ABSTRACT
Generic medicines play a key role in managing the financial resources for pharmaceuticals in every country. This study analysed the generic policy legislative framework in Bulgaria with the aim to identify whether the policy implementation can be considered successful in the light of an international review of such policies introduced in other countries, or on the contrary, it has failed to deliver the main benefits. Legislative analysis, desktop study and macroeconomic overview of the Bulgarian pharmaceutical market were included. The study showed that only 3 out of 11 important policy elements are implemented in the country. Bulgaria has one of the highest shares of generics, an average of 81.39% (volume), for the studied period (2006–2014). However, further research is needed to evaluate the success of the existing generic policy in Bulgaria, as the market share of generic drugs is not the only measure of the policy efficiency.
Introduction
The expenditures on pharmaceuticals have grown fast in all European countries in the last 20 years.[Citation1–4] Generic medicines, as essentially similar to reference medicines with expired patents, play a key role in managing the financial resources for pharmaceuticals in every country.[Citation5–11] They offer the same quality, safety and efficacy as the reference products do, but at more affordable prices.[Citation12–15] Thus, they increase the access to affordable medicines and increase the number of treatment days. According to the European Generic Association, for seven key therapy areas, 60% price decrease leads to 200% increase in treatment days.[Citation16] In the light of constantly increasing pharmaceutical expenditures, the question of successful implementation of generic policy is of crucial importance for every country.[Citation17,Citation18] Many countries have adopted generic policies setting specific measures on different levels: marketing authorization, patent protection, pricing, reimbursement, prescribing, use, etc. In Bulgaria, the generic competition is not strongly stimulated and this fact does not correspond to the trends around the world. The creation of a sustainable generic pharmaceutical market requires active regulatory and marketing measures at all levels, including incentives for manufactures, physicians and dispensers.[Citation19,Citation20] Two previously published studies demonstrated the influence of the entrance of generics on the prescribing patterns on their prices and utilization in Bulgaria and provided more evidence of the important influence of the competition within the same therapeutic group.[Citation21,Citation22]
The aim of this study was to analyse the generic policy legislative framework in Bulgaria and to investigate whether its implementation can be considered successful in the light of an international review of such policies introduced in other countries, or on the contrary, it has failed to deliver the main benefits. The analysis was based on: a review of specific national legislation related to promotion of use of generic medicinal products; a review of international literature concerning the instruments implemented for promotion of generics; analysis of the dynamics of sales of generic medicinal products in Bulgaria in 2006–2014 and analysis of the effect of the implementation of national legislation on the use of generics in Bulgaria.
Materials and methods
Legislative analysis
Legislative changes in the Law on Medicinal Products in Human Medicine and related regulations in the area of marketing authorization, patent protection of medicines, prescribing and use of generic medicinal products, implemented on a national level in the observed period (2006–2014) were identified and discussed.[Citation23–27]
Desktop study
Review of the international literature was done by searching the databases PubMed,[Citation28] EMBASE [Citation29] and Cochrane Library [Citation30] with chosen search terms, such as ‘generic policy’, ‘generic medicines’, ‘substitution’, ‘international nonproprietary name (INN),’ etc. We selected the work of Simoens,[Citation31] which describes the generic drug policy accepted measures in all European countries, to benchmark the national legislation measures with these ones.
Macroeconomic analysis of the pharmaceutical market
Data on the dynamics of the sales of generic medicinal products in Bulgaria (2006–2014) were retrieved from IMS Health (personal communication, 2015; unreferenced). A review of international literature on implemented the instruments of generic policy was performed and the specific Bulgarian legislation related to promotion of the use of generic medicinal products was analysed. Legislative changes in the Law on Medicinal Products in Human Medicine and related regulations [Citation23–27] in the area of marketing authorization, patent protection, prescribing and use of generic medicinal products, implemented during the observed period were identified and analysed in the light of the dynamics of the volume of sales of generic products. A review of the international literature was done by searching the databases PubMed,[Citation28] EMBASE [Citation29] and Cochraine Library [Citation30] with selected search terms, such as “generic policy”, ‘generic medicines’, ‘substitution’, ‘INN,’ etc. The review of generic policies was limited to the member states of the European Union only.
Results and discussion
Legislation analysis and desktop study
The national legislation related to the generic policy in Bulgaria is outlined in . No explicit statement of the generic policy approval is available in the national drug policy draft document. So far, more than 20 years after the decentralization of the health sector in Bulgaria, there is still no national drug policy officially adopted, implemented and monitored. The current draft [Citation23] does not describe the conceptual frame of generic policy but rather comments on different elements of its. The only improvement which will lead to further promotion of generic medicines that the draft recognizes is inclusion of the requirement for marketing authorization holders of generic products to update their Summary of Product Characteristics and Patient Information Leaflets accordingly in a timely manner following changes in the originators’ data. This will help penetration of generics for reimbursement for the new indications already approved for the originals.
Table 1. Bulgarian legislation related to generics.
In general, the generic products enjoy a facilitated procedure for marketing authorization, as the preclinical and clinical data are not needed for submission. As far as the reimbursement is concerned, generic products’ marketing authorization holders are not required to present the relevant pharmacoeconomic evaluations and are, therefore, included in the Positive Drug List faster than the originators (30 days vs. 90 days). Due to the internal reference pricing, the payer pays the lowest price within the group of medicines with the same INN, which promotes the use of lower-priced generic products in most groups. For some therapeutic groups, it is possible to have formation of jumbo reimbursement groups without clear rules.
According to the ordinance on the conditions for prescribing and dispensing of medicinal products,[Citation26] physicians in Bulgaria can prescribe medicines as per their convenience: by their trade names, INNs, according to the pharmacopeia, etc.[Citation26] In non-narcotic medicines, there is a check-box to thick if the physician agrees with a generic substitution. As of 21 February 2014, a new paragraph requires generic prescribing in case the prescriptions are intended for another EU member state. The same regulation forbids a generic substitution in case the product is prescribed with its trade name; the pharmacist is obliged to dispense exactly the prescribed medicine and in case it is not available in the pharmacy, it shall be ordered within the next 24 hours (paragraph 34,(1),2 and (2)). The same is also the requirement of the National Health Insurance Fund.
The generic drug policy approach is focused mainly on flexible marketing authorization procedures, shorter deadline for obtaining reimbursement status and patient stimulation to purchase lower-priced generic medicines. This last point could be considered a rather questionable measure, since, in case the physician has not allowed for a substitution, neither the pharmacists, nor the patient can change their choice.
summarizes the issues that, according to Simoens,[Citation31] promote/inhibit the access to generic medicines and benchmarks their status in Bulgaria. According to Simoens,[Citation31] the education of professionals and patients in the quality, safety and efficacy of generic medicines and interchangeability of generics and originators, reference pricing systems and medicine prices may increase the use of generics. Out of 11 elements, only three ones are implemented in Bulgaria: namely, faster reimbursement procedure for generic medicines, external reference pricing and lower co-payment.
Table 2. Generic policy elements [Citation31]: implementation in Bulgaria.
Market analysis
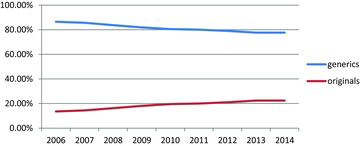
The national pharmaceutical market is small mainly due to the population size in Bulgaria, the gross domestic product, low average income of Bulgarian citizens, as well as the small public spending for reimbursement of medicinal products. The total market size in units for 2014 [Citation29] was 268.54 mln (). The dynamics of sales of medicines for the period 2006–2014 reveal a positive trend over the studied period. This is applicable for both generics and originals. Regarding the market share (out of the total market), there has been a trend for a decrease in the market share of generic medicinal products in Bulgaria from 86.45% in 2006 to 77.63% in 2014 ().
Table 3. Sales of generic and originator medicinal products (mln units) in Bulgaria in 2006–2014*.
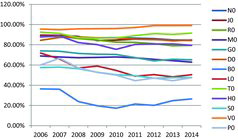
An in-depth look into the figures on the distribution of sales amongst different Anatomical Therapeutic Chemical (ATC) groups () revealed that 58.78% of the generics in 2014 were marketed for treatment of various diseases of the cardiovascular system, nervous system and alimentary tract and metabolism. The biggest increase was observed in the groups of dermatologicals (from 7.31 mln units to 11.91 mln units), hospital solutions (from 10.23 mln units to 14.35 mln units), respiratory system (from 18.31 mln units to 22.39 mln units), blood and blood-forming organs (from 1.29 mln units to 2.59 mln units), etc.
Table 4. Sales of generics (mln units) by ATC groups*.
Further investigation revealed that the generics hold a significant market share for all ATC groups (), except for the groups of sensory organs (an average of 51.79% in the studied period), parasitology (an average of 52.19%) and blood and blood-forming organs (an average of 25%). The average market share of the generics in the studied period was 81.39%.
Final remarks
Taken together, our analysis revealed two basic trends in the Bulgarian market of generics: a significant market share of generics, on the one hand (possibly due to the reference pricing system applied), versus a downward trend in the share of generics in the studied period, on the other hand. Seeking and identifying the underlying factors that could possibly explain these trends could facilitate future optimization of generic penetration and management of budget on medicines and could help to identify the areas which future efforts should focus on.
Generally, the market shares of generics vary from country to country within the European Union: from 4% in Italy to 86.5% in Poland. In our study, we found a significant average market share of generics in Bulgaria, 81.39% (volume) for the studied period. In comparison, in many EU member states, the generics account for more than 50% in the volume of total medicine prescriptions: 49.3% in the United Kingdom, 54% in Slovenia, 65% in Denmark, etc.[Citation32–35] This ranks Bulgaria as a EU member state with one of the highest market shares of generics.
One of the underlying factors for a high market share of genetics could be expected to be a smart implementation of generic policy. This, however, could less likely be the case in Bulgaria, since the only elements which we identified in the local legislation are limited to facilitated reimbursement procedures, external reference pricing and lower co-payments for patients. In contrast, other EU countries apply a broader portfolio, which is documented by Simoens [Citation31] as well as in other studies.[Citation32] Such policies are implemented in Canada, the United States, but also in some EU member states, such as the United Kingdom, the Netherlands, Germany, Denmark, Belgium, etc.[Citation33–35]
Thus, the high share of generic products in Bulgaria could be considered to be more likely due to the external and internal reference pricing, since other essential elements are not implemented in the country, as pointed out in some previous studies.[Citation36,Citation37] This phenomenon should be studied in-depth, as some authors, i.e. Simoens and de Coster,[Citation35] share the opinion that ‘penetration of generic medicines is more successful in countries that permit (relatively) free medicine pricing.’ In Bulgaria, however, the tight pricing system in the light of scarce resources seems to be the main reason for the significant generic penetration. Both mechanisms, generic policy and reference pricing, are part of the local regulation on pricing and reimbursement, according to which the lowest manufacturer price amongst 17 countries is submitted as a base price for price formation. In fact, it has been poorly, if at all, explored whether physicians shift their prescribing habits to lower-priced generics or have preferences for some brand products.
The other trend, i.e. the decrease in the share of generics over the studied period, needs further in-depth investigation. It could be speculated that this trend might be associated with originators’ actions in an effort to balance the market (promotion of new therapeutic options (switch from generics to innovative treatment), subsidizing patient's co-payment, etc.).
Overall, the results of the present investigation indicate that, despite of the high penetration of generic medicines, Bulgaria needs to implement the missing elements of the generic policy, since it is only the complete concept that could best serve the society, patients and payer's needs. Further research is also needed for a more comprehensive evaluation of the success of the existing generic policy in Bulgaria, as the market share of generic drugs is not the only measure of the policy efficiency. Other attributes of the success of a generic drug policy are the quality and efficacy of generic medicines, supply reliability and health outcomes.
Conclusions
A successful generic policy is needed in every country facing a lack of resources. Bulgaria is not an exception. The share of generic drugs on the Bulgarian market is significant and it is actually one of the highest within the European Union by volume. This significant share in Bulgaria is unlikely due to a strong generic policy, but rather to reference pricing and low or no co-payment for generic medicines. The tight pricing system in Bulgaria in the light of scarce resources seems to be the main reason for the significant generic penetration. Despite of the high penetration of generic medicines, Bulgaria needs to implement the missing generic policy elements to best serve the society, the patients and payers’ needs. It is of crucial importance to introduce substitution rights for pharmacists, which need to be supplemented by training of health professionals and laypeople about the generic concept. Further research needs to focus on other attributes of the success of a generic drug policy, such as the quality and efficacy of generic medicines, supply reliability and health outcomes.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Ess SM, Schneeweiss S, Szucs TD. European healthcare policies for controlling drug expenditure. Pharmacoeconomics. 2003;21(2):89–103.
- Jönsson B. Pricing and reimbursement of pharmaceuticals in Sweden. Pharmacoeconomics. 1994;6(S1):51–60.
- Rigter H. Recent public policies in the Netherlands to control pharmaceutical pricing and reimbursement. Pharmacoeconomics. 1994;6(S1):15–21.
- Fattore G, Jommi C. The new pharmaceutical policy in Italy. Health Policy. 1998;46:21–41.
- UK Department of Health. Options for the future supply and reimbursement of generic medicines for the NHS: a discussion paper [Internet]. London: Department of Health; 2001 [updated 2001 Jul 23; cited 2016 Apr 16]. Available from: http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_4019990.pdf.
- Giuliani G, Selke G, Garattini L. The German experience in reference pricing. Health Policy. 1998;44:73–85.
- Mossialos E, Le Grand J. Cost containment in the EU: an overview. In: Mossialos E, Le Grand J, editors. Health care and cost containment in the European Union. Aldershot: Ashgate; 1999. p. 128–129.
- Hensley S. Another war on drugs: European countries turn to ‘reference pricing’ in battle against soaring cost of pharmaceuticals. Mod Healthc. 1999;29:38–39.
- Garattini L, Tediosi F. A comparative analysis of generic markets in five European countries. Health Policy. 2000;51:149–162.
- Tele P, Groot W. Cost containment measures for pharmaceuticals expenditure in the EU countries: a comparative analysis. Open Health Serv Pol J. 2009;2:71–83.
- Kaló Z, Holtorf AP, Alfonso-Cristancho R, et al. Need for multicriteria evaluation of generic drug policies. Value Health. 2015;18(2):346–351.
- Cameron A, Ewen M, Ross-Degnan D, et al. Medicine prices, availability, and affordability in 36 developing and middle-income countries: a secondary analysis. Lancet. 2009;373:632–635.
- Kanavos P. Financing pharmaceuticals in transition economies. Croat Med J. 1999;40:244–259.
- Cameron A, Laing R. Cost savings of switching private sector consumption from originator brand medicines to generic equivalents. Background Paper 35 [Internet]. Geneva: World Health Organization; 2015; c2010 [cited 2015 Apr 16]. Available from: http://www.who.int/healthsystems/topics/financing/healthreport/35MedicineCostSavings.pdf.
- Dylst P, Simoens S. Generic medicine pricing policies in Europe: current status and impact. Pharmaceuticals. 2010;3:471–481.
- van de Hoven A. Improving access to generic & biosimilar medicines in Bulgaria and in the EU [Presentation] Nov 14. Sofia (Bulgaria): European Generic Medicines Associations (EGA); 2013. Available from: http://static.framar.bg/filestore/Adrian_van_den_Hoven.pdf.
- Kanavos P. Pharmaceutical coverage and resource allocation: lessons for the US from pharmaceutical policies in the UK. Paper presented at: Annual Research Meeting of the Academy of Health Services Research and Health Policy; 2002 June 23–25; Washington, DC, USA.
- Hassali MA, Wong ZY. Challenges of developing generics substitution policies in low- and middle-income countries (LMICs). GaBI J. 2015;4(4):171–172.
- Manova M, Stoimenova A, Clerfeuille F, et al. Impact of the generic competition on usage and process of cardiovascular medicines on the Bulgarian pharmaceutical market. J Public Health. 2011;19(1):91–100.
- Zeng W. A price and use comparison of generic versus originator cardiovascular medicines: a hospital study in Chongqing, China: BMC Health Serv Res [Internet]. 2013 [cited 2016 Apr 16];13:390. Available from: http://bmchealthservres.biomedcentral.com/articles/10.1186/1472-6963-13-390.
- Manova M, Stoimenova A, Savova A, et al. Utilization and price trends in some reimbursed cardiovascular medicines. Biotechnol Biotechnol Equip. 2011;25(2):2424–2431.
- Petrova G, Manova M, Stoimenova A, et al. Cardiovascular medicines prescribing in Bulgaria. Compt Rend Acad Bulg Sci. 2011;64(2):285–292.
- Ministry of Health of the Republic of Bulgaria. National drug policy [Internet]. Sofia: Ministry of Health; 2015; c2015 [ cited 2015 Apr 16]. Available from: http://www.mh.government.bg. Bulgarian.
- Law on Medicinal Products in Human Medicine. State Gazette [Internet]. №15 [cited 2013 Feb 15]. Available from: http://en.bda.bg/index.php?option=com_content&view=article&id=21&Itemid=10.
- Legislatiive Document. Ordinance № 27 of 15 Jun 2007 for the requirements and documentation for authorization and registration of medicinal products. Bulgarian State Gazette [Internet]. №54; 2007 [cited 2015 Apr 16]. Available from: http://www.bda.bg/index.php?option=com_content&view=article&id=68&Itemid=60&lang=bg.
- Legislatiive Document. Ordinance № 4 of 4 Mar 2009 on the conditions for prescribing and dispensing of medicinal products. Bulgarian State Gazette [Internet]. №21; 2009 [cited 2015 Apr 2016 ]. Available from: http://www.mh.government.bg/articles.aspx?lang=bgbg&pageid=391&categoryid=1246.
- Legislatiive Document. Ordinance on the terms, rules and procedure for and registration of prices for medicinal products. Bulgarian State Gazette [Internet]. №40; 2013 [Amended and supplemented, SG №66; 2014 Aug 8. Amended and supplemented, SG №92; 2014 Nov 7. Amended, SG №107; 2014 Dec 24. Amended and supplemented, SG № 92; 2015 Nov 27. Cited 2015 Apr 16]. Available from: http://www.ncpr.bg. Bulgarian.
- PubMed Health [Internet]. Bethesda (MD):National Center for Biotechnology Information. 2005 [cited 2016 Feb 14]. Available from: http://www.ncbi.nlm.nih.gov/pubmed.
- Embase (Excerpta Medica Database) [Internet]. Amsterdam: Elsevier B.V; 2015; c2015 [cited 2015 Apr 16]. Available from: http://www.embase.com.
- Cochrane Library [Internet]. Chichester: Wiley; 2016; c1999–2016 [ cited 2016 Feb 14]. Available from: www.cochranelibrary.com.
- Simoens S. Sustainable provision of generic medicines in Europe [Internet]. Brussels: European Generic Medicines Association; 2013 [cited 2015 Feb 25]. Available from: http://www.quotidianosanita.it/allegati/create_pdf.php?all=3090824.pdf.
- Kaplan WA, Ritz LS, Vitello M, et al. Policies to promote use of generic medicines in low and middle income countries: a review of published literature, 2000–2010. Health Policy. 2012;106(3):211–224.
- King DR, Kanavos P. Encouraging the use of generic medicines: implications for transition economies. Croatian Med J. 2002;43(4):462–469.
- Simoens S, De Bruyn K, Bogaert M, et al. Pharmaceutical policy regarding generic drugs in Belgium. Pharmacoeconomics. 2005;23(8):755–766.
- Simoens S, De Coster S. Sustaining generic medicines markets in Europe. J Generic Med. 2006;3(4):257–268.
- Mitkova Z, Manova M, Petrova G. Impact of the generic competition on reference prices of cardiovascular medicines. Pharmacia. 2014;61(3):9–17.
- Mitkova Z, Manova М, Petrova G. Influence of the generic competition on reference prices of cardiovascular fixed dose combinations. Sci Pharmacol. 2014;2(9):27–37.