes of Jatropha curcas: a candidate biodiesel plant. Ind Crops Prod. 2012;39:62–68.
- Zhang C, Fu SP, Tang GJ, et al. Factors influencing direct shoot regeneration from mature leaves of Jatropha curcas, an important biofuel plant. In Vitro Cell Dev Biol Plant. 2013;49:529–540.
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–479.
- Kumar N, Vijayanand KG, Reddy MP. In vitro plant regeneration of non-toxic Jatropha curcas L.: direct shoot organogenesis from cotyledonary petiole explants. J Crop Sci Biotech. 2010;13:189–194.
- Liu Y, Yu L, Zhang Q, et al. High concentration short duration treatment of benzyladenine stimulates adventitious bud regeneration from hypocotyl explants in soybean. Adv Mater Res. 2013;647:331–335.
- Huetteman CA, Preece JE. Thidiazuron: a potent cytokinin for woody plant tissue culture. Plant Cell Tissue Organ Cult. 1993;33:105–119.
- Lin HS, De Jeu MJ, Jacobsen E. Direct shoot regeneration from excised leaf explants of in vitro grown seedlings of Alstroemeria L. Plant Cell Rep. 1997;16:770–774.
- Magioli C, Rocha APM, de Oliveira DE, et al. Efficient shoot organogenesis of eggplant (Solanum melongena L.) induced by thidiazuron. Plant Cell Rep. 1998;17:661–663.
- Murthy BNS, Murch SJ, Saxena PK. Thidiazuron: a potent regulator of in vitro plant morphogenesis. In Vitro Cell Dev Biol Plant. 1998;34:267–275.
- Feyissa T, Welander M, Negash L. In vitro regeneration of Hagenia abyssinica (Bruce) J.F. Gmel. (Rosaceae) from leaf explants. Plant Cell Rep. 2005;24:392–400.
- Jha T, Mukherjee P, Datta MM. Somatic embryogenesis in Jatropha curcas Linn., an important biofuel plant. Plant Biotechnol Rep. 2007;1:135–140.
- Rajore S, Batra A. An alternative source for regenerable organogenic callus induction in Jatropha curcas. Indian J Biotechnol. 2007;6:545–548.
- Sotirpoulos TE, Dimassi KN, Therios IN. Effects of L-arginine and L-cysteine on growth, and chlorophyll and mineral contents of shoots of the apple rootstock EM 26 cultured in vitro. Biol Plantarum. 2005;49:443–445.
- Sarina P, Beniwal VS, Laura JS. An efficient plant regeneration protocol from petiole explants of physic nut (Jatropha curcas L.). Afr J Biotechnol. 2012;11:12652–12656.
- Vuylasteker C, Dewaele S, Rambour S. Auxin induced lateral root formation in chicory. Ann Bot. 1998;81:449–454.
- Gunes T. Peroxidase and IAA oxidase activities during rooting of poplar species. Turk J Bot. 2000;24:97–101.
- Nandagopal S, Ranjitha Kumari BD. Effectiveness of auxin induced in vitro root culture in chicory. J Cent Eur Agric. 2007;8:73–79.
- Kochhar S, Singh SP, Kochhar VK. Effect of auxins and associated biochemical changes during clonal propagation of the biofuel plant Jatropha curcas. Biomass Bioenergy. 2005;32:1136–1143.
- Datta MM, Mukherjee P, Ghosh B, et al. In vitro clonal propagation of biodiesel plant (J. curcas L.). Curr Sci. 2007;93:1438–1442.
- Thepsamran N, Thepsithar C, Thongpukdee A. In vitro induction of shoots and roots from J. curcas L. explants. J Hortic Sci and Biotechnol. 2008;83:106–112.
- Singh A, Reddy MP, Chikara J, et al. A simple regeneration protocol from stem explants of Jatropha curcas-a biodiesel plant. Ind Crops Prod. 2010;31:209–213.
- Daud N, Faizal A, Geelen D. Adventitious rooting of Jatropha curcas L. is stimulated by phloroglucinol and by red LED light. In Vitro Cell Dev Biol Plant. 2013;49:183–190.
- Ozel C, Khawar K, Mirici S, et al. Induction of ex vitro adventitious roots on softwood cuttings of Centaurea tchihatcheffii tchihatcheffii Fisch. et Mey using indole-3-butyric acid and naphthalene acetic acid. Int J Agric Biol. 2006;1:66–69.
- Joshi M, Mishra A, Jha B. Efficient genetic transformation of Jatropha curcas L. by microprojectile bombardment using embryo axes. Ind Crops Prod. 2011;33:67–77.