ABSTRACT
Vascular development has a great significance in the osteogenic process and in bone tissue engineering (BTE). BTE is based on various combinations of three principal types of components: biomaterials as scaffolds, regulatory signals and cells. The aim of our study was to evaluate, at gene expression and histological level, the effect of BTE triad components on the vascularization process in an ectopic bone-forming model in mice. Bone mineral matrix (BMM) was used as a scaffold and a carrier, platelet-rich plasma (PRP) as a source of regulatory signals and adipose stem cells (ASCs) as a source of cells for endothelial differentiation, in order to show how and to what extent the biological enrichment of BMM influences the outcome of the osteogenic process and its key precondition, vascularization. Implants composed of BMM, PRP and ASCs in vitro induced into endothelial cells (EPB implants) and implants composed of BMM and PRP (PB implants) were compared with implants composed of BMM only (B implants). More pronounced endothelial-related gene expression and stronger VCAM-1 (vascular cell adhesion molecule-1) immunoexpression were observed in EPB implants in comparison with PB and B ones at later time points of the in vivo experimental period. Osteopontin gene expression and immunoexpression of osteopontin were significantly higher in EPB compared to PB and B implants. Therefore, addition of ASCs combined with PRP to BMM improved the vascularization process in the ectopic bone-forming model, which makes this BTE composition the most favourable among the examined types of implants for application in BTE.
Introduction
Vascular development is a prerequisite for the osteogenic process: when the vascular network is normally developed, osteoblasts (OBs) produce osteoid, differentiate into osteocytes and build a healthy bone.[Citation1] The bone is a remarkably vascularized organ with high regenerative capacity so most of the fractures heal spontaneously, but in case of critical-sized bone defects, surgical interventions are necessary.[Citation2] These defects can be reconstructed by using autografts, allografts or a bone tissue engineering (BTE) approach.[Citation3] BTE is based on various combinations of three principal types of components: biomaterials as scaffolds, regulatory signals and cells.[Citation3]
Due to a similarity with the structure and function of autologous bones,[Citation4] calcium phosphate-based biomaterials have a great potential for application in BTE as bone substitutes alone [Citation5] or as implant components.[Citation6–9] Incorporation of active biomolecules including growth factors (GFs) into bone substitutes can improve their osteogenic and angiogenic potential.[Citation10] Artificial recombinant GFs require synthetic or animal proteins as carriers, but platelet-rich plasma (PRP) is a natural carrier itself.[Citation11] Addition of PRP [Citation12,Citation13] or blood [Citation14] enhances the osteoinductivity [Citation12] and osteoconductivity [Citation13] or leads to increased vascularization of implants,[Citation14] whereas blood clots combined with macrophages have a beneficial effect on angiogenesis and organic bone matrix formation.[Citation9] Stem cells isolated from adipose tissue and in vitro induced into endothelial cells (ECs) [Citation7,Citation15] can be used as bioactive implant components.
The aim of our study was to evaluate, at gene expression and histological level, the effect of BTE triad components on the vascularization process in an ectopic bone-forming model in mice. Bone mineral matrix (BMM) was used as a scaffold and a carrier, PRP as a source of regulatory signals and adipose stem cells (ASCs) as a source of cells for endothelial differentiation, in order to show how and to what extent biological enrichment of BMM could influence the outcome of the osteogenic process and its key precondition, vascularization. To the best of our knowledge, this is the first study in which ectopically implanted BMM alone was evaluated regarding the expression pattern of endothelial-related genes and compared with BMM enriched with PRP, and BMM enriched with PRP plus ASCs.
Materials and methods
Animals
We used male, syngeneic Balb/c mice, 22–24 g in weight, 8 weeks old, held in standard laboratory conditions and allowed to eat and drink ad libitum. Animal procedures were approved by the Local Ethical Committee of the Faculty of Medicine, University of Niš (No. 01-2857-8), Serbia, in accordance with Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes.[Citation16]
Isolation, expansion and endothelial differentiation of adipose stem cells
Mice epididymal adipose tissue was extracted, washed, macerated and digested (45 min, at 37 °C) using Collagenase type I (Sigma–Aldrich, Hamburg, Germany) at a concentration of 2000 I.U. per millilitre of low glucose Dulbecco's Modified Eagles Minimal Essential Medium (DMEM, PAA Laboratories GmbH, Pasching, Austria). The homogenate was filtered, centrifuged (10 min, at 300 × g, 4 °C) and the pellet containing the stromal vascular fraction cells (SVFs) was resuspended in complete DMEM, which contained low glucose DMEM, 10% fetal bovine serum, 2 mmol/L of L-glutamine and 1% antibiotic-antimycotic solution (all from PAA Laboratories GmbH, Pasching, Austria). SVFs were seeded at a density 1 × 106 per 25 cm2 flask. At the third passage (P03), ASCs were seeded into 24-well plates (0.5 × 104 cells per well) and were either induced into ECs by using an EndoPrime Kit (PAA Laboratories GmbH, Pasching, Austria), or cultured in complete DMEM (non-induced ASCs).
Immunocytochemistry
One day after isolation of SVFs and one day after P03, the stem cell phenotype was assessed by using positive stem cell marker anti-CD44 antibody (1:500, ab65829, Abcam, Cambridge, USA). At these two time points and at the 7th, 12th and 15th day of endothelial induction, ECs’ phenotype was examined by using antibodies to vascular cell adhesion molecule-1 (anti-VCAM-1, 1:200, ab106777, Abcam, Cambridge, USA). Cells were incubated with these primary antibodies overnight, at 4 °C. Negative controls were prepared by omitting the primary antibodies. Rabbit-specific horseradish peroxidase/diaminobenzidine (HRP/DAB) detection Kit (ab64261, Abcam, Cambridge, USA) was used for visualization, according to the manufacturer's instructions. Specimens were counterstained (Mayer's Haematoxylin, 1 min, at room temperature), mounted (VectaMount®, Vector Laboratories, Burlingame, CA, USA) and analysed on a LEICA DMR light microscope equipped with a LEICA DC 300 camera (Leica Microsystems, Solms, Germany). Immunoreactivity was visualized as brown colour and represents immunopositivity for applied antibodies.
In vivo experimental design
Bio-Oss®, size S (Geistlich-Pharma, Wolhusen, Switzerland) was used as BMM for the implant construction. PRP was prepared by two-step method [Citation17] and it had 4–6 times higher concentration of platelets compared to the physiological one. The number of platelets established in a Malassez chamber (Paul Marienfeld, Lauda-Königshofen, Germany) was 1.89 ± 0.5 × 106/µL. The final concentration of PRP in the liquid component of the implant was 10% (v/v), which is optimal for combining with ASCs.[Citation18]
Three types of implants were constructed (). Before implantations, the cells were allowed to attach to the BMM surface for 15 min and fibrin fibres to form within implants. Implants were placed ectopically into the interscapular subcutaneous tissue of anaesthetized mice. In every group, each mouse had four implants of the same type. Implants extracted 1, 2, 4 and 8 weeks after implantations were placed in RNAlater® (Ambion, Life Technologies, Paisley, UK) at −80 °C, whereas implants extracted 2 and 8 weeks after implantations were fixed in 10% neutral buffered formalin (NBF).
Table 1. Experimental groups of mice used in this study.
Gene expression
The concentration of total RNA isolated from the implants by using an RNeasy Mini Kit® (Qiagen, Hilden, Germany) was measured with a Qubit® RNA assay Kit and a Qubit® 2.0 fluorometer (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA), according to the manufacturers’ recommendations. Residual DNA was digested with DNase I RNase-free set (Qiagen, Hilden, Germany). Isolated RNA was reversely transcribed into cDNA with a High-capacity cDNA Reverse Transcription Kit (Applied Biosystems®, Thermo Fisher Scientific, Waltham, MA, USA), according to the manufacturer's instructions, in a thermal cycler SureCycler 8800 (Agilent Technologies, Santa Clara, CA, USA). Relative gene expression was evaluated in a Stratagene MxPro-Mx3005P Real-Time thermal cycler (Agilent Technologies, Santa Clara, CA, USA). The reactions were prepared by using QuantiTect primer assays (Qiagen, Hilden, Germany) for endothelial-related genes and the osteopontin gene () and Kapa Sybr® Fast Universal 2 × qPCR Master Mix (Kapa Biosystems, Wilmington, MA, USA), according to the manufacturers’ instructions. The results were expressed relative to these genes’ expression in PRP (calibrator sample) and normalized to the expression of the housekeeping gene β-actin.
Table 2. Primers used for qPCR.
Histology
Implants fixed in 10% NBF were decalcified in 10% ethylenediaminetetraacetic acid solution (pH 7.4). The tissue was processed, embedded in paraffin and paraffin blocks were sliced at 4 µm on a Leica RM2235 microtome (Leica Microsystems, Solms, Germany). Toluidine blue staining was performed and stained sections were analysed on a LEICA DMR light microscope.
Immunohistochemistry
Implants fixed with 10% NBF and processed as described in the ‘Histology’ procedure above were subjected to heat-induced antigen-retrieval, using 10 mmol/L of sodium citrate buffer (pH 6.0) prior to incubation with primary antibodies (4 °C, overnight) anti-VCAM-1 (1:1000) and anti-osteopontin (1:1000, ab8448, Abcam, Cambridge, USA). Primary antibodies were omitted in negative controls. HRP/DAB detection Kit was used for visualization. Sections were counterstained (Mayer's Haematoxylin, 5 min, room temperature), mounted (VectaMount®) and analysed on a LEICA DMR light microscope. Immunoreactivity was visualized as brown colour and specifies immunopositivity for the applied antibodies.
Statistics
Statistics were conducted in SPSS version 15.0 (SPSS Inc., Chicago, IL, USA) using the Kruskal–Wallis analysis of variance (ANOVA) and a post hoc Mann–Whitney test to determine statistically significant differences (P < 0.05) between groups.
Results and discussion
SVFs expressed CD44 ((A)) and VCAM-1 ((C)) one day after isolation. VCAM-1 expression can be related to microvascular ECs (mECs) contained in SVF.[Citation19] A day after P03, ASCs retained CD44 expression ((B)), whereas the VCAM-1 expression was weaker ((D)) compared to SVFs probably due to elimination of mECs through passages.[Citation20] VCAM-1, a protein representative of ECs functions,[Citation21] was expressed in induced ASCs at each time point ((A–C)), but the most pronounced expression was at the 12-day time point. Non-induced ASCs were mostly VCAM-1 negative (Figure (D)–(F)).
Figure 1. CD44 (A and B) and VCAM-1 (C and D) immunoexpression in ASCs one day after isolation (A, C and E) and one day after passage 3 (P03) (B, D and F). Negative controls (E and F).
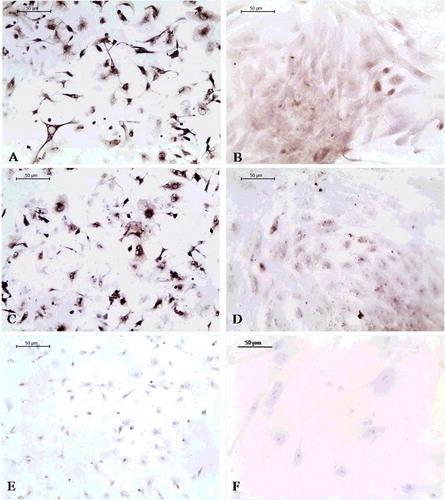
Figure 2. VCAM-1 immunoexpression in ASCs induced into ECs 7 (A), 12 (B) and 15 days (C) after P03 and in non-induced ASCs: 7 (D), 12 (E) and 15 days (F) after P03. Negative controls: 7 (G), 12 (H) and 15 (I) days after ASCs induction.
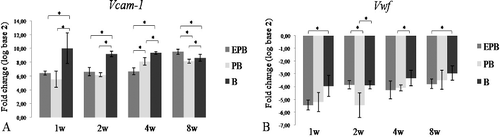
The Egr-1 gene encodes a transcription factor for Flt-1 and Vcam-1 [Citation22,Citation23] and its relative expression was significantly higher in EPB ((A)) than in PB (8-week time point) and B implants (2- and 8-week time point). Since GFs can rapidly activate Egr-1,[Citation24] Egr-1 activation in the EPB and PB group probably happened because of GFs released from PRP. In the EPB group, the relative expression of Egr-1 increased from the 4-week to the 8-week time point (P < 0.05), whereas in the PB and the B group, the relative expression of Egr-1 decreased between these time points. In EPB implants, the relative expression of Egr-1 ((A)) and Flt-1 ((B)) had the same expression patterns: the relative expression of Flt-1 also decreased from the 1-week to the 4-week time point and increased (P < 0.05) again at the 8-week time point ((B)). The relative expression of Vcam-1 was significantly higher in EPB than in the PB and the B group at the 8-week time point ((A)). Unlike in the EPB and the PB group where it had an increasing trend from the first to the last time point, in the B group, the relative expression of Vcam-1 had a decreasing trend towards the last time point ((A)). The initially higher relative expression of Egr-1, Flt-1 and Vcam-1 in B than in EPB and PB implants could be associated with the feature of osteoconductive biomaterials’ to mechanically support ingrowth of hosts’ blood vessels.[Citation25] The relative expression of Vwf was highest at the 8-week time point in all groups ((B)), which is in accordance with its role in late angiogenesis.[Citation26] The generally lower endothelial-related relative gene expression in the PB group than in the EPB and the B one could be explained by high initial burst release and short lifespan of GFs released from PRP.[Citation18]
Figure 3. Egr-1 (A) and Flt-1 (B) relative expression in EPB, PB and B implants at 1-, 2-, 4- and 8-week (w) time points.
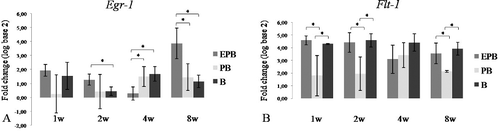
Figure 4. Vcam-1 (A) and Vwf (B) relative expression in EPB, PB and B implants at 1-, 2-, 4- and 8-week (w) time points.
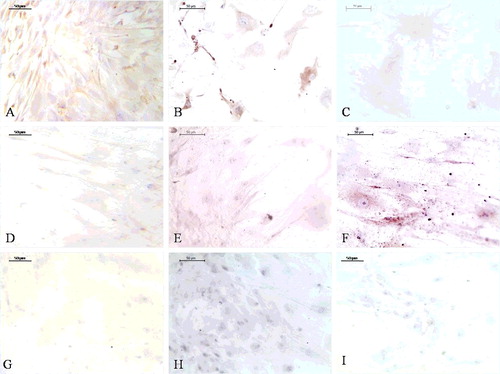
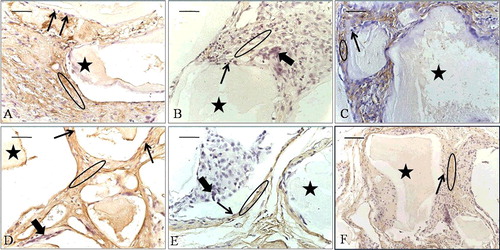
At the 2-week time point, the EPB ((A)) and the PB ((B)) implants had VCAM-1 positive cells in the tissue between the BMM granules, and the B implants in the blood vessel wall cells ((C)). At the 8-week time point, the immunoexpression of VCAM-1 in the cells between the granules and in the blood vessel wall cells was stronger in the EPB group ((D)) than in the PB ((E)) and the B one ((F)). There was no immunoreactivity in negative controls ((A–F)). After stimulation by cytokines, VCAM-1 is expressed in blood vessel cells and binds to its receptor on leukocytes, very late activation antigen-4, thus mediating leukocyte binding to ECs.[Citation27] Therefore, VCAM-1 immunoexpression ((A–F)) can be associated with an inflammation process that precedes the activation phase of angiogenesis.[Citation9] It has also been found that VCAM-1 is expressed on stromal cells and its corresponding ligand on osteoclast precursor cells and that their interaction is of crucial importance for osteoclast formation.[Citation28] Since multinucleated giant osteoclast-like cells (OCLs) were seen in all groups at the 8-week time point ((D–F)), the VCAM-1 immunoexpression in the implants might as well be related to a preceding osteoclastogenesis process within them.
Figure 5. VCAM-1 immunoexpression (ellipse) at the 2-week time point (A–C) and at the 8-week time point (D–F). EPB (A and D), PB (B and E) and B (C and F) implants. Bio-Oss (star); blood vessels (arrowhead).
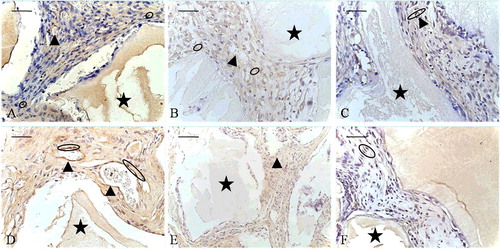
Figure 6. Negative controls for immunohistochemistry: at the 2-week time point (A–C) and the 8-week time point (D–F). EPB (A and D), PB (B and E) and B (C and F) implants.
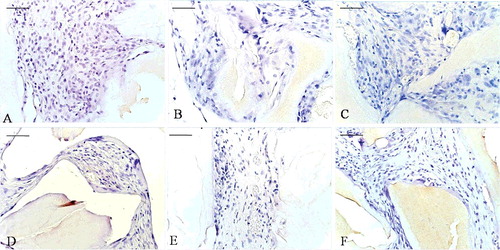
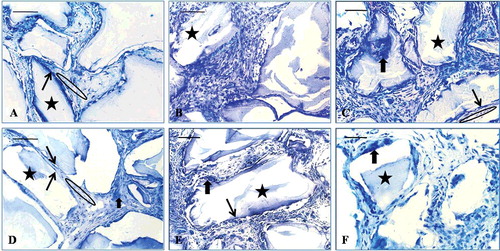
Figure 7. Toluidine blue staining: at the 2-week time point (A–C) and at the 8-week time point (D–F). EPB (A and D), PB (B and E) and B implants (C and F). Bio-Oss (star), osteoblast-like cells (long, thin arrow), multinucleated giant cells (short, thick arrow), osteoid-like tissue (ellipse).
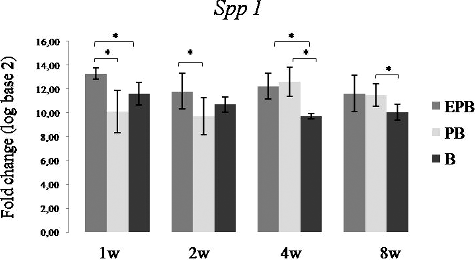
At the first two time points, the relative expression of Spp1 was significantly higher in the EPB group than in the PB one, and at the 1- and the 4-week time point the relative expression of Spp1 was significantly higher in the EPB than in the B group (). The relative expression of Spp1 was significantly higher in PB compared to B implants at the 4- and the 8-week time point, but at the first two time points, its relative expression was higher in B implants compared to PB ones. The initially higher relative expression of Spp1 in the B group could be attributed to the osteoconductive nature of Bio-Oss®: it ‘attracts’ resident progenitors,[Citation29] thus building a ‘framework’ for blood vessels and bone-forming cells.[Citation30] However, the relative expression of Spp1 was more sustainable in EPB implants compared to PB and B ones, which might be related to interaction that occurs between secreted osteopontin and ECs. Soluble osteopontin has an anti-apoptotic effect on adherent ECs [Citation31] and it can, therefore, be presumed that an impact on sustainable vascularization and osteogenic process in EPB implants can be achieved through this mechanism.
Figure 8. Relative expression of Spp1 in EPB, PB and B implants at 1-, 2-, 4- and 8-week (w) time points.
Osteopontin immunoexpression was detected in all groups but its distribution was different. At the 2-week time point, osteopontin was expressed in the EPB group next to BMM granules and in the tissue more distant from the granules ((A)). In the PB group ((B)) and the B group ((C)), osteopontin immunoexpression appeared in the tissue near BMM granules. At the 8-week time point, osteopontin immunoexpression in the EPB implants ((D)) was more pronounced than in PB ((E)) and B ((F)) implants. Multinucleated giant OCLs were seen next to BMM granules and between them in all groups, at each time point, but they were more abundant at week 8 ((D–F)) than at the 2-week time point ((A–C)). In normal bone tissue, OBs and osteoclasts (OCs) express osteopontin, but at different time.[Citation32] Thus, the osteopontin immunoexpression observed in our implants was probably the result of coordinated activity of these cell types. Osteopontin secreted by OBs at early stages of bone formation promotes OBs’ attachment to the bone matrix, whereas osteopontin secretion by OCs occurs at bone resorption sites.[Citation32] OB-like cells (OBLs), attached and lined onto BMM particles, and osteoid-like tissue were more abundant in the EPB group ((A,D)) than in the PB ((B,E)) and the B ((C,F)) group, at both time points. Mesenchymal cells that aggregate and constitute loose mesenchymal tissue during intramembranous bone formation may differentiate into OBs only in an adequate vascular environment.[Citation33] Since heterotopic bone formation induced by biomaterials has an intramembranous pathway,[Citation34] the appearance of numerous OBLs in EPB implants could be associated with the presence of ECs in these implants’ composition. A finding that perycites, mural cells of the abluminal side of blood vessels,[Citation34] can differentiate into many different cell types, including OBs, thus playing a role in ectopic calcification,[Citation35] suggest the possibility that OBs in our implants may at least partly have a vascular origin, which is one more way of association between vascularization and the osteogenic process.
Figure 9. Osteopontin immunoexpression (ellipse) at the 2-week time point (A–C) and at the 8-week time point (D–F). EPB (A and D), PB (B and E) and B implants (C and F). Bio-Oss (star), osteoblast-like cells (long, thin arrow), multinucleated giant cells (short, thick arrow).
Our present and recent studies and the studies of other authors regarding the effects of BTE triad components, individually or joint, on vascularization and the osteogenic process, are performed on different models (orthotopic/ectopic), with various types of implant components (e.g. adipose or bone marrow stem cells, platelet-rich plasma/platelet-rich fibrin, natural/synthetic biomaterials) and in different animal species. Therefore, it is difficult to compare all data and bring definitive conclusions about the most favourable solution to the problem of vascularization in critical-sized bone defects. Large interspecies differences are another difficulty for extrapolating data obtained in animal models into clinical application. That is why additional clinical trials are necessary before clinical implementation of different BTE triad components.
Conclusions
Implant composition that included addition of ASCs combined with PRP to BMM induced more pronounced endothelial-related gene expression and stronger VCAM-1 immunoexpression at later time points of the in vivo experimental period in comparison with addition of PRP to BMM, and BMM alone. Enrichment of BMM with ASCs combined with PRP also led to significantly higher osteopontin gene and protein expression in EPB than in PB and B implants. Considering the crucial importance of vascularization for osteogenic process and that the addition of ASCs and PRP to BMM in our study improved the vascularization process in an ectopic bone-forming model, we could conclude that this BTE composition is the most favourable among the examined types of implants for possible application in BTE. On the way to clinical applications of these findings, it is necessary to do research on other models, such as orthotopic ones.
Acknowledgments
The authors would like to thank Esma Đinđić, technician at the Faculty of Medicine, University of Niš, for performing the microtome-slicing procedure.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Zhou J , Dong J . Vascularization in the bone repair. In: Lin Y , editor. Osteogenesis. Rijeka : InTech; 2012. p. 286–296. Available from: http://www.intechopen.com/books/osteogenesis/vascularization-in-the-bone-repair
- Stevens MM . Biomaterials for bone tissue engineering. Mater Today. 2008;11:18–25.
- Healy KE , Guldberg RE . Bone tissue engineering. J Musculoskelet Neuronal Interact. 2007;7:328–330.
- Mauney JR , Jaquiéry C , Volloch V , et al. In vitro and in vivo evaluation of differentially demineralized cancellous bone scaffolds combined with human bone marrow stromal cells for tissue engineering. Biomaterials. 2005;26:3173–3185.
- Ignjatović N , Savić V , Najman S , et al. A study of HAp/PLLA composite as a substitute for bone powder, using FT-IR spectroscopy. Biomaterials. 2001;22:571–575.
- Cvetković VJ , Najdanović JG , Vukelić-Nikolić MĐ , et al. Osteogenic potential of in vitro osteo-induced adipose-derived mesenchymal stem cells combined with platelet-rich plasma in an ectopic model. Int Orthop. 2015;39:2173–2180.
- Najdanović JG , Cvetković VJ , Stojanović S , et al. The influence of adipose-derived stem cells induced into endothelial cells on ectopic vasculogenesis and osteogenesis. Cell Mol Bioeng. 2015;8:577–590.
- Rajković J , Stojanović S , Đorđević Lj , et al. Locally applied cholecalciferol and alfacalcidol act differently on healing of femur defects filled with bone mineral matrix and platelet-rich plasma in ovariectomized rats. Biotechnol Biotecnol Equip. 2015;29:963–969.
- Živković JM , Najman SJ , Vukelić MĐ , et al. Osteogenic effect of inflammatory macrophages loaded onto mineral bone substitute in subcutaneous implants. Arch Biol Sci. 2015;67:173–186.
- Allo BA , Costa DO , Dixon SJ , et al. Bioactive and biodegradable nanocomposites and hybrid biomaterials for bone regeneration. J Funct Biomater. 2012;3:432–463.
- Anitua E , Andia I , Ardanza B , et al. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91:4–15.
- Hanna R , Trejo PM , Weltman RL . Treatment of intrabony defects with bovine-derived xenograft alone and in combination with platelet-rich plasma: a randomized clinical trial. J Periodontol. 2004;75:1668–1677.
- Torres J , Tamimi F , Martinez PP , et al. Effect of platelet-rich plasma on sinus lifting: a randomized-controlled clinical trial. J Clin Periodontol. 2009;36:677–687.
- Barbeck M , Najman S , Stojanović S , et al. Addition of blood to a phycogenic bone substitute leads to an increased in vivo vascularization. Biomed Mater [Internet]. 2015 [cited 2015 Sep 13];10:055007. Available from: http://iopscience.iop.org/1748-605X/10/5/055007
- Yang P , Huang X , Shen J , et al. Development of a new pre-vascularized tissue-engineered construct using pre-differentiated rADSCs, arteriovenous vascular bundle and porous nano-hydroxyapatide-polyamide 66 scaffold. BMC Musculoskelet Disord [Internet]. 2013 [cited 2015 Apr 24];14:318. Available from: http://www.biomedcentral.com/1471-2474/14/318
- The European Parliament and the Council of the European Union. Directive 2010/63/EU of the European Parliament and of the council of 22 September 2010 on the protection of animals used for scientific purposes. Off J Eur Union [Internet]. 2010 [cited 2015 Sep 15];L276:33–79. Available from: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:276:0033:0079:EN:PDF
- Intini G , Andreana S , Intini FE , et al. Calcium sulfate and platelet-rich plasma make a novel osteoinductive biomaterial for bone regeneration. J Transl Med [Internet]. 2007 [cited 2015 Aug 18];5:13. Available from: http://translational-medicine.biomedcentral.com/articles/10.1186/1479-5876-5-13
- Liu Y , Zhou Y , Feng H , et al. Injectable tissue-engineered bone composed of human adipose derived stromal cells and platelet-rich plasma. Biomaterials. 2008;29:3338–3345.
- Yoshimura K , Shigeura T , Matsumoto D , et al. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J Cell Physiol. 2006;208:64–76.
- Planat-Benard V , Silvestre JS , Cousin B , et al. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004;109:656–663.
- Culmes M , Ecksteina HH , Burgkartb R , et al. Endothelial differentiation of adipose-derived mesenchymal stem cells is improved by epigenetic modifying drug BIX-01294. Eur J Cell Biol. 2013;92:70–79.
- Vidal F , Aragonés J , Alfranca A , et al. Up-regulation of vascular endothelial growth factor receptor Flt-1 after endothelial denudation: role of transcription factor Egr-1. Blood. 2000;95:3387–3395.
- Fu M , Zhu X , Zhang J , et al. Egr-1 target genes in human endothelial cells identified by microarray analysis. Gene. 2003;315:33–41.
- Gashler A , Sukhatme VP . Early growth response protein 1 (Egr-1): prototype of a zinc finger family of transcription factors. Prog Nucleic Acid Res Mol Biol. 1995;50:191–224.
- Oryan A , Alidadi S , Moshiri A , et al. Bone regenerative medicine: classic options, novel strategies, and future directions. J Orthop Surg Res [Internet]. 2014 [cited 2015 Aug 14];9:18. Available from: http://josr-online.biomedcentral.com/articles/10.1186/1749-799X-9-18
- Starke RD , Ferraro F , Paschalaki KE , et al. Endothelial von Willebrand factor regulates angiogenesis. Blood. 2011;117:1071–1080.
- Michiels C . Endothelial cell functions. J Cell Physiol. 2003;196:430–443.
- Feuerbach D , Feyen JH . Expression of the cell-adhesion molecule VCAM-1 by stromal cells is necessary for osteoclastogenesis. FEBS Lett. 1997;402:21–24.
- Scherberich A , Müller AM , Schäfer DJ , et al. Adipose tissue-derived progenitors for engineering osteogenic and vasculogenic grafts. J Cell Physiol. 2010;225:348–353.
- Kim SS , Kim BS . Comparison of osteogenic potential between apatite-coated poly (lactide-co-glycolide)/hydroxyapatite particulates and Bio-Oss. Dent Mater J. 2008;27:368–375.
- Khan SA , Lopez-Chua CA , Zhang J , et al. Soluble osteopontin inhibits apoptosis of adherent endothelial cells deprived of growth factors. J Cell Biochem. 2002;85:728–736.
- Denhardt DT , Noda M , O'Regan AW , et al. Osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling, and cell survival. J Clin Invest. 2001;107:1055–1061.
- Van Gaalen S , Kruyt M , Meijer G , et al. Tissue engineering of bone. In: van Blitterswijk C , Thomsen P , Lindahl A , Hubbell J , Williams DF , Cancedda R , de Bruijn JD , Sohier J , editors. Tissue engineering. Amsterdam : Elsevier Inc; 2008. p. 559–610. Available from: 10.1016/B978-0-12-370869-4.00019-7
- Barradas AM , Yuan H , van Blitterswijk CA , et al. Osteoinductive biomaterials: current knowledge of properties, experimental models and biological mechanisms. Eur Cell Mater. 2011;21:407–429.
- Collett GDM , Canfield AE . Angiogenesis and pericytes in the initiation of ectopic calcification. Circ Res. 2005;96:930–938.
