ABSTRACT
The present work investigated the antibacterial activity of Ptaeroxylon obliquum leaves (POL) extracts in the presence or absence of ciprofloxacin by the broth microdilution method and time–kill assay against bacterial strains associated with wound infections. Free-radical–scavenging activity (FRSA) was determined using the stable 2,2-diphenyl-2-picrylhydrazyl hydrate (DPPH) bioassay method. The chemical composition of the most active antioxidant extract was analysed using a gas chromatograph interfaced with a mass spectrometer (GC-MS). All POL extracts showed good antibacterial activity against the bacterial strains, with a minimum inhibitory concentration (MIC) ranging from 4 to 128 µg/mL. The exposure of bacterial strains to POL extracts resulted in 2–64-fold reductions in the MIC of ciprofloxacin. Correspondingly, the time–kill curves showed that combined POL extracts and ciprofloxacin treatment inhibited bacterial growth below the lowest detectable limit after 24 h of incubation. Furthermore, the ethanol extract from P. obliquum (POE) had the highest total flavonoids content (TFC: 62.7 mg/quercetin equivalent/g), while the methanol extract of P. obliquum (POM) had the best total phenolic content (TPC: 275 mg/quercetin equivalent/g) and DPPH*-scavenging activity having 50% inhibitory concentration (IC50) of 0.125 mg/mL. The chemical composition indicated the presence of aromatic and aliphatic compounds that are known to have a wide biological effect. The findings from this study suggest that POL extracts could be a source of pharmaceutical agents for treatment of skin diseases, wound infections and as putative candidates to modulate the multidrug resistance mechanism.
| Abbreviations | ||
| CFU | = | colony-forming unit |
| DPPH | = | 2,2-diphenyl-2-picrylhydrazyl hydrate |
| DMSO | = | dimethyl suphuroxide |
| FCR | = | Folin–Ciocalteu reagent |
| FRSA | = | free-radical–scavenging activity |
| GAE | = | gallic acid equivalents |
| GC-MS | = | gas chromatography–mass spectrometry |
| IC | = | inhibitory concentration |
| MHB | = | Mueller–Hinton broth |
| MIC | = | minimum inhibitory concentration |
| MTT | = | 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide |
| MW | = | molecular weight |
| POA | = | Ptaeroxylum obliquum acetone |
| POC | = | Ptaeroxylum obliquum chloroform |
| POL | = | Ptaeroxylum obliquum leaf |
| POM | = | Ptaeroxylum obliquum methanol |
| ROS | = | reactive oxygen species |
| SIC | = | sub-inhibitory concentration |
| TFC | = | total flavonoids content |
| TPC | = | total phenolic content |
Introduction
A wound is a form of injury that disrupts the epithelial integrity of the skin or any of the underlying body tissues. It is mostly caused by physical, thermal, chemical and immunological insult to the tissue.[Citation1] The healing of a wound is a dynamic process that involves various biochemical and physiological phenomena for the restoration of the structural and functional integrity of injured tissues via inflammation, wound contraction, remodelling and granulation of tissue with angiogenesis.[Citation2] However, the process of wound healing may be hindered or delayed due to high free radicals generation with proven detrimental effects to wound-surrounding cells or by the invasion of multidrug-resistant bacteria resulting in chronic wounds.[Citation3,Citation4] This type of wound affects a large number of patients and at the same time reduces their quality of life. The current estimate shows that about 6 million people are suffering from chronic wounds worldwide.[Citation5] In South Africa, the prevalence of patients living with chronic wounds is high and rapidly increasing conceivably due to growing number of people living with human immunodeficiency virus, tuberculosis, ageing population, antibiotic resistance and metabolic syndromes with limited treatment options amongst the population.[Citation6] Presently, the burden of chronic wound treatment is escalating and becoming worrisome due to financial implications arising from the demand on health care systems.[Citation7] Herbal drugs have been recommended for primary health care to promote the rate of wound healing in patients and to improve their well-being [Citation8] but only a small number of these plants have received rigorous scientific investigation.
Ptaeroxylon obliquum (Rutaceae), also known as sneezewood, is a small tree that is endemic to South Africa. It was discovered as one of the most commonly used plants in the Eastern Cape Province of South Africa for the treatment of wounds sustained during male circumcision rite, cattle wound and mysiasis in livestock.[Citation9] In addition, the infusion of the leaves, stem bark and the root of this folk medicine is mostly used in the region for the management of diverse diseases such as headaches, cough, dysentery, malaria, itching, colic, tick control and chest pain.[Citation10] The same authors reported a mild antibacterial potential of aqueous extract from P. obliquum leaf (POL) against certain bacterial strains. Nielsen et al. [Citation11] also reported a considerable antibacterial activity of heartwood and stem bark extracts of POL against some bacterial strains. Chemical compounds such as peucenin, ptaeroxylinol acetate (chromones) and scopoletin along with prenyletin (coumarins) have been isolated from this botanical using different chromatographic techniques.[Citation12,Citation13] A new meroterpenoid known as ptaerobliquol was isolated from this plant having a moderate activity towards Toxoplasma gondii replication but appeared to be toxic, suggesting a low therapeutic index.[Citation13] Presently, there is a dearth of scientific data on the bacterial resistance modulation and time–kill effect of POL extracts against a panel of bacterial strains. Therefore, the present study aimed at investigating the antibacterial activity of POL extracts against bacterial strains associated with wound infections and the chemical composition of the most active extract using gas chromatography coupled with mass spectrometry.
Materials and methods
Plant collection and preparation
The plant materials were collected from the Campus area of the University of Fort Hare in June 2014 and authenticated by Prof. DS Grierson of Botany Department, the University of Fort Hare. The voucher specimen (Dele01) was deposited in the Giffen herbarium of the University. The POLs were washed with sterilized water and were air-dried at room temperature for 14 days. They were ground with a blender to fine powdery form and stored in an airtight container for further use. Fifty grams (50 g) of the dried powdered materials were extracted with 500 mL of 100% methanol or ethanol or chloroform or acetone on a mechanical shaker (Stuart Scientific Orbital Shaker 20.2, SOSI, Essex, UK) for 24 h, and the extract was filtered using a Buchner funnel and Whatman No.1 filter paper. The extract was concentrated using a rotary evaporator at 40°C to recover the solvent and then dried over the air in a fume chamber to give a yield of 3 and 4.5 g. The extract was stored in the refrigerator at 6 °C for future use.
Total flavonoids content (TFC) determination
TFC in the leaf extract was determined by using the aluminium colorimetric assay method.[Citation14] A volume of 1 mL of 2% AlCl3 ethanol solution was added to 1 mL of the sample solution. The yellow colour formation after 1 hour of incubation at room temperature was measured at 420 nm using an AJI-C03 UV–VIS spectrophotometer. The TFC present in POL extracts was calculated as mg/g of quercetin and was expressed as quercetin equivalent (QE).
Total phenolic content (TPC) determination
TPC in POL extracts was determined by the Folin–Ciocalteu reagent (FCR), using the method of Lister and Wilson as described in Kim et al. [Citation15] with slight modification. Briefly, 0.5 mL of plant extract solution (1 mg/mL) was added to the reaction mixture consisting of 2.5 mL of 10% FCR and 2 mL of Na2CO3 (2% w/v). The resulting mixture was incubated at 45 °C with shaking for 15 min and the absorbance at 765 nm was measured. A standard curve was prepared by mixing ethanol solution of gallic acid (1 mL; 0.025–0.400 mg/mL) with 5 mL of 10% FCR and sodium carbonate (4 mL, 0.7 mol/L). The experiment was carried out in triplicate, and the results are presented as mean values with standard deviation (±SD). The TPC in the extract was extrapolated using the standard gallic acid equivalents (GAE), calculated by the following formula: T = C×V/M, where T is the TPC, (mg/g) of extract, in GAE; C is the concentration of gallic acid established from the calibration curve; V is the volume of the extract, mL; M is the dry weight (g) of the leaf powder from which the extract was obtained. The TPC present in POL extracts were expressed as GAE.
Free-radical–scavenging activity (FRSA) using DPPH assay
Free-radical–scavenging activity (FRSA) of P. obliquum (POL) extracts was measured in vitro by the DPPH (2,2-diphenyl-2-picrylhydrazyl hydrate) assay, according to the method described by Tariq et al. [Citation16]. Twenty-five milligrams (25 mg) of the crude extracts were dissolved in 5 mL of methanol as stock solution and serially diluted to working concentrations (1, 0.5, 0.25, 0.125, 0.0625 and 0.03125 mg/mL); 1.5 mL of this solution was mixed with 1.5 mL of 0.1 mmol/L DPPH prepared in DMSO (dimethyl sulfoxide, Sigma). The reaction mixture was vortexed thoroughly and left in the dark at room temperature for 30 min. The absorbance was read at 517 nm after 2 min. The percentage FRSA was calculated as [1−TAi−Aj)/Ac]×100, where Ai is the absorbance of 1.5 mL of the crude extract solution mixed with an equal volume of the DPPH solution; Aj is the absorbance of 1.5 mL of the crude extract solution mixed with an equal volume of DMSO and Ac is the absorbance of a blank sample (1.5 mL of DPPH with an equal volume of methanol). Here, we calculated the concentration of plant samples needed to decrease the absorbance of DPPH by 50% (IC50). Rutin (Sigma-Aldrich, ≥94%, HPLC grade) at the same working concentrations of the plant extracts was used as standard drug. The 50% inhibitory concentration (IC50) was calculated as the concentration at which 50% of the free radical was scavenged.
Bacterial strains
The following bacterial strains Escherichia coli (ATCC 8739), Staphylococcus aureus (OKOH1), Pseudomonas aeruginosa (ATCC 19582), Proteus vulgaris (CSIR 0300), Streptococcus pneumoniae (ATCC 49619), P. mirabilis (ATCC 43071), Aspergillus brazilensis (ATCC 0322) and S. sonnei (ATCC 29930) were obtained from the Department of Nature Conservation and Ethnobotany, Mangosuthu University of Technology, South Africa. The inocula of these bacteria were prepared using the colony suspension method. The bacterial strains and isolates were grown at 37°C overnight and maintained on nutrient agar (Basal media) and then standardized following the McFarland turbidity of 0.5 at 600 nm to achieve 5×105 colony-forming units per millilitre (CFU/mL).
Minimum inhibitory concentration (MIC) determination
The antibacterial activity of the leaf extracts from P. obliquum was determined following the micro-dilution assay procedure as described by Shiu and Gibbons.[Citation17] Inocula of the test organisms were diluted in normal saline (9 mg/mL) and the suspensions were adjusted to 5×105 (CFU/mL). A volume of 100 µL Mueller–Hinton broth (MHB) was dispensed into the sterilized 96-well plates. A stock solution of ciprofloxacin (Sigma-Aldrich, Johannesburg, South Africa) or herbal extracts was prepared in 1% DMSO (Sigma-Aldrich, Johannesburg, South Africa) and serially diluted into each well, mixed with 100 μL of standardized bacterial inocula to give a final concentration ranging from 2048 to 1 μg/mL for the extract and 128 to 0.08 μg/mL for the antibiotic. Then, 20 μL of a 5 mg/mL methanol solution of 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT; Sigma-Aldrich, Johannesburg, South Africa) was added to each well and incubated for 30 mins. A dark blue coloration indicated bacterial growth. The MIC value was recorded as the lowest concentration at which no visible growth was observed.
Bacterial resistance modulation assays
The modulation assay was performed using sub-inhibitory concentration (SIC) of the plant extracts with ciprofloxacin against our panel of bacterial strains.[Citation18] Briefly, a volume of 100 µL of MHB was dispensed in each well of a 96-well microplate except the wells in column 1, which contained 100 µL of the plant extracts (stock). The samples at the stock concentrations (0.5, 1, 2, 4, 8 and 16 µg/mL) were introduced into the wells of columns 2–10, whereas 100 µL of ciprofloxacin (MICs) was added into well 1 and serially diluted (0.039 to 64 µg/mL) across the plate, leaving column 11 empty for absolute growth control and column 12 as uninoculated controls. All strains were cultured on nutrient agar slope before MIC determination. Overnight cultures of each strain were prepared in 9 mg/mL saline to an inoculum density of 5×105 CFU/mL. Then, 100 µL standardized inocula were added to the wells but the wells in row 7 and 8 were maintained free from the extracts. The plates were incubated at 37 °C for 24 h, after which 20 µL of MTT was added to each well and then incubated for additional 30 min. Inhibition of bacterial growth was visible as a clear well, and the presence of growth was detected by the blue colour in the well. All experiments were performed in triplicate under aseptic conditions.
Time–kill curve method
The combinatorial effect of the plant extracts and ciprofloxacin against E. coli, S. aureus, S. pneumoniae and P. aeruginosa was assessed using the time–kill assay as a method of investigation as described by Oluwatuyi et al. [Citation18] with slight modification. For time–kill assays, bacteria were freshly sub-cultured in MHB, incubated overnight at 37 °C until exponential phase was reached and were standardized to give 5×105 CFU/mL. Tests were carried out in 25-mL flasks containing 10 mL of bacterial culture with the antibiotic or plant extracts at their SIC. The flasks were incubated at 37 °C for 24 h on a mechanical shaker (Stuart Scientific Orbital Shaker 20.2, SOSI, Essex, UK), at 1200 r/min. After incubation, aliquots were taken from controls and the test samples. The aliquots were transferred to a recovery medium containing 3% Tween 80, to prevent drug carry-over, and later plated on nutrient agar in duplicates. The plates were incubated at 37 °C for 24 h under aseptic and aerobic conditions. The surviving microorganisms after treatment with combination therapy were scored, and the mean counts (CFU/mL) for each treatment were expressed in logarithm reduction. Bacteriostatic or bactericidal activities were defined as < 3-log10 reductions and ≥ 3 – log10 reductions in CFU/mL after 24 h of incubation, respectively, about the starting inoculums.[Citation19]
Gas chromatography–mass spectrometric (GC-MS) analysis
The analysis of the components in the methanol or ethanol or chloroform extracts from P. obliquum leaves was carried out using GC-MS (Hewlett-Packard 6890, Palo Alto, CA, USA) coupled with an Agilent mass selective detector, driven by Agilent Chemstation software (Agilent Technologies, Palo Alto, CA, USA). A DB-5SIL MS capillary column was used (30 m×0.25 mm×0.25 µm film thickness). The carrier gas was ultra-pure helium at a flow rate of 0.7 mL/min and a linear velocity of 37 cm/s. The injector temperature was set at 250 °C and the oven temperature at 60 °C, which was programmed to 280 °C at a rate of 10 °C min−1 with a hold time of 3 min. Injections of 1 µL were made in the splitless mode with a manually split ratio of 20:1. The mass spectrometer operated in the electron ionization mode at 70 eV and electron multiplier voltage at 1859 V. Other MS operating parameters were as follows: ion source temperature 230 °C, quadrupole temperature 150 °C, solvent delay 4 min and scan rage 50–700 a.m.u. Compounds were identified by direct comparison of the retention times (RT) and mass fragmentation pattern with those from the National Institute of Standards and Technology (NIST) library.
Data analysis
Data analysis was done on Microsoft Excel v. 12 Windows to obtain descriptive statistics. Means were separated by the Duncan multiple test using SAS 9.4 version. The different levels of significance within the separated groups were analysed using one-way analysis of variance (ANOVA). Values were considered significant at P < 0.05.
Results and discussion
TFC and TPC content
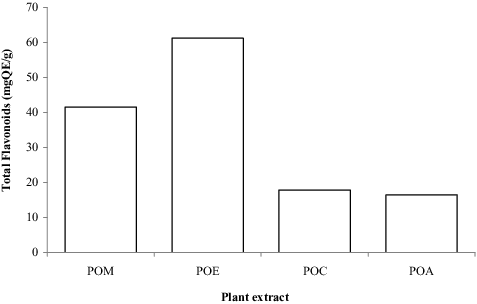
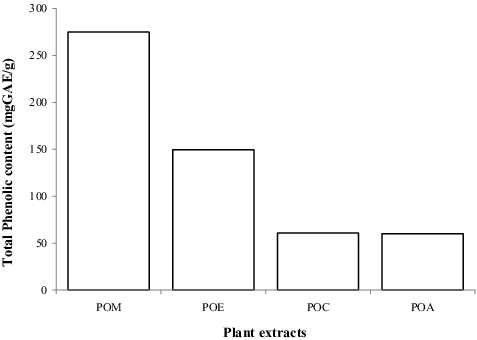
Research has shown that any agent capable of eliminating or reducing the growth of microorganisms associated with wound infection and reducing the levels of reactive oxygen species (ROS), may facilitate the wound-healing process.[Citation20] Polyphenols such as phenols and flavonoids are plant secondary metabolites employed in defence responses against ultraviolet radiation or pathogen attack. Epidemiological studies conducted in recent times strongly recommend long-term consumption of diets rich in plant polyphenols for their therapeutic purposes.[Citation21–24] Hubbard et al. [Citation25] showed that flavonoids increase the viability of collagen fibrils via wound contraction and increased rate of epithelialization due to its free-radical scavenging, astringent and antibacterial effects. The TFC and TPC present in POL extracts were determined and expressed as QE and GAE, respectively ( and ). The highest concentration of TFC and TPC expressed for POE (62.7275 mgQE/g) and POM (275 mgGAE/g) was higher than the 29.17 mgQE/g and 155 mgGAE/g reported by Moyo and Masika [Citation26] for TFC and TPC in methanol leaf extract, respectively. The variation is perhaps due to the time of harvest, climate change, processing and the method used. The higher concentration of these compounds recorded in POL extracts could support its traditional use in the region for the prevention and treatment of wound infections.
FRSA using DPPH assay
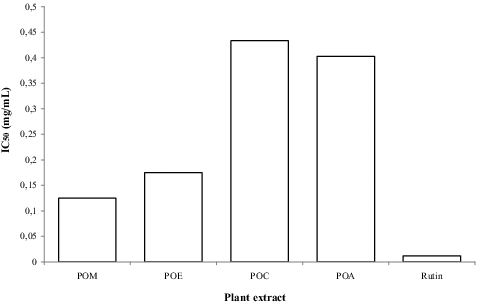
Chronic wounds are associated with excessive production of ROS at the site of the wound as a defence mechanism against invading bacteria.[Citation27] Conversely, at a high concentration, ROS can induce tissue damage particularly in cellular membrane and thereby interfere with the healing process.[Citation28] DPPH free-radical scavenging is a well-known global conventional mechanism for screening antioxidant activity of plant-derived products. The principle involves changing the violet colour of the DPPH radical solution to yellow diphenyl picryl hydrazine upon addition of antioxidant agents in a concentration-dependent manner via electron donation. Under the assay conditions, the POM extract exhibited strong antioxidative capacity having an IC50 of 0.115 mg/mL, followed by POE (0.185 mg/mL), POA (0.41 mg/mL) and POC (0.43 mg/mL) extracts, but this capacity was considered lower as compared to the standard rutin (IC50; 0.02 mg/mL) as shown in . The concentration of flavonoid and phenolic compounds observed earlier in these extracts showed a good correlation with the strong antioxidative potential of POL extracts and hence support its traditional use in the treatment of stress-related diseases and wound-healing processes.
Antibacterial activity of POL extracts
The colonization by pathogenic bacteria such as Staphylococcus, Pseudomonas, E. coli and Streptococcus spp. plays a significant role as the primary cause of the microbial infection that impedes the healing process of wounds.[Citation29] Although S. aureus is part of the normal skin microflora, it is also one of the most problematic nosocomial bacteria identified in the treatment of wound infections. Pseudomonas spp. are opportunistic bacteria that trigger environmental enteropathy in malnourished condition.[Citation30] These bacteria have been affiliated with multidrug resistance genes that pose a serious challenge in both hospital and community settings, thus necessitating discovery of new drugs.[Citation31] Similarly, Streptococcus spp. or beta-haemolytic streptococci have been implicated in most studies as the primary cause of delay or serious threat in the healing of chronic wound infections.[Citation29] Other bacteria are E. coli identified with post-operative wound infections and notorious for their resistance mechanism to the existing antibiotics. These bacteria and other aerobic bacteria directly invade wounds and interfere with the healing process via tissue damage, inflammation, fluid exudation and delay fibroplasia and collagen synthesis.[Citation32] The plant extracts exhibited good antibacterial activity having MIC ranging from 4 to 128 µg/mL compared to 0.016 to 25 µg/mL for ciprofloxacin () by the classification of Salvat et al. [Citation33]. The MIC value for ciprofloxacin against the test bacterial strains was much lower as compared to the extract due to the complexity of phytochemicals. Out of the bacteria tested, S. pneumoniae (TE 10) was more susceptible to the POL extracts. The findings in this study, therefore, concurred with a previous study vis-à-vis the antibacterial properties reported by Nielsen et al. [Citation11] against certain microorganisms. This observation together with the antioxidative capacities of the studied POL extracts provides support at least in part for the Ethno-therapeutic uses of this folk medicine in the treatment of wound infections.
Table 1. Minimum inhibitory concentration of different solvent extracts from Ptaeroxylon obliquum leaves or ciprofloxacin against bacterial strains associated with wound infections.
Antibiotic potentiation activity of POL extracts
The use of multiple drugs with potentials to reverse multidrug-resistance has been proposed as a new alternative approach to mitigate the spread of resistant bacteria by changing the phenotype of resistant pathogens to certain antibiotics. Here, we demonstrated the bacterial-resistance modifying effect of P. obliquum leaf extract in the presence of ciprofloxacin (at SIC) against selected bacterial strains associated with wound infections (). All the POL extracts reduced the MIC values of ciprofloxacin by 2–64-fold against the bacterial strains, whilst the POM extract was indifferent against S. pneumoniae (TE 10). An interesting finding was that the lower concentrations of POC showed better activity (2–16-fold) against certain bacteria than more concentrated extracts. The POE extract demonstrated a 4- to 16-fold potentiation of ciprofloxacin activity against the test bacteria. Correspondingly, a remarkable reduction in the MIC of ciprofloxacin was recorded in the presence of acetone extracts (4–64-fold). It is interesting to note that all the POL extracts showed a strong potential to modulate ciprofloxacin activity against multidrug-resistant bacterial strains. The basis of synergy between ciprofloxacin and POL at present is clearly unknown. Though ciprofloxacin is capable of penetrating P. aeruginosa (ATCC 19582) and E. coli (ATCC 8739) strains according to Chalkley and Koornhof [Citation34], a pronounced synergistic interaction was exerted when combined with POL extracts. This observation is a notable finding, suggesting that POL extracts could be a potential source of active compounds which could be useful in the development of new antimicrobial agents or in modulating the existing old antibiotics.[Citation35]. Further bio-guided isolation and characterization of active compounds is recommended for probable new drug discovery.
Table 2. Minimum inhibitory concentration (µg/mL) of ciprofloxacin in the presence or absence of Ptaeroxylon obliquum leaf extracts at sub-inhibitory concentration (SIC).
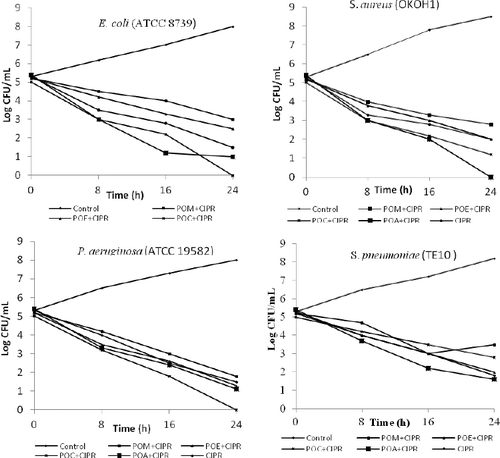
Time–kill effect of POL extracts against selected bacterial strains
The time–kill effect of POL extracts in the presence of ciprofloxacin at 24-h time point against the four bacterial strains is presented in . This study is imperative to know whether the extract–antibiotic combination could exert a bacteriostatic or bactericidal effect in the treatment of certain infections.[Citation36] The combination of plant extracts with ciprofloxacin at SIC showed bactericidal activity in the range of 3- and 6-log10 reductions of CFU/mL of more than 70% of the test bacteria. With a combination of POM and ciprofloxacin against E. coli, a bacteriostatic effect was observed and indifference towards S. pneumoniae (TE10). Ciprofloxacin in combination with POE or POC or POA extract exhibited a dramatic bactericidal activity against the four bacterial strains with varying degree of growth inhibition. The POA extract in the presence of ciprofloxacin completely inhibited the growth of P. aeruginosa (ATCC 19582) and E. coli (ATCC 8739). This observation was corroborated by the MIC and modulatory effect observed earlier in this work. This outstanding bactericidal activity of POL extracts in the presence of ciprofloxacin suggested that this botanical can inhibit the resistance mechanism present in the tested pathogens. In this work, POL extracts could be proposed as a potential source of novel molecules for the treatment of microbial infections; using them may be more cost-effective and may engender fewer side effects compared with Western medicine.
GC-MS analysis of POM extracts
Phenolic compounds are known to play a broad range of biological activities such as inflammatory, antidiabetic, cardioprotective, antibacterial, antioxidant and anticarcinogenic effects ascribed to their functional groups and ability to inhibit membrane-bound enzymes.[Citation21] Based on its antioxidative potential, the methanol extract of P. obliquum leaves was selected for GC-MS analysis. Six major peaks: 1-fluoro-dodecane; hentriacontane; sulphurous acid, 2-ethylhexyl hexadecyl ester; cyclotetradecatetraene, 3,7,11-trimethyl-14-(1-methylethyl)-; hexacosyl acetate; and 2,5-bis(1,1-dimethylethyl) phenol, were identified by GC-MS analysis via their fragmentation pattern in conjunction with the data available in the NIST library (). The active principles and their retention time (RT), molecular weight (MW), percentage composition as a percentage by peak area and their structures are presented in . The strong antioxidative capacities noted in POM extract may be due to the presence of 2,5-bis(3,3-dimethyl ethyl)phenol. This cyclic compound is unsaturated characterized with a hydroxyl group responsible for proton/electron donation to the unpaired electron of a free radical. The degree of unsaturation has been reported to be greater than that of enediol (–COH = COH–) found in L-ascorbic acid. Another constituent that was identified to be abundantly present in POM is hentriacontane, which is known as a potent anti-inflammatory agent [Citation37] but its detailed therapeutic targets have not been elucidated. So far, the biological activities of some of these main compounds have not been documented. The robust antibacterial activity displayed by the POA extract may perhaps be due to the presence of sydnone, 3-(3,3-dimethyl butyl) (data withheld), a mesoionic compound having the 1,2,3-oxadiazole skeleton bearing an oxygen atom attached to the 5-carbon atom. This compound has been reported to have a wide spectrum of biological and pharmacological activities including antibacterial and antioxidative capacity.[Citation38] It is evident that high phenol and flavonoid concentration in the methanol extract (POM) coupled with the presence of phenol derivative (2,5-bis(1,1-dimethylethyl)phenol) as a major compound could significantly contribute to the robust antioxidative potential observed in the POM extract. Phenolic compounds are well known as primary antioxidants against free radicals that are responsible for neurodegenerative diseases, suggesting that the POM extract may serve as a potential herbal remedy in the treatment of radical-related diseases in man.
Figure 5. GC–MS chromatogram of the most active antioxidant extract of Ptaeroxylon obliquum leaves (POM).
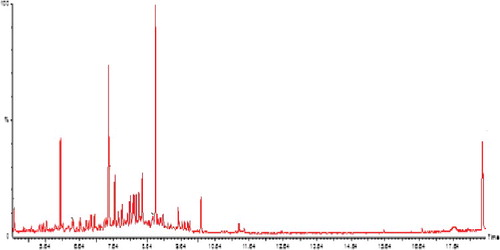
Table 3. Identified major components of the methanol extract from Ptaeroxylon obliquum leaves in the order of retention time by GC–MS and their structure.
Conclusions
To the best of our knowledge, we have shown for the first time that extracts of P. obliquum leaves have a strong antibacterial effect against multidrug-resistant bacteria associated with wound infections. All POL extracts were found to enhance the therapeutic efficacy of ciprofloxacin, showing bactericidal activity against most bacterial strains tested in this work. Similarly, a remarkable antioxidative capacity of POL extracts, in particular, methanol and ethanol extracts (POM and POE) was demonstrated. This study at least in part gives support to the folkloric use of POL by the local dwellers in the Eastern Cape Province of South Africa for the treatment of wound infection and mysiasis in livestock. Further studies are needed to isolate, purify and characterize active principles responsible for this observation and to determine the toxic potential as well as the probable mechanism of action. We proposed that potential applications of P. obliquum extracts or active compounds might serve as natural and cheaper phytomedicine for promoting the well-being of man and livestock.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Jalalpure S, Agrawal N, Patil MB, et al. Antimicrobial and wound healing activities of leaves of Alternanthera sessilis Linn. Int J Green Chem. 2008;2:141–148.
- Mustoe TA, O'Shaughnessy K, Kloeters O. Chronic wound pathogenesis and current treatment strategies: a unifying hypothesis. Plast Reconstr Surg. 2006;117:35–41.
- Dissemond J. Praktische Konsequenzen durch den Nachweis von MRSA in chronischen Wunden [ Practical consequences after MRSA identification in chronic wounds]. Hautarzt. 2007;12:1–6. German.
- Houghton PJ, Hylands PJ, Mensah AY, et al. In vitro tests and ethnopharmacological investigations: wound healing as an example. J Ethnopharmacol. 2005;100:100–107.
- Alam H, Sehgal L, Kundu S, et al. Novel function of keratins 5 and 14 in proliferation and differentiation of stratified epithelial cells. Mol Biol Cells. 2011;22:4068–4078.
- Statistics South Africa. Mortality and causes of death in South Africa, 2007: findings from death notification. Statistical Release PO309.3. Pretoria: Statistics South Africa; 2009.
- Howell-Jones RS, Price PE, Howard AJ, et al. Antibiotic prescribing for chronic skin wounds in primary care. Wound Repair Regen. 2006;14:387–393.
- Mackay D, Miller AL. Nutritional support for wound healing. Altern Med Rev. 2003;8:359–377.
- Van Wyk BE, Van Oudtshoorn B, Gericke N. Medicinal plants of South Africa. 1st ed. Pretoria: Briza Publications; 1997. p. 1–304.
- Soyelu OT, Masika PJ. Traditional remedies used for the treatment of cattle wounds and mysiasis in Amatola Basin, Eastern Cape Province, South Africa. Onderstepoort J Vet Res. 2009;76:393–397.
- Nielsen TRH, Kuete V, Jager AK, et al. Antimicrobial activity of selected South African medicinal plants. BMC Complement Altern Med [Internet]. 2012 [ cited 2012 Jun 14];12:74. Available from: http://dx.doi.org/10.1186/1472-6882-12-74.
- Mulholland DA, Kotsos M, Mahomed HA, et al. The chemistry of Ptaeroxylaceae. Niger J Nat Prod Med. 1999;3:15–18.
- Agostinho D, Boudesocque L, Thery-Kone I, et al. A new meroterpenoid isolated from roots of Ptaeroxylon obliquum Radlk. Phytochem Lett. 2013;6:560–566.
- Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–559.
- Kim D, Kim Y, Choi U. Optimization of antibacterial activity of Perilla frutescens var. acuta leaf against Staphylococcus aureus using evolutionary factorial design technique. Int J Mol Sci. 2011;12:2395–2407.
- Tariq A, Athar M, Ara J. Biochemical evaluation of antioxidant activity and polysaccharides fractions in sea weeds. Global J Environ Sci Manage. 2015;1:47–62.
- Shiu WKP, Gibbons S. Anti-staphylococcal acylphloroglucinols from Hypericum beanii. Phytochemistry. 2006;67:2568–2572.
- Oluwatuyi M, Kraatz GW, Gibbons S. Antibacterial and resistance modifying activity of Rosmarinus officinalis. Phytochemistry. 2004;6:3249–3254.
- Standards NCCL. Methods for determining bactericidal activity of antimicrobial agents: approved guideline. Villanova, PA: National Committee for Clinical Laboratory Standards (NCCLS); 1999.
- Hollinworth H. The management of patients’ pain in wound care. Nurs Stand. 2005;20(7):65–73.
- Oyedemi SO, Afolayan AJ. In vitro and in vivo antioxidant activity of aqueous leaves extract of Leonotis leonurus (L.) R. Br. Int J Pharmacol. 2011;7:248–256.
- Moyo B, Oyedemi SO, Masika PJ, et al. Polyphenolic content and antioxidant properties of Moringa oleifera leaf extracts and enzymatic activity of liver from goats supplemented with Moringa olifera leaves/sunflower seed cake. Meat Science. 2012;91:441–447.
- Oyedemi SO, Coopoosamy RM. Preliminary studies on the antibacterial and antioxidative potentials of hydroalcoholic extract from the whole parts of Artemisia vulgaris L. Int J Pharmacol. 2015;11:561–569.
- Ozken G, Kamiloglu S, Ozdal T, et al. Potential use of Turkish medicinal plants in the treatment of various diseases. Molecules [Internet]. 2016 [ cited 2016 Jun 28];21:257. Available from: http://www.mdpi.com/1420-3049/21/3/257.
- Hubbard GP, Wolffram S, Lovegrove JA, et al. Ingestion of quercetin inhibits platelet aggregation and essential components of the collagen stimulated platelet activation pathway in humans. J Thromb Haemost. 2004;2:2138–2145.
- Moyo B, Masika PJ. Tick control methods used by resource-limited farmers and the effect of ticks on cattle in rural areas of the Eastern Cape Province, South Africa. Trop Anim Health Prod. 2009;41:517–523.
- Reddy BS, Reddy RKK, Naidu VGM et al. Evaluation of antimicrobial, antioxidant and wound healing potentials of Holoptelea integrifolia. J Ethnopharmacol. 2008;115:249–256.
- Jorge MP, Madjarof C, Ruiz ALTG, et al. Evaluation of wound healing properties of Arrabidaea chica Verlot extract. J Ethnopharmacol. 2008;118:361–366.
- Bowler PG, Deurden BI, Armstrong DG, et al. Wound microbiology and associated approaches to wound management. Clin Microbiol Rev. 2001;14:244–269.
- Brown EM, Wlodarska M, Willing BP, et al. Diet and specific microbial exposure trigger features of environmental enteropathy in a novel murine model. Nat. Commun [Internet]. 2015 [ cited 2015 Aug 4];6:7806. Available from: http://dx.doi.org/10.1038/ncomms8806.
- Mayhall GC. The epidemiology of burn wound infections: then and now. Clin Infect Dis. 2003;37:543–550.
- Thomas JC, Howes PR. Effect of bacterial contamination on wound healing. J Ethnopharmacol. 1997;64:191–194.
- Salvat A, Antonacci L, Fortunato RH, et al. Antimicrobial activity in methanolic extracts of several plant species from Northern Argetina. Phytomedicine. 2004;11:230–234.
- Chalkley LJ, Koornhof HJ. Antimicrobial activity of ciprofloxacin against Pseudomonas aeruginosa. Escherichia coli and Staphylococcus aureus determined by the killing curve method: antibiotic comparisons and synergistic interactions. Antimicrob Agents Chemother. 1985;28:331–342.
- Finberg RW, Moellering RC, Tally FP, et al. The importance of bactericidal drugs: future directions in infectious disease. Clin Infect Dis. 2004;64:191–194.
- Oyedemi SO, Oyedemi BO, Prieto JM. In vitro assessment of antibiotic-resistance reversal of a methanol extract from Rosa canina. South Africa J Bot. 2016:105;337–342.
- Kim SJ, Chung WS, Kim SS, et al. Anti-inflammatory effect of Oldenlandia diffusa and its constituent, Hentriacontane, through suppression of Caspase-1 activation in mouse peritoneal macrophages. Phytother Res. 2011;25:1537–1546.
- Dunckley CS, Thoman CJ. Synthesis and biological evaluation of a novel phenyl substituted sydnone series as potential antitumour agents. Bioorg Med Chem Lett. 2013;13:2899–2901.