ABSTRACT
Leucine-rich repeat receptor-like kinase protein (LRR-RLK) is involved in a wide range of biological pathways. So far, the function of LRR-RLK in the growth, developmental processes and various external stimuli has still not been clearly elucidated in rice (Oryza sativa L.). To understand the mechanism(s) underlying stress response and to discover novel stress-tolerance genes in rice, we analysed a global genome expression profiling of the indica cultivar Pei'ai 64S subjected to cold, drought or heat stresses. Expression profiles were obtained for leaf and panicle tissues at seedling, booting and heading stages from plants under no stress, or cold, drought or heat stress, using the GeneChip Rice Genome Array (Affymetrix) representing 51279 transcripts from japonica and indica rice. We identified a gene, OsLRR2 (Oryza sativa L. leucine-rich repeat receptor-like kinase 2, GenBank accession: EAZ02952.1), which was highly up-regulated under cold and drought stress. In order to study its function in stress tolerance, we cloned the cDNA of the gene through amplification by reverse-transcription polymerase chain reaction. Sequence analysis showed that the cDNA encodes a protein of 375 amino-acid residues with molecular weight of ≈40.62 kD and pI of ≈5.75. The sequence databases search found that the open reading frame of OsLRR2 contained a leucine-rich repeats domain. Analysis of the putative promoter region for candidate cis-regulatory elements identified five matches to cis-elements related to stress responses, suggesting that OsLRR2 could be considered a new candidate gene involved in stress tolerance in rice.
Introduction
Rice (Oryza sativa L.) is an essential crop cultivated in arable land worldwide and serves as a source of carbohydrates for more than one-third of the world's population [Citation1]. A lot of rice varieties are severely damaged by various abiotic stresses, with strong economic and social impact [Citation2]. Abiotic stresses, such as cold, heat, drought and high salinity adversely affect the plants' growth and yield potential [Citation3]. Plants have developed the ability to respond to environmental stresses in order to survive in the stressful conditions. That is why the molecular and cellular processes underlying the acclimation of higher plants to environmental stresses have attracted much attention [Citation4,Citation5]. A large number of genes induced by environmental stresses have been identified recently by using molecular and genetic approaches, e.g. by microarray technology [Citation6,Citation7]. The products of these genes are believed to promote the stress tolerance potential [Citation8,Citation9]. It has been shown that changing the expression of a single gene can enhance the resistance to drought, freezing and salinity [Citation8–10]. Therefore, better understanding of the molecular signal-transduction pathways involved in activating the adaptive responses is significant for ensuring stable and high rice yield.
The leucine-rich repeat receptor-like kinase proteins (LRR-RLKs) are a large superfamily of proteins in animals, yeast, bacteria and plants [Citation11]. LRR-RLKs are composed of extracellular domains and cytoplasmic domains, and so they often occur as a conjugation of LRR domains as well as RLK domains. The LRRs consist of 20–29 amino-acid residue sequence motifs and one of their functions is regarded as protein identification [Citation12]. A lot of protein sequences containing LRR have been identified by automatic annotation methods [Citation13]. The LRR proteins superfamily can be subdivided into at least six subfamilies based on different lengths and hallmark sequences of the repeats [Citation11]. In plants as in other eukaryotes, LRR proteins carry out diverse functions in a wide range of developmental processes and signal-transduction as well as defence-related pathways [Citation14,Citation15].
The RLK domain was first found in maize [Citation16] and has then been characterized in rice [Citation17] and Arabidopsis [Citation18–20]. In plants, RLKs play a key role in many abiotic stress and physiological processes such as regulating gene expression responses and sensing external signals at the cellular environment level [Citation21,Citation22]. However, a large part of the biological functions of LRR-RLK genes in plants, especially in rice, have not been clearly elucidated.
In this study, GeneChip Rice Genome Array was used to screen a stress tolerance candidate gene, OsLRR2, from cultivar Pei'ai 64S, which is the maternal parent of the super-hybrid rice Liang-You-Pei-Jiu (LYP9). The expression of the OsLRR2 gene was studied under cold, drought and heat stress at different developmental stages.
Materials and methods
Stress treatments and sample preparation
Stress treatments and sample preparation were based on protocols previously described by Dong et al. [Citation23], Jiang et al. [Citation24] and Cao et al. [Citation25]. The germination seeds of cultivated rice Pei'ai 64S (O. sativa) were obtained from the Institute of Subtropical Agriculture (The Chinese Academy of Sciences, Changsha, Hunan, China.). The seeds were suspended in a sterile solution of 0.1% HgCl2 for 10 min and were then washed three times with running water. Next, they were immersed in tap water for three days at 25 °C, with the water being changed once a day. Then they were germinated and grown in distilled water at 37 °C for two to three days under a physiological photoperiod of 12 h light and 12 h darkness. The plants were divided into one control and three treatment groups (10 plants in each group). All the rice seedlings were incubated in a PGC-15.5 plant growth chamber (Percival Scientific, Perry, IA, USA), while the control group was kept in another chamber at 28 °C. The test groups and the control group were all kept in darkness. For the drought tests, the water was poured away from the basin and the treatment groups were put in a scaffold to dry out; meanwhile the water level of the control group was kept the same. The leaves were harvested when they started curling after 16 h. For the heat tests, the treatment group was exposed to 45 °C for 2 h, after which the plants were harvested. For the cold stress tests, the treatment group was harvested after incubation at 4 °C for 12 h at the seedling stage and at 12 °C for 16 h at the booting and flowering stages.
Microarray data analysis
The procedure was done according to protocols previously described by Dong et al. [Citation23]. Briefly, microarray analysis was performed according to the GeneChip® Expression Analysis Technical Manual (2005 version) provided by Affymetrix (Affymetrix, Inc., Santa Clara, CA, USA). The main steps were as follows: (1) Extraction and purification of total RNA, (2) cDNA synthesis and purification, (3) synthesis and segmentation of cRNA, (4) array hybridization and washing, (5) scan chips and (6) data analysis. Expression profiles were obtained for leaf and panicle tissues at seedling, booting and heading stages from plants under no stress, or cold, drought or heat stress, respectively, using the GeneChip Rice Genome Array (Affymetrix) representing 51,279 transcripts.
cDNA cloning
Special primers were designed by using the Primer Premier 5.0 software after searching for homologous cDNA sequences. The primers were OsLRR2-F: 5′-ACGATGCTTGATTGAGTGATTGAC-3′, and OsLRR2-R: 5′-ACCGCGAGACACATGACGA-3'. The full-length of the OsLRR2 cDNA was amplified to use high fidelity (HiFi) Taq DNA polymerase (Transgen, Beijing, China). The polymerase chain reaction (PCR) cycler (Bio-Rad C1000 PCR, Bio-Rad, Irvine, CA, USA) was programmed as follows: an initial denaturation step for 5 min at 94 °C, 30 amplification cycles (30 s at 94 °C (denaturation), 30 s at 62 °C (annealing) and 30 s at 72 °C (polymerization)), followed by a final elongation step for 10 min at 72 °C. All the PCR products were purified by using a Gel Extraction Mini Kit (Biomed, Shanghai, China) and the amplified product was ligated into a pMD18-T vector (TaKaRa, Dalian, China), then transformed into Escherichia coli strain Top 10 (Transgen). The positive transformants were screened by using ampicillin selection; restriction enzymes, HindIII and BamHI (TaKaRa), were used for double cuts for confirmation. Restricted fragments were analysed in a 1.0% agarose gel. The positively screened clone was sequenced by TSINGKE (Chengdu, China).
Sequence analysis
The genomic sequence and chromosome location of OsLRR2 were determined by comparing the cloned cDNA with the genomic DNA sequences in Gramene (http://www.gramene.org/) and GenBank (https://www.ncbi.nlm.nih.gov/genbank/). Promoter analysis of 1500 bp upstream of the OsLRR2 gene was performed with PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/). The analysis and comparison of the deduced amino-acid sequence with published sequences were performed with NetPhos 3.1 Server (http://www.cbs.dtu.dk/services/NetPhos/) and BLASTp (Standard Protein-Protein Basic Local Alignment Search Tool) on the NCBI (National Center for Biotechnology Information) server (http://www.ncbi.nlm.nih.gov/). Conserved domains in OsLRR2 were identified by the SMART (http://www.learnmarketing.net/smart.htm) and InterProScan online protein structure prediction software (http://www.ebi.ac.uk/InterProScan/). The OsLRR2 gene was aligned with other protein-encoding genes from different species by using DNAMAN (http://www.lynnon.com/). The phylogenetic relationship with other protein-encoding genes from different species was constructed by using Mega 4.1 [Citation26].
Results and discussion
Microarray analysis OsLRR2 in plants subjected to different abiotic stress
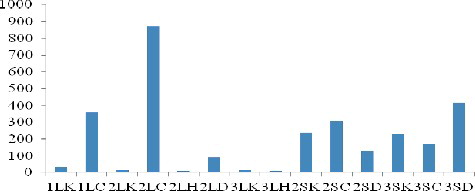
A large number of genes up-regulated or down-regulated under stress were identified. One of these genes, OsLRR2, coding for a LRR-RLK, showed significant changes at the expression level at different developmental phases subjected to different abiotic stress. The results showed that there occurred changes in the expression level of OsLRR2 in different tissues of rice at different stages under cold stresses. The minimum expression level was almost three-fourths of the initial level, whereas the maximum increase was 66.17-fold and the average increase, 19.7-fold. Under drought conditions, the expression level of OsLRR2 was up-regulated 3.1-fold on average; the minimum and maximum expression levels were almost one-half and 6.91-fold of the initial level, respectively ().
Sequence and structural analyses of gene OsLRR2
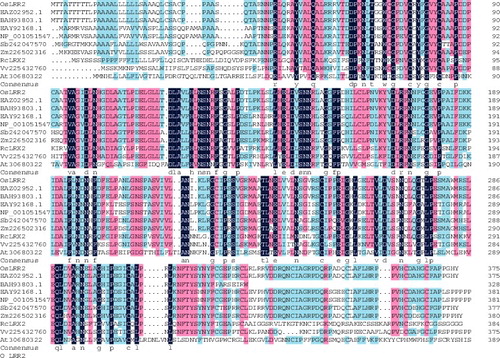
Based on the conserved region of the LRR gene sequence of rice in Gramene and GenBank, two specific primers (OsLRR2-F, OsLRR2-R) were designed and synthesized for the amplification of OsLRR2. After cloning the full-length cDNA sequence of OsLRR2 from rice Pei'ai 64S through reverse-transcription PCR, the sequence analysis showed that the cloned cDNA contained an 1128-bp open reading frame encoding a putative protein of 375 amino acids that has a calculated molecular weight of 40.62 kDa with a pI of 5.75. A signal peptide sequence was predicted to be localized at the N-terminus and four LRR motifs were found in the putative protein (). To understand the organization of the regulatory region of the OsLRR2 gene, several putative cis-elements related to stress responses were identified (PlantCARE) as the putative promoter region of OsLRR2 about 1.5 kb upstream of the transcriptional start site (). The presence of putative cis-acting elements indicated that the OsLRR2 gene might be regulated by the interaction between the cis-acting elements in the promoter and the corresponding trans-acting factors. There were six cis-elements related to stress responses in the predicted promoter region: ABRE (ACGT-containing abscisic acid (ABA) response element), CE3 (coupling element 3), MBS (myeloblastosis (MYB) binding site involved in drought-inducibility), CGTCA-motif (cis-acting regulatory element involved in the methyl–jasmonate–responsiveness), TC-rich repeats (cis-acting element involved in defence and stress responsiveness) and TCA-element (cis-acting element involved in salicylic acid responsiveness). The existence of these stress-related cis-elements provided evidence that the promoter region of OsLRR2 responds to various kinds of stress signals and the expression of OsLRR2 is regulated by several stress factors.
Phylogenetic tree analysis of OsLRR2
Based on the predicted protein sequence of OsLRR2 and analysis with BLASTp, the full-length protein sequence of OsLRR2 was found to be in consensus with a hypothetical protein (EAZ02952.1) encoded by OsI_25092 from Guang Lu Ai 4. The OsLRR2 protein sequence was shown to share highest similarity (85.33%) with a putative protein (BAH93803) encoded by Os07g0176400 from Nipponbare, and 73% similarity with a putative protein (NP_001051547.1) encoded by Os03g0795300 from Nipponbare, as well as a putative protein (EAY92168.1) encoded by OsI_13881 from Guang Lu Ai 4. BLASTp analysis showed that OsLRR2 also shares high similarity with genes from other plant species (). The percentages of similarity were from 57% to 79%, e.g. 57% (putative protein NP_849339.1 encoded by At. 30680322 from Arabidopsis), 58% (XP_002279192.1 encoded by Vv.225432760 from Vitis vinifera), 60% (RcLRX2), 78% (XP_002461531.1 encoded by Sb.242047570) and 79% (NP_001147644.1 encoded by Zm.226502316).
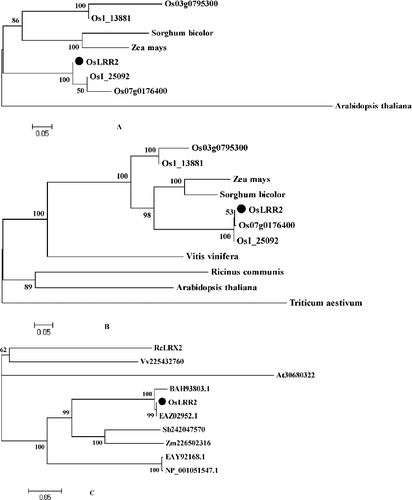
To generate a phylogenetic tree (), the full-length amino acid sequences, cDNA and gDNA of OsLRR2, several putative rice LRR gene family members and the corresponding genes in other plant species were used. The obtained dendrogram indicates that OsLRR2 has the highest homology with OsI_25092 (Guang Lu Ai 4, EAZ02952.1), Zea mays and Arabidopsis ((A)). Moreover, OsLRR2 was also revealed to have high homology with Z. mays, Sorghum bicolor and Arabidopsis ((B)). In addition, OsLRR2 also showed high homology with BAH93803 (Nipponbare), EAZ02952.1 (Guang Lu Ai 4), Sb242047570 (S. bicolor) and Zm226502316 (Z. mays) ((C)). The phylogenetic tree showed that OsLRR2 has very high homology with Nipponbare, Guang Lu Ai 4, Z. mays, S. bicolor and Arabidopsis.
Figure 5. Phylogenetic tree of OsLRR2, its deduced protein and other similar proteins. Phylogenetic tree of genomic DNA (A), cDNA (B) and protein (C).
Signalling proteins have a role in stress resistance when plants or crops are subjected to adverse environmental stresses. The results from the GeneChip Rice Genome Array analysis showed that OsLRR2 was highly expressed in different growth periods and different tissues and organs shortly after exposure to cold treatments and drought treatments as part of the plant response to these stress factors. We found some cis-elements related to plant stress responses by analysing the promoter region. Among these cis-elements, CE3 is involved in drought responses, ABRE has been reported to respond to cold stress, the CGTCA-motif, TC-rich repeats and TCA-element have also been associated with stress responses according to previous reports [Citation27–29]. The existence of these stress-related cis-elements indicated that OsLRR2 might be involved in the adaptation process of tolerating abiotic stresses in rice. By analysing the putative protein sequence encoded by OsLRR2, we found that the protein encoded by OsLRR2 is LRR-RLKs albuminoid. All the proteins, which have high similarities with it, contain the domain of LRR-RLKs. They belong to the LRR-RLKs protein family or proteins analogous to LRR-RLKs. SMART analysis showed that the protein encoded by OsLRR2 had only one signal peptide and four LRR sequences, with no transmembrane domain or intracellular kinase domain, which was in 60% consistency with the protein encoded by RcLRX2. Moreover, similarities were also identified with the LRX-like proteins in Arabidopsis and rice. The phylogenetic tree () also showed that they have close relationship. These results indicated that LRR-RLKs might belong to the LRX subfamily; however, their C-terminus does not have a C-terminal extension [Citation30,Citation31]. LRR motifs are involved in protein–protein interactions and particularly play an important role in molecular recognition processes such as signal transduction, cell adhesion, cell growth, DNA repair and RNA processing [Citation32]. Some reports have shown that the expression of OsRLK1 (rice LRR-RLK 1) is induced by cold and salt stress [Citation33]; pepper CALRR2 could be induced not only by the anthrax pathogen, but also by other abiotic stresses such as salt, ABA and so on [Citation34]; tobacco NtLRR2 and NtLRR2 are also related to resistance to a variety of abiotic stresses [Citation35]. The NetPhos 3.1 Server analysis showed that LRR-RLKs may contain 11 phosphorylation sites, which indicated that the protein encoded by OsLRR2 may be modified by phosphorylation after transcription.
Regarding the mechanism of action of LRR-RLKs, there is a generally accepted view that ligands bind to the extracellular domain of LRR-RLKs and then activate signal-transduction pathways by phosphorylation or dephosphorylation of receptors, eventually leading plants to correctly respond to the external stimulus [Citation36]. Although the mechanism of action of the LRR-RLK encoded by OsLRR2 is still unclear, we could speculate that it could possibly be involved in signal-transduction pathways to regulate plant growth and development and induce defence reactions based on the biochemical functions of LRR-RLKs.
Conclusions
This study identified and analysed a new abiotic stress regulated gene, OsLRR2, from cultivar Pei'ai 64S, which is the maternal parent of the super-hybrid rice Liang-You-Pei-Jiu (LYP9). The expression of OsLRR2 in the leaves at the seeding, booting and flowering stage were markedly up-regulated after cold and drought treatment. Six cis-elements related to stress responses were found in the predicted promoter region, which provided further evidence that OsLRR2 is most likely related to stress tolerance.
Acknowledgment
We thank Wei Xu and Xiaomei Jia for offering help for revising this manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Jain M, Nijhawan A, Arora R, et al. F-box proteins in rice. Genome-wide analysis, classification, temporal and spatial gene expression during panicle and seed development, and regulation by light and abiotic stress. Plant Physiol. 2007;143(4):1467–1483.
- Almeida DM, Almadanim MC, Lourenço T, et al. Screening for abiotic stress tolerance in rice: salt, cold, and drought. Methods Mol Biol. 2016;1398:155–182.
- Singh A, Jha SK, Bagri J, et al. ABA inducible rice protein phosphatase 2C confers ABA insensitivity and abiotic stress tolerance in Arabidopsis. PloS One [Internet]. 2015 [cited 2016 Apr 13];10(4):e0125168. Available from: http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0125168
- Cushman JC, Bohnert HJ. Genomic approaches to plant stress tolerance. Curr Opin Plant Biol. 2000;3(2):117–124.
- Mittler R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006;11(1):15–19.
- Kreps JA, Wu Y, Chang HS, et al. Transcriptome changes for Arabidopsis in response to salt, osmotic, and cold stress. Plant Physiol. 2002;130(4):2129–2141.
- Seki M, Narusaka M, Ishida J, et al. Monitoring the expression profiles of 7000 Arabidopsis genes under drought, cold and high-salinity stresses using a full-length cDNA microarray. Plant J. 2002;31(3):279–292.
- Xiong L, Schumaker KS, Zhu JK. Cell signaling during cold, drought, and salt stress. Plant Cell. 2002;14(Suppl 1):S165–S183.
- Shinozaki K, Yamaguchi-Shinozaki K, Seki M. Regulatory network of gene expression in the drought and cold stress responses. Curr Opin Plant Biol. 2003;6(5):410–417.
- Zhu JK. Cell signaling under salt, water and cold stresses. Curr Opin Plant Biol. 2001;4(5):401–406.
- Park SJ, Moon JC, Park YC, et al. Molecular dissection of the response of a rice leucine-rich repeat receptor-like kinase (LRR-RLK) gene to abiotic stresses. J Plant Physiol. 2014;171(17):1645–1653.
- Hwang SG, Kim DS, Jang CS. Comparative analysis of evolutionary dynamics of genes encoding leucine-rich repeat receptor-like kinase between rice and Arabidopsis. Genetica. 2011;139(8):1023–1032.
- Bella J, Hindle KL, McEwan PA, et al. The leucine-rich repeat structure. Cell Mol Life Sci. 2008;65(15):2307–2333.
- Vernon DM, Forsthoefel NR. Leucine-rich repeat proteins in plants: diverse roles in signaling and development. In: Pandali SG, editor. Recent research developments in plant biology, Vol. 2. Trivandrum: Research Signpost; 2002. p. 202–214.
- Forsthoefel NR, Cutler K, Port MD, et al. PIRLs: a novel class of plant intracellular leucine-rich repeat proteins. Plant Cell Physiol. 2005;46(6):913–922.
- Walker JC, Zhang R. Relationship of a putative receptor protein kinase from maize to the S-locus glycoproteins of Brassica. Nature. 1990;345:743–746.
- Becraft PW. Receptor kinases in plant development. Trends Plant Sci. 1998;3(10):384–388.
- Chang C, Schaller GE, Patterson SE, et al. The TMK1 gene from Arabidopsis codes for a protein with structural and biochemical characteristics of a receptor protein kinase. Plant Cell. 1992;4(10):1263–1271.
- Kohorn BD, Lane S, Smith TA. An Arabidopsis serine/threonine kinase homologue with an epidermal growth factor repeat selected in yeast for its specificity for a thylakoid membrane protein. Proc Natl Acad Sci. 1992;89(22):10989–10992.
- Walker JC. Receptor-like protein kinase genes of Arabidopsis thaliana. Plant J. 1993;3(3):451–456.
- Stone JM, Walker JC. Plant protein kinase families and signal transduction. Plant Physiol. 1995;108(2):451–457.
- Lease K, Ingham E, Walker JC. Challenges in understanding RLK function. Curr Opin Plant Biol. 1998;1(5):388–392.
- Dong JL, Jiang YY, Chen RJ, et al. Isolation of a novel xyloglucan endotransglucosylase (OsXET9) gene from rice and analysis of the response of this gene to abiotic stresses. Afr J Biotechnol. 2013;10(76):17424–17434.
- Jiang YY, Chen RJ, Dong JL, et al. Analysis of GDSL lipase (GLIP) family genes in rice (Oryza sativa). Plant Omics. 2012;5(4):351–358.
- Cao XF, Liao YR, Rong SH, et al. Identification and characterization of a novel abiotic stress responsive sulphotransferase gene (OsSOT9) from rice. Biotechnol Biotechnol Equip. 2016;30(2):227–235.
- Liu S, Cao X, Liao Y, et al. Identification and characterization of a novel abiotic stress responsive ATPase gene from rice. Plant Omics. 2015;8(2):169–177.
- Merlot S, Gosti F, Guerrier D, et al. The ABI1 and ABI2 protein phosphatases 2C act in a negative feedback regulatory loop of the abscisic acid signalling pathway. Plant J. 2001;25(3):295–303.
- Rowland O, Ludwig AA, Merrick CJ, et al. Functional analysis of Avr9/Cf-9 rapidly elicited genes identifies a protein kinase, ACIK1, that is essential for full Cf-9–dependent disease resistance in tomato. Plant Cell. 2005;17(1):295–310.
- Diévart A, Clark SE. LRR-containing receptors regulating plant development and defense. Development. 2004;131(2):251–261.
- Baumberger N, Steiner M, Ryser U, et al. Synergistic interaction of the two paralogous Arabidopsis genes LRX1 and LRX2 in cell wall formation during root hair development. Plant J. 2003;35(1):71–81.
- Baumberger N, Doesseger B, Guyot R, et al. Whole-genome comparison of leucine-rich repeat extensions in Arabidopsis and rice. A conserved family of cell wall proteins form a vegetative and a reproductive clade. Plant physiol. 2003;131(3):1313–1326.
- Kobe B, Deisenhofer J. A structural basis of the interactions between leucine-rich repeats and protein ligands. Nature. 1995;374:183–186.
- Lee SC, Kim JY, Kim SH, et al. Trapping and characterization of cold-responsive genes from T-DNA tagging lines in rice. Plant Sci. 2004;166(1):69–79.
- Jung EH, Jung HW, Lee SC, et al. Identification of a novel pathogen-induced gene encoding a leucine-rich repeat protein expressed in phloem cells of Capsicum annuum. Biochim Biophys Acta. 2004;1676(3):211–222.
- Xu ZS, Xiong TF, Ni ZY, et al. Isolation and identification of two genes encoding leucine-rich repeat (LRR) proteins differentially responsive to pathogen attack and salt stress in tobacco. Plant Sci. 2009;176(1):38–45.
- Tilman D, Cassman KG, Matson PA, et al. Agricultural sustainability and intensive production practices. Nature. 2002;418(6898):671–677.