 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Currently, different methods for air clean-up from chemical pollutants are applied worldwide: adsorption, absorption, and thermal and catalytic oxidation. One of the most promising methods is biological air cleaning. The aim of this study was to test the performance of a developed biofilter with packing material of activated pine bark for biological air cleaning and to mathematically model the biofiltration processes. Comparative analysis of the modelling results for individual pollutants (butyl acetate, butanol and xylene) showed strongest dependence of the efficiency of xylene removal from the air on the amount and ratio of other substances (from 20% to 70%). Thus, the process of removal of pollutants (butanol and butyl acetate) that are easier to biologically decompose was obtained to be influenced to a lesser extent by the amount and ratio (%) of other components. The results also showed that the efficiency of butyl acetate removal mostly depended on the ratio of other substances in the mixture (from 15% to 100%), whereas the efficiency of butyl acetate and xylene removal mostly depended on the amount of other substances in the mixture (from 20% to 100%). With the parameters of the biofilter (height of packing material, incoming air flow velocity) and the pollutants to be removed known, the mathematical expression of the filter efficiency was found, which would allow to make theoretical calculation and selection of the most appropriate parameters of the device as well as to achieve maximum efficiency of air cleaning.
Introduction
Currently, different methods for air clean-up from chemical pollutants (butanol, butyl acetate, ethyl acetate, xylene, etc.) are applied worldwide: adsorption, absorption, and thermal and catalytic oxidation. Each of them has its advantages and disadvantages; for example, the high efficiency of removal of chemical substances by thermal decomposition, the simplicity of a sorption method, etc. The downsides cover generation of secondary pollutants (decomposition products) when applying the absorption method for air cleaning, wastewater, etc.
Therefore, when choosing a method for air cleaning, the following key characteristics should be taken into account: high level of cleaning, generation of non-harmful decomposition products and low costs of the method. One of the most promising air-cleaning methods is biological air cleaning using cultures of self-contained micro-organisms [Citation1–Citation7]. To that end, the Department of Environmental Protection of Vilnius Gediminas Technical University (VGTU) developed a biofilter with packing material of activated pine bark. This biofilter was tested for biological air cleaning and the biofiltration processes were mathematically modelled.
The experimental research revealed it was a rather efficient method for air cleaning. The efficiency of the filter for cleaning the air from a mixture of volatile organic compounds (VOCs) (butilacetate, butanol and xylene) at the permitted concentrations reached up to 70%–98% [Citation8–Citation21].
However, this does not complete the final evaluation the functioning of the filter. A mathematical model of the biofilter has been developed on the grounds of the results gained in the course of the research and after full consideration of physical, chemical and biological processes occurring while the filter is functioning. The aim of the present study was to mathematically model the biofiltration and to obtain data from experimental tests to determine the dependence of the efficiency of removal of the pollutants in question (butyl acetate, butanol and xylene) on their amount (%) and ratio in the mixture.
Materials and methods
The experimental biofilter with activated packing material of pine bark was developed at the VGTU [Citation12]. The biofilter (0.5 × 0.48 × 2.0 m) with the biologically activated packing material contains five discrete layers of biomedium.
When purifying air from the mixture of volatile compounds of organic nature, the flow of polluted air was blown through all five layers of biomedium by means of a ventilator. There were dampers installed with control handles in the inlet and outlet ducts of the filter to adjust the air flow rate (56.7–144.69 m3/h) and flow velocity (0.8–2.0 m/s). To increase the amount of polluted air, the inlet airduct had a funnel shape at the front end. Purified air was exhausted through the flexible duct from the filter.
The air flow velocity during the experiments was measured by the velocity meter ‘TESTO–452’ with a thermopair. When increasing the velocity of the injected air flow up to 2.0 m/s, the measurement error also became larger. Each measurement was repeated five times.
During the test, the filter efficiency when changing the mixture of VOCs (butanol, butyl acetate and xylene) and their concentrations in the air to be purified, the mixture composition, the velocities of injected air flow, the duration of contact between the packing material, and the pollutants and its biological activation were experimentally determined.
Different concentrations of the mixture of organic substances were obtained by heating them (to 55 °C) in a pot on a regulated electric range. The temperature of the air flow injected into the equipment varied in the range of 20–55 °C. In order to compare the biodegradation of selected pollutants in the packing material of activated pine bark, five different concentrations (5, 15, 50, 70 and 90 mg/m3) of pollutants (butanol, butyl acetate and xylene) up to 100 mg/m3 were taken in approximately 15–20 mg/m3 increments (–).
Figure 1. Dependence of the efficiency of butyl acetate removal on the amount of other components (butanol and xylene) in the mixture and their ratio in the mixture. I, amount (%) of butanol and xylene in the mixture; II, ratio of butanol and xylene to butyl acetate in the mixture.
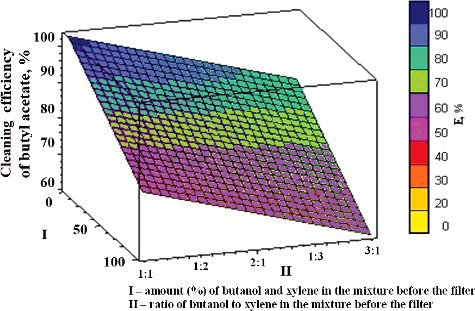
Figure 2. Dependence of the efficiency of butanol removal on the amount of other components (butyl acetate and xylene) and their ratio in the mixture. I, amount (%) of butyl acetate and xylene in the mixture; II, ratio of butyl acetate and xylene to butanol in the mixture.
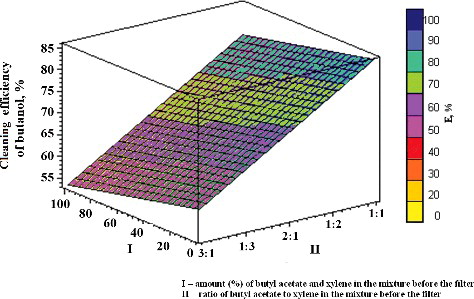
Figure 3. Dependence of the efficiency of xylene removal on the amount of other components (butyl acetate and butanol) and their ratio in the mixture. I, amount (%) of butyl acetate and butanol in the mixture; II, ratio of butyl acetate and butanol to xylene in the mixture.
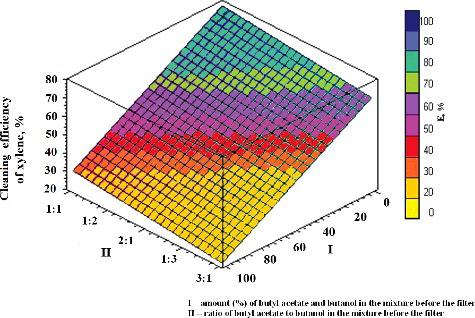
Figure 4. Dependence of the efficiency of removal of pollutants (butyl acetate, butanol and xylene) on their amount and ratio in the mixture. I, amount (%) of pollutants in the mixture; II, ratio of pollutants in the mixture.
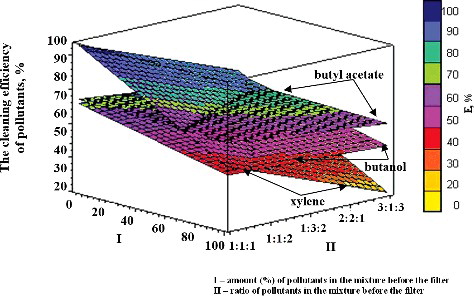
Figure 5. Dependence of the efficiency of removal of pollutants (butyl acetate, butanol and xylene) on the ratio of the other two components in the mixture.
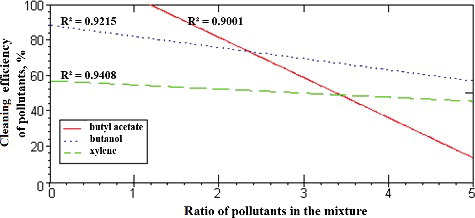
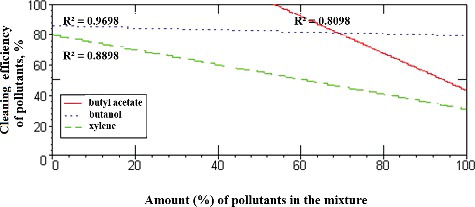
Figure 6. Dependence of the efficiency of removal of pollutants (butyl acetate, butanol and xylene) on the amount (%) of the other two components in the mixture. .
Air samples before and after the filter and after each layer were taken at measurement points by using glass sampling tubing (Ø 4.0 ± 0.1 mm, length of 7.0 ± 0.01 m) filled with activated coconut carbon (0.3–0.85 mm) produced by SKC Inc. (Morgan Hill, CA, USA) and a universal sampler Airchek 224-PCXR8 (SKC, Inc., Fullerton, CA, USA) for 15 min at 0.2 L/min velocity.
The sampling tubing had two sections and each section was filled with a different amount of activated carbon (100 and 50 mg, correspondingly). Air samples from the sampling tubes were desorbed with 1 cm3 of carbon disulphide for 30 min in two (separate from each section) 2-cm3 bottles.
The concentration of the mixture of compounds in question in the air samples was determined by gas chromatographic analysis (Hewlett Packard Model 5890 (GMI, Inc., Ramsey, MN, USA)). The operating conditions were as follows: sensitivity of the flame ionisation sensor of 4 × 10−9 mg/s, glass column (Ø 3.0 ± 0.1 mm, length 2.0 ± 0.01 m) filled with Chromosorb® WHP (0.18–0.15 mm) and 10% OV-101 (GMI, Inc.). The column temperature was 90 °C; that of the injector and detector, 250–260 °C and of the evaporator, 200 °C. The temperature programme was: 70 °C (0.5 min), 70–200 °C (15 °C/min), 200 °C (2 min), 200–250 °C (15 °C/min), 250 °C (10 min), 250–260 °C (10 °C/min), ionizing voltage of 70 eV and injection (splitless) volume, 1 or 2 µL.
After performing the chromatographic analysis, the arithmetic mean values of the peak area of the VOC mixture were calculated on the grounds of three measurements of the sample.
After determining the concentrations of the organic substances in the mixture by chromatographic analysis, the efficiency () of the biological air purifier was evaluated, using the following equation:
(1)
(1) where
is the efficiency of the filter (%);
is the initial concentration of the pollutant in question in the mixture (mg/m3) and
is the concentration of the pollutant in the mixture after the air purification (mg/m3).
Each analysis was performed in triplicates. Data analysis of the modelling results (means, standard errors of the means, standard deviations and relative standard deviations) was done using Microsoft Excel 2003. The effect of the studied factors was tested at a probability of 0.95 and their influence, by the coefficient of determination ().
Results and discussion
Mathematical modelling was used to determine the dependence of the efficiency of removal of each pollutant (butanol, butyl acetate and xylene) on the amount of the other components and their ratio in the mixture. San-Valero et al. [Citation22] have modelled the kinetic study of a pollutant (VOC) biodegradation. Their approach was adapted in our study, as this model was calibrated and validated with data from laboratory-scale biotrickling filters. The laboratory experiments exhibited peaks of pollutants in the outlet of the biotrickling filter during spraying periods. For example, in our experiments, when the other organic substances amounted to 50% and their ratio was 1:1, the efficiency of butyl acetate removal was up ∼95% ().
Therefore, in our experiments, when the amount of the other components (butanol and xylene) (%) was lower and their ratio was even (1:1), the air purification from butyl acetate was nearly 100%. However, as the percentage of the other studied initial concentrations of VOCs increased (to nearly 100%) and their ratio in the mixture became more uneven (1:3 or 3:1), the efficiency of butyl acetate removal decreased to 57%. Like the model of Ikemoto et al. [Citation23], our model represents the full kinetic behaviour of the system and simplified steady-state reactor performance. Solutions are developed by finite difference approximation.
According to the results from our experiments, the dependency of the efficiency of butyl acetate removal () on the amount and ratio of other pollutants (butanol and xylene) was expressed using the following equation:
(2)
(2) where
is the height of the filter (m);
is the initial concentration of the pollutant in question (in this case, butyl acetate) in the mixture (mg/m3);
is the amount (%) of other pollutants in question (butanol and xylene) in the mixture and
is the ratio of pollutants in question (butanol and xylene) in the mixture. The equation of the coefficient of determination was
.
Using the above equation, when we use the primary data (the initial concentration of butyl acetate in the mixture and the height (750 mm) of the packing material in the filter), the following was obtained:(3)
(3) When the results from the experimental tests were taken into account, the dependence of the efficiency (
) of removal of butanol from the air was obtained in the same way ():
(4)
(4)
The coefficient of determination was . At optimal conditions, i.e. 50 mg/m3 initial concentration of butanol in the mixture and height of 750 mm (preliminary experiments; data not shown), the following equation was obtained:
(5)
(5)
The latter dependence is displayed in a graphical form (). In a similar way, the increase in the amount of pollutants (to nearly 100%) and the unevenness of their proportion in the total mixture (ratios of 1:3 or 3:1) resulted in lower efficiency of butanol removal from the air (to 53%).
According to the modelling results, the dependence of the efficiency () of removal of xylene from the air (
) was also found:
(6)
(6)
When the initial concentration of xylene in the mixture is 50 mg/m3 and the height of the packing material in the filter is 750 mm (optimal conditions, data not shown), the following equation was obtained:(7)
(7)
With the amount (5%) and ratio (from 1:1 to 3:1) of other pollutants (butyl acetate and butanol) known, the diagram of this function allows to determine the efficiency of removal of xylene ().
Compared with all the analysed substances (butyl acetate and butanol), the efficiency of xylene removal showed the greatest dependency on the amount and the ratio of the other components in the gas mixture. When the other organic substances (butyl acetate and butanol) accounted for a considerable concentration (%) and uneven ratio in the mixture, the removal of xylene decreased considerably (to 20%). Our results correspond well with the model presented by Lu et al. [Citation24].
The comparison of the obtained dependences of the efficiency of removal of the studied VOC pollutants (butyl acetate, butanol or xylene) on the amount (%) and ratio of the other two components () showed that the efficiency of butanol and butyl acetate removal from the air was highest, whereas that of xylene was lowest (from 20% to 80%), . Substantially, such different dependences are predetermined by differences in the solubility and biodegradability of these VOCs. Thus, the process of removal of pollutants (butanol and butyl acetate), which are easier to biologically decompose, was observed to be influenced to a lesser extent by the amount (%) and ratio of other components.
Mathematical modelling was used in order to reveal how the efficiency of removal of the studied VOCs (butyl acetate, butanol and xylene) from the air depends on the initial concentrations of the pollutant in the mixture. Thus, a mathematical expression was derived for each studied VOC as a function of the ratio of the pollutants in the mixture. The expression for butyl acetate removal was as follows:(8)
(8)
For butanol:(9)
(9)
For xylene:(10)
(10)
The dependence of the efficiency of removal of these substances on their ratio in the mixture is given in a diagram (). First, each particular VOC was taken to be 50% in the mixture; then its removal efficiency was determined in the mixture of three pollutants (butyl acetate, butanol and xylene) when the other two gases, which constituted together the remaining 50% of the mixture, were fed in different ratios to each other: 1:1, 1:2, 2:1, 1:3 or 3:1. The results presented in illustrate that, of all the substances included in this study (butyl acetate, butanol and xylene), the removal of butyl acetate was shown to be most strongly dependent on the ratio of other substances in the mixture. For example, the efficiency of butyl acetate removal decreased (from 100% to 60%) as the ratio of the other two pollutants, namely butanol to xylene, in the mixture changed from 1:1 to 3:1.
The efficiency of removal of the three studied VOCs was also studied as a function of their amount (%) in the mixture (). In this case, if the ratio of two VOCs is taken to be 1:1, then the dependency of the efficiency of removal of the third substance on the amount (%) of these two other substances in the mixture can be found. The following dependence was, respectively, derived for butyl acetate:(11)
(11)
For butanol:(12)
(12)
For xylene:(13)
(13)
Of all the substances included in this study (butyl acetate, butanol and xylene), the removal efficiencies of butyl acetate and xylene were shown to be most strongly dependent on the amount (%) of other components in the mixture (). However, due to the high water solubility of butanol, its removal efficiency showed weak dependence (up to 10%) on the amount of other components in the mixture, .
Thus, with the parameters of the biofilter (height of packing material, air flow velocity) and pollutants to be removed (their initial concentration, biodegradation, ratio, amount of individual pollutants in the mixture, etc.) known, the mathematical expression of the filter efficiency derived here would allow to make theoretical calculations and selection of the most appropriate parameters of the device in order to achieve maximum efficiency of air cleaning.
Conclusions
The results from the modelling of the removal efficiency of individual pollutants (butyl acetate, butanol and xylene) showed that the efficiency of xylene removal from the air was most strongly dependent on the amount and ratio of other substances (from 20% to 80%; ). The efficiency of butyl acetate removal, however, was found to be mostly dependent on the ratio between the other two substances in the mixture (from 15% to 100%;
). The observed differences in the extent of dependence of the removal efficiency could be attributed to different solubility and biodegradability of the studied organic pollutants. Thus, based on the derived mathematical expression of the filter efficiency, the optimal parameters of the device can be determined and adjusted in advance so as to achieve maximum efficiency of air cleaning.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Paca J , Weigner P . Biofilter characteristics during degradation of xylene and toulene mixture. In: Reynolds FE , editor. Proceedings of USC-TRG conference on biofiltration; 1998 Oct 22–23; University of Southern California, Los Angeles. Scotts Valley (CA) : Reynolds Group; 1998. p. 249–256.
- Hodge DS . Determination of mathematical model constants using specially designed mini-column biofilters. In: Reynolds FE , editor. Proceedings of USC-TRG conference on biofiltration; 1998 Oct 22–23; University of Southern California, Los Angeles. Scotts Valley (CA) : Reynolds Group; 1998. p. 53–70.
- Baltzis BC , Wojdyla SM . Towards a better understanding of biofiltration of VOC mixtures. In: Reynolds FE , editor. Proceedings of USC-TRG conference on biofiltration; 1998 Oct 22–23; University of Southern California, Los Angeles. Scotts Valley (CA) : Reynolds Group; 1998. p. 131–138.
- Mirpuri R , Sharp WA . Predictive model for toulene degradation in a flat plate vapor phase reactor. In: Reynolds FE , editor. Proceedings of USC-TRG conference on biofiltration; 1998 Oct 22–23; University of Southern California, Los Angeles. Scotts Valley (CA) : Reynolds Group; 1998. p. 71–84.
- Plessis CA , Straus JM , Sebapalo EMT , et al . Empirical model for methane oxidation using a composted pine bark biofilter. Fuel. 2003;82(11):1359–1365.
- Li C , Moe WM . Sequencing batch biofilter operation for treatment of methyl ethyl ketone (MEK) contaminated air. Environ Technol. 2003;24(5):531–544.
- Delhomenie MC , Heitz M . Elimination of chlorobenzene vapors from air in a compost-based biofilter. J Chem Technol Biotechnol. 2003;78(5):588–595.
- Baltrenas P , Spakauskas V , Vaiskunaite R . Experimental study and mathematical modelling of biofiltration of volatile organic compound mixtures using biofilters. J Environ Lands Manag. 2003;9(3):87–92.
- Vaiskunaite R , Baltrenas P , Spakauskas V . Mathematical modelling of biofiltration in activated pine-bark charge of a biofilter. Environ Sci Pollut Res Int. 2005;12(5):297–301.
- Baltrenas P , Vaiskunaite R , Spakauskas V . Experimental study and mathematical modelling of biofilter aerodynamic resistance. J Environ Lands Manag. 2005;12(3):79–84.
- Zagorskis A , Vaiskunaite R . An investigation on the efficiency of air purification using a biofilter with activated bed of different origin. Chem Petrol Eng. 2005;35(4):435–445.
- Baltrenas P , Vaiskunaite R . A biofilter containing a biologically active layer of pine bark for removing volatile hydrocarbons from air. Chem Petrol Eng. 2005;40(7–8):417–420.
- Nausėdienė A , Vaiskunaite R. Formaldehyde removal from the air by biofilter packed with reed charge. In: Čygas D , Tollazzi T , editors. Proceedings of 9th International conference, environmental engineering; 2014 May 22–23; Vilnius Gediminas Technical University, Vilnius. Vilnius ( Lithuania ): Technika; 2014. p. 1–6.
- Vaiskunaite R . Analysis and assessment of biofilter packed with different wood waste charges for toluene removal. In: Čygas D , Tollazzi T , editors. Proceedings of 9th International conference, environmental engineering; 2014 May 22–23; Vilnius Gediminas Technical University, Vilnius. Vilnius ( Lithuania ): Technika; 2014. p. 1–7.
- Vaiskunaite R . Mathematical modelling of biofilter effectiveness in pH regimes. In: Čygas D , Froehner KD , editors. Proceedings of 8th International conference, environmental engineering; 2011 May 19–20; Vilnius Gediminas Technical University, Vilnius. Vilnius ( Lithuania ): Technika; 2011. p. 408–414.
- Vaiskunaite R , Navickaitė R. Evaluation of the performance with biofilter effectiveness treating volatile organic compounds under different pH value. In: Čygas D , Froehner KD , editors. Proceedings of 8th International conference, environmental engineering; 2011 May 19–20; Vilnius Gediminas Technical University, Vilnius. Vilnius ( Lithuania ): Technika; 2011. p. 416–424.
- Vaiskunaite R , Miškinytė D. Temperature effects on biofiltration by varying biofilters parameters. In: Čygas D , Froehner KD , editors. Proceedings of 7th International conference, environmental engineering; 2008 May 22–23; Vilnius Gediminas Technical University, Vilnius. Vilnius ( Lithuania ): Technika; 2008. p. 423–432.
- Vaiskunaite R . Mathematical modelling of biofilter's temperature regimes. In: Čygas D , Froehner KD , editors. Proceedings of 7th International conference, environmental engineering; 2008 May 22–23; Vilnius Gediminas Technical University, Vilnius. Vilnius ( Lithuania ): Technika; 2008. p. 433–441.
- Baltrėnas P , Vaiškūnaitė R , Zagorskis A. Experimental studies of air flow rate and aerodynamic resistance of biological air purification device that contains activated fir bark charge. In: Čygas D , Froehner KD , editors. Proceedings of 6th International conference, environmental engineering; 2005 May 26–27; Vilnius Gediminas Technical University, Vilnius. Vilnius ( Lithuania ): Technika; 2005. p. 25–29.
- Zigmontienė A , Vaiškūnaitė R. Application of the biological air purification technologies. In: Čygas D , Froehner KD , editors. Proceedings of 6th International conference, environmental engineering; 2005 May 26–27; Vilnius Gediminas Technical University, Vilnius. Vilnius ( Lithuania ): Technika; 2005. p. 311–315.
- Baltrėnas P , Zagorskis A , Misevičius A . Research into acetone removal from air by biofiltration using a biofilter with straight structure plates. Biotechnol Biotechnol Equip. 2015;29(2):404–413.
- San-Valero P , Penya-roja JM , Alvarez-Hornos Javier F , et al. Dynamic mathematical modelling of the removal of hydrophilic VOCs by biotrickling filters. Int J Environ Res Public Health. 2015;12(1):746–766.
- Ikemoto S , Jennings AA , Skubal KL . Modelling hydrophobic VOC biofilter treatment in the nutrient stimulation and hydrophobic VOC inhibition. Environ Modell Softw. 2006;21(10):1387–1401.
- Lu C , Chang K , Hsu S . A model for the biofiltration of butyl acetate and xylene mixtures by a trickle-bed air biofilter. Eng Life Sci. 2005;5(1):46–53.
