ABSTRACT
In the present study, 13 Salmonella Infantis strains, which have been originated from Turkey, were selected due to their clinical and industrial relevance, sufficient biofilm producing capability and multidrug resistance. Although all tested strains were built up of thin pellicle, optimum pellicle formation has occurred at 28 °C. All S. Infantis biofilms were categorized as ‘bdar’ morphotype following the incubation at both 20 and 28 °C, while they were categorized as ‘saw’ morphotype at 37 °C. Under a certain incubation temperature (28 °C), 84.62% of strains have formed strong biofilm structures. By using the disk diffusion method, high levels of resistance have been observed among tested bacteria against nalidixic acid (100%), spectinomycin (100%), streptomycin (92.3%), tetracycline (92.3%), kanamycin (76.9%) and neomycin (76.9%). Further studies were performed with S. Infantis DMC 12 strain, due to its capability to produce biofilm and multidrug resistance phenotype. Gentamycin (>64 µg/mL, 2 × MIC) and tetracycline (>128 µg/mL, 4 × MIC) were determined as the most effective antibiotics against biofilm formation. The biofilm forms have showed increased antimicrobial resistance when it was compared to the planktonic bacteria. The highest resistance rates of the biofilm bacteria were observed to neomycin (12 × MIC) followed by spectinomycin (10 × MIC) and streptomycin (10 × MIC). Biofilm structure was induced as a result of nalidixic acid, spectinomycin, tetracycline and neomycin treatment at sub-MIC concentrations of tested antibiotics.
Introduction
Salmonella species are enteric pathogens and the leading cause of bacterial foodborne illness. Salmonella species can form multicellular structures, called biofilms, on various surfaces such as polymer, stainless steel and glass, as well as biotic surfaces such as gallstones and epithelial cells [Citation1,Citation2]. Salmonella may also form biofilms (pellicles) in the air–liquid interphase [Citation3].
Bacterial biofilms are described as bacterial communities that can be attached to abiotic or biotic surfaces and enclosed with self-produced extracellular matrix substances [Citation1]. Generally, the bacterial cells in biofilm, embedded within a matrix structure, are well protected due to a chemical and physical barrier against various undesirable environmental conditions.
Around the world, antibiotic resistance in Salmonella sp. is a re-emerging problem. Determination of the antibiotic resistance profiles of Salmonella sp. from different serovars is important in order to apply suitable treatment against Salmonella infection. Biofilms are resistant to eradication by antibiotics at concentrations that are 10–1000 times greater than concentrations needed to eradicate free-living cells [Citation4]. Though sub-minimal inhibitory concentrations (sub-MIC) of some antibiotics can inhibit biofilm formation, it is known that they are not able to kill bacteria. On the other hand, numerous studies have shown that, when present at concentrations below the MIC, some antibiotics can significantly induce biofilm formation in a variety of bacterial species in vitro.
In the present study, antibiotics used for the treatment of Salmonella infections were tested against Salmonella Infantis serovar, including biofilm formation, morphotype determination, and we also determined the biofilm inhibition or induction at sub-MICs of tested antibiotics. For the first time, this report shows that certain antibiotics induce biofilm development of S. Infantis serovar.
Materials and methods
Salmonella Infantis isolates
The 13 DMC coded (DMC 7, 12, 20, 23, 40, 41, 57, 58, 70, 71, 75, 76, 77) Salmonella Infantis strains, isolated from raw chicken samples collected from Turkey, were all obtained from Prokaryotes Genetics Laboratory, Biology Department, Ankara University. All isolates were stored at −80 °C in a glycerine freezing medium and recovered in Luria Bertani (LB; Merck KGaA, Germany) at 37 °C overnight. LB without NaCl (LBwo/NaCl; bacto-tryptone 10 g/L, yeast extract 5 g/L) was used as a test broth in the biofilm assays [Citation5,Citation6].
Screening of biofilm morphotype
All 13 S. Infantis strains were screened at least three times for colony morphology by using the method described previously [Citation6]. Briefly, colonies were inoculated onto LB agar without NaCl containing 40 µg/mL Congo red (Sigma-Aldrich, Germany). The CR agar plates were incubated at 20, 28 and 37 °C, and were examined using dissection microscope (Leica, Germany) under 1× magnification. Isolates were grouped into three distinct morphotypes: (1) red, dry and rough, indicating curli and cellulose production (rdar); (2) brown, dry and rough, indicating curli production but a lack of cellulose synthesis (bdar); and (3) smooth and white, indicating a lack of both curli and cellulose production (saw).
Quantification of biofilm formation
Biofilm formation was quantified in LBwo/NaCl as previously described [Citation6]. Briefly, cells were cultured overnight in the appropriate medium and then diluted in LBwo/NaCl to OD595 = 0.2 (∼108 CFU/mL) (Shimadzu, Japan) and inoculated into 96-well polystyrene microtitre plates. Plates were incubated overnight at 20, 28 and 37 °C under static conditions, and were gently washed twice with sterile physiologic sodium chloride solution (0.85% NaCl). The plates were dried in room temperature before adding 130 µL 33% glacial acetic acid (v/v) (Sigma-Aldrich, Germany) and incubated for 30 min in room temperature. Following the incubation, solubilized dye from the adherent cells was measured at OD595. For each strain, the result was calculated by subtracting the median OD595 of the three parallels of the control (test broth only) from the median of OD595 of the three parallels of sample. The cut-off value (ODc) for a biofilm producer and the classification of the isolates as non-biofilm producers were determined as (OD ≤ ODc), for weak biofilm producers (ODc < OD ≤ 2 × ODc), for moderate biofilm producers (2 × ODc < OD ≤ 4 × ODc), and for strong biofilm producers (4 × ODc) [Citation7].
Biofilm formation in liquid–air interface
To analyse biofilm formation in liquid, i.e. pellicle formation at the liquid–air interface, 4.5 mL of LBwo/NaCl was inoculated with 0.5 mL of an overnight culture and incubated at 20, 28 and 37 °C for eight days. At the end of the incubation process, the strains were also evaluated by their appearance, to be able to see if they showed elastic brittle and rigid features [Citation3].
Antibiotic susceptibility testing
The following antimicrobial agents were selected due to their common use for the treatment of Salmonella infections. Therefore, the study evaluated the bacterial resistance against kanamycin (30 µg), neomycin (10 µg), nalidixic acid (30 µg), tetracycline (30 µg), spectinomycin (10 µg), trimethoprim (5 µg), ampicillin (10 µg), amoxicillin/clavulanic acid (30 µg), ceftiofur (30 µg), chloramphenicol (30 µg), florfenicol (30 µg), gentamicin (10 µg), ciprofloxacin (5 µg), trimethoprim/sulfamethoxazole (25 µg) and streptomycin (10 µg). All solutions were sterilized by using a 0.2 µm cellulose syringe filter (Sartorious, Germany). Agar disk diffusion and MIC were performed using Mueller-Hinton agar (MH-Agar) and Mueller–Hinton Broth (MHB, Oxoid), respectively, according to CLSI guidelines [Citation8]. Escherichia coli ATCC 25922 strain was used as a control.
Effect of sub-inhibitory concentrations (sub-MICs) of tested antibiotics on biofilm formation
The activities of antimicrobial agents were tested at a concentration ranged from 0.5 to 512 µg/mL to determine their effects on biofilm formation. Various concentrations of antibiotics, prepared in 200 µL of MHB, were added to wells of 96-flat bottom plates containing 20 µL of diluted (OD595 = 0.2) overnight culture of bacteria. After incubation at 28 °C for 24 h, quantification of biofilm was measured as previously described and control wells contained only drug-free medium [Citation9].
Effects of antimicrobial agents on the reduction of Salmonella biofilms on stainless steel coupons
Stainless-steel-type 304 (1 × 8 × 9 mm3) coupons were cleaned with 100% acetone, dried and cleaned with 70% alcohol (v/v). Cleaned coupons were autoclaved at 121 °C for 15 min. Previously proposed biofilm formation model was used to develop biofilm structure on stainless steel coupons and they were individually placed in sterile tubes that contain 3.5 mL of LBwo/NaCl and 1 mL of overnight culture, adjusted to 108 CFU/mL, and then incubated at 28 °C for 24 h [Citation10]. Following the incubation, coupons were removed with sterile forceps, washed with sterile physiologic sodium chloride solution (to remove loosely attached cells), transferred to a sterile tube and air dried for 10 min. Subsequently, coupons were individually transferred into tubes containing 2 mL of various antibiotic concentrations with a serial twofold dilution of each antibiotic; (MIC/2 to MIC/1024 µg/mL) and incubated at 37 °C for 24 h. The positive control tubes only contained MHB. After incubation, stainless steel coupons were removed, washed and dried as described above. Five sterile glass beads (r = 3 mm) and dried coupons were placed in a 10-mL tube which contained 4.5 mL of sterile physiologic sodium chloride solution and tubes were vortexed for 1 min. CFU counts were enumerated by spread plating in LB agar and incubated at 37 °C for 24 h. Antibiotic concentrations eradicate 99.9% of biofilm structure compared to control group was determined as Minimum Biofilm Eradication Concentration (MBEC). MBEC experiments were performed by using multidrug-resistant and biofilm producer S. Infantis DMC 12 strain.
Statistical analysis
All statistical analyses were performed by using SPSS (version 18, USA). The assays for inactivation of performed biofilms were replicated three times independently. One-way ANOVA test was preferred for microtitre plate assay data and MBEC data.
Results and discussion
Even though S. Infantis serovar, which was used in the present study, is not the common serovar in Salmonella epidemiology, it is one of the most frequently isolated serovar from clinical and food environment. Because of the widespread usage of antimicrobial agents in both developed and developing countries, antibiotic resistance in some types of Salmonella infections is increasing. Increasing antimicrobial resistance in non-typhoid Salmonella species has been a serious problem for public health [Citation11,Citation12]. Antibiotic resistance profiles of food-originated DMC coded 13 S. Infantis isolates, determined in the present study are compatible with the previous findings [Citation13–Citation15]. The most prevalent resistances, found in the multidrug-resistant DMC coded S. Infantis strains, were to nalidixic acid (100%) and spectinomycin (100%). Previous reports indicated the potential emergence of quinolone resistance in Salmonella [Citation14,Citation16–Citation18]. It is known that isolates with nalidixic acid resistance have commonly decreased susceptibility to ciprofloxacin. Similar to this knowledge, as a result of antimicrobial susceptibility assays obtained from the present study, ciprofloxacin resistance was determined in 76.9% of tested S. Infantis isolates.
A total of 13 isolates studied, 10 (76.9%) were sensitive to ciprofloxacin, 1 (7.69%) was sensitive to tetracycline. By using the disk diffusion method, high levels of resistance among tested bacteria were observed against nalidixic acid (100%), spectinomycin (100%), streptomycin (92.3%), tetracycline (92.3%), kanamycin (76.9%) and neomycin (76.9%). The concentrations of antibiotic that were required to inhibit planktonic (MIC) and kill biofilm bacteria (MBEC) are summarized in and . Among seven tested antibiotics, the highest resistance was observed against nalidixic acid (≥128) and spectinomycin (≥128), followed by kanamycin (≥64). The incidence of multidrug resistance (MDR to three or more antibiotics) in Salmonella strains was 100%. All MBEC levels were determined higher than the MIC values. The most effective antibiotic, against biofilm formation, was gentamycin (>64 µg/mL, 2 × MIC), followed by tetracycline (>128 µg/mL, 4 × MIC).
Table 1. MIC values of tested S. Infantis strains.
Table 2. MBEC values of tested S. Infantis strains.
World Health Organization has classified quinolones as critically important antimicrobial agents for human medicine. Unfortunately, the incidence of resistance to quinolones in bacteria from food-producing animals has been recognized as a primary public health problem [Citation19]. Quinolones and fluoroquinolones are broad-spectrum antimicrobial agents that target DNA gyrase (gyrA and gyrB) and topoisomerase IV genes (parE and parC), and the resulting drug–enzyme–DNA complex inhibits DNA synthesis [Citation13]. Mobile genetic elements such as plasmids, transposons, and the more recently explored integrons, which are able to disseminate antibiotic resistance genes by horizontal or vertical transfer, have played an important role in the evolution and dissemination of MDR in Gram-negative bacteria [Citation20,Citation21]. Further investigations are required to identify the genetic nature of antibiotic resistance of the 13 S. Infantis strains.
A biofilm is a structured consortium of bacteria embedded in a self-produced polymer matrix which consists of polysaccharide, protein and DNA. Bacteria in biofilms are known to be more resistant to antimicrobials and drying [Citation22]. Curli fimbriae, proteinaceous extracellular fibres and cellulose are major components of the extracellular matrix of S almonella t yphimurium biofilms [Citation5,Citation23,Citation24]. It was reported that the contribution of the extracellular matrix was variable in environmental conditions [Citation24]. In the present study, DMC coded 13 Salmonella isolates displayed two different biofilm morphotypes; ‘bdar’ morphotype after incubation at 20 and 28 °C, ‘saw’ morphotype after incubation at 37 °C in LBwo/NaCl supplemented with Congo Red (40 µg/mL).
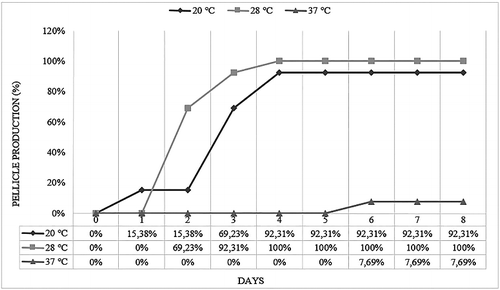
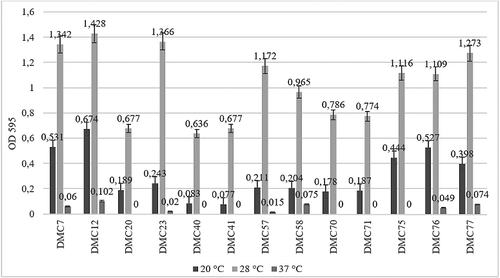
In the screen of food-originated 13 Salmonella strains, OD595 values were found to range from 0.07 to 0.67 at 20 °C, 0.67 to 1.42 at 28 °C and 0 to 0.1 at 37 °C (). Statistically significant differences between serovars were observed as a result of one-way ANOVA, p < 0.01. Since the highest intensity of biofilm formation on polystyrene was produced by DMC 12 coded Salmonella Infantis strain at 28 °C, further analyses were performed by using DMC 12. At 20 and 28 °C, 24.8% and 76.9% of S. Infantis strains produced strong biofilm, respectively. Also, all strains were tested for their biofilm-forming abilities at the liquid–air interface. Although all tested strains were built up of thin pellicle after growth at 20, 28 and 37 °C, maximum pellicle formation occurred at 28 °C ().
Figure 1. Biofilm production levels of DMC coded 13 S. Infantis strains following the incubation at 20, 28 and 37 °C on polystyrene plates.
Researchers reported that the ‘bdar’ morphotype is rarer in natural isolates and lacking only the cellulose component of rdar morphotypes [Citation6]. The bdar morphotype has been suggested to be linked to Salmonella groups with a narrower host range that may not need to survive for long periods in the environment and are potential commensals with their hosts [Citation22,Citation25]. As it was previously mentioned, S serovar is not the common serovar in Salmonella epidemiology and all tested isolates formed ‘bdar’ morphotype at 20 and 28 °C, our findings match with the previous reports [Citation22,Citation25].
Curli and cellulose production behaviour of 51 S almo nella t yphimurium strains in different conditions of the biofilm formation has been analysed [Citation24]. They concluded that biofilm forming behaviour is not only affected by environmental conditions such as temperature and the culture medium, but also it was associated with strain origin. Steponovic et al. [Citation26] reported that 1.6% of the tested 122 Salmonella strains produced strong biofilm on polystyrene at 28 °C [Citation26]. De Oliveira et al. [Citation27] also assessed biofilm production abilities of 171 Salmonella spp. on stainless steel, glass and PVC (the most similar to polystyrene material) [Citation27]. They observed that 1.7% of strains growth on stainless steel and glass, but PVC was not colonized at 28 °C. In our previous study, we tested biofilm-producing abilities of Salmonella strains at different environmental conditions such as temperature, pH and NaCl concentrations [Citation28]. The highest amount of biofilm was formed by all tested strains on polystyrene at 20 °C, and it was produced at similar levels by S. t yphimurium (rdar, OD595 = 3.481) and S. Infantis (bdar, OD595 = 3.478) strains. Biofilm contents and physiology are highly heterogeneous and varies depending on the material of the surface, growth conditions, environmental conditions and gene expression levels that are affected by these factors. It can be concluded that, since suitable conditions and gene expressions occurred at 28 °C, we determined optimum production for S. Infantis serovar at this incubation temperature.
Bacteria protected within biofilms are 10–1000 times more resistant to antibiotics than planktonic cells [Citation4]. There are numerous factors such as contact time, the surrounding environment and the concentration of the antimicrobial agent to determine its effectiveness. Although MIC has been used as a gold standard to determine antimicrobial sensitivities for bacteria, the MIC value is not predictive of clinical efficacy of a particular antibiotic [Citation29]. The MBEC assay was developed for rapid and reproducible antimicrobial susceptibility testing for bacterial biofilms. It has been reported that MBEC values are greater than MIC against isolated strains from Salmonella infections [Citation30,Citation31]. In the present study, at first, we determined the MBEC levels of seven different antibiotics on S. Infantis serovar biofilms. Also, few publications are available on MBEC values of several antibiotics on other Salmonella serovars. Olson et al. [Citation29] determined antibiotic sensitivities of certain bacteria including Salmonella Bredeney, S. t yphimurium and Salmonella sp.[Citation29]. Parallel to our findings, there was considerable variability among the MICs of the Salmonella spp. isolates; similar variability between the MIC and MBEC values was observed. Besides Salmonella strains, they also tested other Gram-negative livestock pathogens such as bovine, porcine and avian Pasteurella spp., as well as the Haemophilus somnus, and Mannheimia haemolytica, formed biofilms, but in most cases, there was not any difference between the MIC and MBEC values. Papavasileiou et al. [Citation32] compared antimicrobial susceptibility of biofilm versus planktonic forms of 194 Salmonella enterica strains isolated from children gastroenteritis [Citation32]. The biofilm forms showed increased antimicrobial resistance compared to the planktonic bacteria. The highest resistance rates of the biofilm bacteria were observed with respect to gentamicin (89.9%) and ampicillin (84.4%). S. enterica strains isolated from children with gastroenteritis. Combining our results with the limited previous reports, this observations reflected the complexity in prediction of chemotherapeutic agents for treatment of different Salmonella isolates, since higher dosage of antibiotic requires to combat with biofilm structure.
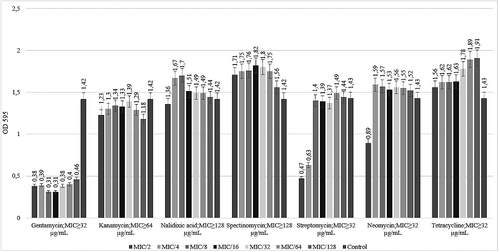
The activities of antimicrobial agents tested at sub-MIC concentrations against DMC 12 S. Infantis biofilm are shown in . Gentamycin, streptomycin and kanamycin significantly (p < 0.05) reduced optical density of the biofilm of DMC 12. Gentamycin (MIC ≤ 2) was the most effective antibiotic in eradication of bacterial biofilm structure. The exposure of bacteria to nalidixic acid, spectinomycin, tetracycline and neomycin induced the biofilm production (p < 0.05) ().
As reviewed by Kaplan [Citation33], although numerous studies have shown that sub-MICs of some antibiotics are not able to kill bacteria, but they can inhibit biofilm formation [Citation33]. In contrast, many studies have shown that some antibiotics, when present at concentrations below the MIC, can significantly induce biofilm formation in variety of bacterial species in vitro. The results of our study, as presented in , show that when S. Infantis strain was grown in the presence of sub-MIC of antibiotics, the maximum inhibition of biofilm formation was observed as a result of gentamycin treatment. The exposure of bacteria to nalidixic acid, spectinomycin, tetracycline and neomycin induced the biofilm production (p < 0.05). As it was performed in the previous study, sub-MIC concentrations of tobramycin-induced Pseudomonas aeruginosa biofilm formation through a mechanism that involves the intracellular second messenger cyclic dimeric guanosine monophosphate (c-di-GMP) [Citation34]. Other researchers have studied the modulation of Salmonella gene expression by sub-MIC concentrations of quinolones [Citation35]. Even at the sub-MICs employed, all tested fluoroquinolones upregulated genes involved in the SOS response, umuD, LexA, sbmC and dinP. They concluded that small molecules can have functions other than growing the inhibition that may affect the establishment and maintenance of community dynamics in complex environments. Wojnicz and Tichaczek-Goska [Citation9] analysed the effect of sub-MIC of ciprofloxacin, amikacin and colistin on biofilm formation of E. coli isolates [Citation9]. Their results showed that all three tested antibiotics, that reduced biofilm production, interfere with curli expression but do not influence motility. They suggested that ciprofloxacin, amikacin and colistin may be useful in the treatment of biofilm-associated infections caused by E. coli strains. As a result of our study, at first, we analysed the effect of sub-MICs of seven antibiotics on biofilm formation of S. Infantis serovar. Consequently, further investigations are required to be able to determine molecular mechanisms that involve the inhibition of S. Infantis biofilms at sub-MIC concentrations.
Conclusion
In conclusion, the present study is the first report that assessed antibiotic susceptibility, besides MBEC and sub-MICs of tested antibiotics on S. Infantis biofilm structure. This study showed the diversity of response to antibiotic treatment in S. Infantis serovars depending on their cell forms, planktonic cells or biofilms. Although S. Infantis serovars were sensitive to gentamycin antibiotic while they formed biofilm, they showed much greater resistance to nalidixic acid in planktonic cultures. This may be due to the same bacterium which is different in point of both genetic and physiologic in the biofilm state than in the planktonic state for which the antibiotic was designed and tested. It is concluded that biofilm-forming S. Infantis bacteria are able to escape in vitro the action of commonly used antibiotics. Kaplan reviewed that ‘this process may have clinical relevance because bacteria are exposed to sub-MIC concentrations of antibiotics at the beginning and the end of a dosing regimen, between doses, or continuously during low-dose therapy’ [Citation33,Citation36,Citation37]. Knowledge about the consequences of the usage of antimicrobial agents at treatment of Salmonella infections is required to be examined and needs to be decided the concentrations considering biofilm structure.
Acknowledgement
We thank Cihan Akın GÜRSOY for proofreading and editing our research paper.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Costerton JW , Stewart PS , Greenberg EP . Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322.
- Moretro T , Vestby LK , Ness LL , et al. Evaluation of efficacy of disinfectants against Salmonella from the feed industry. J Appl Microbiol. 2009;106:1005–1012.
- Scher K , Römling U , Yaron S . Effect of heat, acidification, and chlorination on Salmonella enterica serovar typhimurium cells in a biofilm formed at the air–liquid interface. Appl Environ Microbiol. 2005;71:1163–1168.
- Mah TF , O'Toole GA . Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001;9:34–39.
- Romling U , Sierralta WD , Eriksson K , et al. Multicellular and aggregative behaviour of Salmonella typhimurium strains is controlled by mutations in the agfD promoter. Mol Microbiol. 1998;28:249–264.
- Vestby LK , Moretro T , Langsrud S , et al. Biofilm forming abilities of Salmonella are correlated with persistence in fish meal- and feed factories. BMC Vet Res. 2009;5:1–6.
- Stepanovic S , Cirkovic I , Mijac M , et al. Influence of the incubation temperature, atmosphere and dynamic conditions on biofilm formation by Salmonella spp. Food Microbiol. 2003;20:339–343.
- Performance standards for antimicrobial susceptibility Testing. Proceedings of 16th Informational Supplement M100. Wayne : Clinical and Laboratory Standards Institute; 2006. p. S16.
- Wojnicz D , Tichaczek-Goska D . Effect of sub-minimum inhibitory concentrations of ciprofloxacin, amikacin and colistin on biofilm formation and virulence factors of Escherichia coli planktonic and biofilm forms isolated from human urine. Braz J Microbiol. 2013;44:259–265.
- Giaouris E , Chorianopoulos N , Nychas GJE . Effect of temperature, pH, and water activity on biofilm formation by Salmonella enterica Enteritidis PT4 on stainless steel surfaces as indicated by the bead vortexing method and conductance measurements. J Food Protect. 2005;10:2149–2154.
- Avsaroglu MD , Helmuth R , Junker E , et al. Plasmid-mediated quinolone resistance conferred by qnrS1 in Salmonella enterica serovar Virchow isolated from Turkish food of avian origin. J Antimicrob Chemother. 2007;60:1146–1150.
- Macedo-Vinas M , Cordeiro NF , Bado I , et al. Surveillance of antibiotic resistance evolution and detection of class 1 and 2 integrons in human isolates of multi-resistant Salmonella typhimurium obtained in Uruguay between 1976 and 2000. Int J Infect Dis. 2009;13:342–348.
- Goncagül G , Günaydın E , Çarlı KT . Antibiotic resistance of Salmonella enteritidis of human and chicken origin. Turk J Vet Anim Sci. 2004;28:911–914.
- Nogrady N , Kiraly M , Davies R , et al. Multidrug resistant clones of Salmonella Infantis of broiler origin in Europe. Int J Food Microbiol. 2012;157:108–112.
- Bayramoğlu G , Özgümüş OB , Kolaylı F , et al. Salmonella enterica serotip Paratyphi B klinik izolatlarının moleküler epidemiyolojisi, antimikrobiyal direnci ve genişletilmiş spektrumlu beta-laktamazlarının karakterizasyonu [Molecular epidemiology, antimicrobial resistance and characterization of extended-spectrum beta-lactamases of Salmonella enterica Serotype Paratyphi B clinical isolates]. Mikrobiyol Bul. 2014;48:191–200.
- Dunowska M , Morley PS , Traub-Dargatz JL , et al. Comparison of Salmonella enterica serotype Infantis isolates from a veterinary teaching hospital. J Appl Microbiol. 2007;102:1527–1536.
- Fábrega A , Sánchez-Céspedes J , Soto S , et al. Quinolone resistance in the food chain. Int J Antimicrob Agents. 2008;31:307–315.
- Gal-Mor O , Valinsky L , Weinberger M , et al. Multidrug-resistant Salmonella enterica serovar Infantis, Israel. Emerg Infect Dis. 2010;16:1754–1757.
- Malorny B , Schroeter A , Guerra B , et al. Incidence of quinolone resistance in strains of Salmonella isolated from poultry, cattle and pigs in Germany between 1998 and 2001. Vet Rec. 2003;153: 643–648.
- Carattoli A . Importance of integrons in the diffusion of resistance. Vet Res. 2001;32:243–259.
- Miko A , Pries K , Schroeter A , et al. Molecular mechanisms of resistance in multidrug-resistant serovars of Salmonella enterica isolated from foods in Germany. J Antimicrob Chemother. 2005;56:1025–1033.
- White AP , Gibson DL , Kim W , et al. Thin aggregative fimbriae and cellulose enhance long-term survival and persistence of Salmonella . J Bacteriol. 2006;188:3219–3227.
- Jonas K , Tomenius H , Kader A , et al. Roles of curli, cellulose and BapA in Salmonella biofilm morphology studied by atomic force microscopy. BMC Microbiol. 2007;7:70.
- Castelijn GA , van der Veen S , Zwietering MH , et al. Diversity in biofilm formation and production of curli fimbriae and cellulose of Salmonella typhimurium strains of different origin in high and low nutrient medium. Biofouling. 2012;28:51–63.
- Chia TW , McMeekin TA , Fegan N , et al. Significance of the rdar and bdar morphotypes in the hydrophobicity and attachment to abiotic surfaces of Salmonella sofia and other poultry-associated Salmonella serovars. Lett Appl Microbiol. 2011;53:581–584.
- Stepanović S , Cirković I , Ranin L , et al. Biofilm formation by Salmonella spp. and Listeria monocytogenes on plastic surface. Lett Appl Microbiol. 2004;38:428–432.
- De Oliveira DC , Fernandes Júnior A , Kaneno R , et al. Ability of Salmonella spp. to produce biofilm is dependent on temperature and surface material. Foodborne Pathog Dis. 2014;11:478–483.
- Karaca B , Akcelik N , Akcelik M . Biofilm producing abilities of Salmonella strains isolated from Turkey. Biologia. 2013;68:1–10.
- Olson ME , Ceri H , Morck DW , et al. Biofilm bacteria: formation and comparative susceptibility to antibiotics. Can J Vet Res. 2002;66:86–92.
- Spoering AL , Lewis K . Biofilms and planktonic cells of Pseudomonas aeruginosa have similar resistance to killing by antimicrobials. J Bacteriol. 2001;183:6746–6751.
- Toté K , Horemans T , Berghe DV , et al. Inhibitory effect of biocides on the viable masses and matrices of Staphylococcus aureus and Pseudomonas aeruginosa biofilms. Appl Environ Microbiol. 2010;76:3135–3142.
- Papavasileiou K , Papavasileiou E , Tseleni-Kotsovili A , et al. Comparative antimicrobial susceptibility of biofilm versus planktonic forms of Salmonella enterica strains isolated from children with gastroenteritis. Eur J Clin Microbiol Infect Dis. 2010;29:1401–1405.
- Kaplan JB . Antibiotic-induced biofilm formation. Int J ARTF Organs. 2011;34:737–751.
- Hoffmann LR , D'Argenio DA , MacCoss MJ , et al. Aminoglycoside antibiotics induce bacterial biofilm formation. Nature. 2005;436:1171–1175.
- Yim G , McClure J , Surette MG , et al. Modulation of Salmonella gene expression by subinhibitory concentrations of quinolones. J Antibiot. 2011;64:73–79.
- Odenholt I . Pharmacodynamic effects of subinhibitory antibiotic concentrations. Int J Antimicrob Agents. 2001;17:1–8.
- Dan I. Andersson DI. , Diarmaid Hughes D . Microbiological effects of sublethal levels of antibiotics. Nat Rev Microbiol. 2014;12:465–478.