ABSTRACT
Previous reports suggest that rotensin II (UII) and its G protein-coupled receptor (GPR14) involve in inflammatory mediation, while the effects of UII/GPR14 in liver inflammatory response are not fully illustrated. Thus, in this study, urantide, a special antagonist of GPR14, was used to investigate the effects of UII/GPR14 on carbon tetrachloride (CCl4) induced liver injury in mice. The results showed that CCl4 upregulated liver UII and GPR14 expression. Meanwhile, CCl4 caused liver injury, inflammation and oxidative stress. Injection of urantide alleviated CCl4-induced inflammatory response and oxidative stress, which might further exhibit a hepatoprotective effect. In conclusion, UII/GPR14 may serve as a potential target for therapeutic intervention in liver injury.
KEYWORDS:
Introduction
Urotensin II (UII) is an autocrine/paracrine growth, which is widely expressed in various tissues, such as liver, heart, brain and intestine [Citation1,Citation2]. UII exerts many biological functions under both physiological and pathological conditions via binding to a class of G protein-coupled receptor known as GPR14. UII associated with GPR14 activation increases inositol phosphate turnover and intracellular Ca2+, which further produces vasoconstriction, dilation and ionotropic effects [Citation3]. UII/GPR14 also involves in cell proliferation (i.e. vascular smooth muscle cells, fibroblasts and cancer cells) and foam cell formation (i.e. chemotaxis of inflammatory cells) [Citation3]. Thus, elevated UII concentrations and GPR14 upregulation have been widely observed in numerous un-homeostasis conditions, including hypertension, atherosclerosis, metabolic syndrome, heart failure, renal failure, liver disease and diabetes [Citation3]. Therefore, UII/GPR14 may serve as a potential target for therapeutic intervention in these diseases. For example, urantide, a special antagonist of GPR14, has been demonstrated to exhibit a protective effect on liver inflammatory injury [Citation4], ischemia-reperfusion injury [Citation5], atherosclerosis [Citation6] and pulmonary arterial hypertension [Citation7].
Recently, UII/GPR14 has been identified to mediate pro-inflammatory responses in various inflammation models, including lipopolysaccharide stimulation in mice and Kupffer cells [Citation4,Citation8]. Meanwhile, inhibition of UII/GPR14 has been suggested to relieve inflammatory response via preventing activation of NF-κB pathway [Citation8], which is known to be a major pro-inflammatory transcription factor controlling a wide number of genes, including cytokines [Citation9]. However, UII/GPR14 signal in liver injury and the potential therapeutic function in liver disease were still obscure. Thus, in this study, we investigated the UII/GPR14 signal expression and the effects of inhibition UII/GPR14 via urantide treatment in CCl4-induced liver injury in mice.
Material and methods
Animal model and groups
Thirty female Kunming mice (22.34 ± 1.67 g) were randomly assigned into three groups: a control group (CG, n = 10), a CCl4 group (CC, n = 10) in which mice received intraperitoneal injection of 0.2 mL of CCl4 in olive oil (1:1, v/v) per kg bodyweight twice weekly for up to one week to induce liver inflammation according to previous report [Citation10], and a urantide group (CU, n = 10) in which mice received CCl4 and intravenous injection of 0.6 mg/kg urantide (Peptides, Louisville, KY, USA) dissolved in saline. The control and untreated challenged animals received the same volume of olive oil and saline alone. The dosage of urantide used in this study was according to previous report [Citation11]. All mice were housed in polycarbonate cages in a room with controlled temperature (25 ± 3 °C), humidity (50% ± 5%) and a 12-h cycle of light and dark. They were allowed free access to laboratory strip chows throughout the experimental period.
After the experimental period, each animal was weighed to calculate average weight gain and then each mouse was sacrificed. Liver tissues from each mouse were harvested and immediately frozen in liquid nitrogen and stored at −70 °C for subsequent gene expression and western blotting analyses. This study was conducted according to the guidelines of the Declaration of Helsinki and all procedures involving animal subjects were approved by the animal welfare committee of Qingdao Hospital for Infectious Diseases.
Histomorphometry determination
The morphological evaluation after CCl4 treatment was conducted via haematoxylin and eosin (HE) staining according to previous report [Citation12]. Briefly, one piece of each liver samples (0.5 cm) was kept in 4% neutral buffered 10% formalin, processed using routine histological methods and mounted in paraffin blocks. Six-micrometer-thick sections were cut and stained with HE. All specimens were examined under a light microscope (Nikon, Tokyo, Japan).
Serum alanine aminotransferase (ALT) andaspartate aminotransferase (AST)
Blood samples were collected by cardiac puncture and serum samples were separated by centrifugation of blood at 3000 rpm and 4 °C (CR22 GII, Guangzhou, China) for 15 min. The activities of ALT and AST in serum were estimated spectrophotometrically (PerkinElmer Lambda 25) using commercial diagnostic kits (Jiancheng Institute of Biotechnology, Nanjing, China).
Liver oxidative stress
Livers were homogenized in Tris–HCl buffer (0.01 mol/L, pH = 7.4) to give 10% homogenates. Homogenates were centrifuged at 3000 rpm and 4 °C for 10 min and supernatants were harvested for determining liver catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) activities and malondialdehyde (MDA) concentration.
Real-time PCR
Total RNA was isolated from liquid nitrogen pulverized tissues with TRIZOL regent (Invitrogen, Waltham, MA, USA) and then treated with DNase I (Invitrogen) according to the manufacturer's instructions. Synthesis of the first strand (cDNA) was performed with oligo (dT) 20 and Superscript II reverse transcriptase (Invitrogen). Primers were designed with Primer 5.0 according to the gene sequence of mice to produce an amplification product. The primer sets used are as follows: β-Actin, F:GTCCACCTTCCAGCAGATGT, R:GAAAGGGTGTAAAACGCAGC; IL-1β, F:CTGTGACTCGTGGGATGATG R:GGGATTTTGTCGTTGCTTGT; IL-10, F: ACAGCCGGGAAGACAATAAC, R: CAGCTGGTCCTTTGTTTGAAAG; IL-17, F:TACCTCAACCGTTCCACGTC, R:TTTCCCTCCGCATTGACAC; IFN-γ, F:ATGAACGCTACACACTGCATCTTGGCTT, R:CCTCAAACTTGGCAATACTCATGAATGC; TNF-α, F:AGGCACTCCCCCAAAAGAT, R:TGAGGGTCTGGGCCATAGAA. Real-time PCR was performed according to previous studies [Citation13,Citation14]. Relative expression was normalized and expressed as a ratio to the expression in control group.
Western bolt
Proteins were extracted with protein extraction reagents in accordance with the manufacturer's instructions (Thermo Fisher Scientific Inc., Waltham, MA, USA). Proteins from each sample (20–50 µg) were separated by dodecyl sulfate, sodium salt (SDS)–polyacrylamide gel electrophoresis and electrophoretically transferred to apolyvinylidene difluoride membrane (BioRad, Hercules, CA, USA). Membranes were blocked in 5% evaporated milk, diluted in Tris-buffered saline containing 0.1% Tween 20 (TBS-226 T) at room temperature for at least 2 h, and then incubated overnight at 4 °C with the following primary antibodies: Glutathione peroxidase 1 (Gpx1, ab22604), Superoxide dismutase 1 (SOD1, ab20926), catalase (CAT, ab20926), UII (ab194676) and GPR14 (ab78449) (Abcam, Inc., San Francisco, CA, USA). Mouse β–actin antibody (Sigma) was used for protein loading control. After primary antibody incubation, membranes were washed with Tris Buffered Saline, with Tween-20 (TBS-T) and incubated with alkaline phosphatase-conjugated anti-mouse or anti-rabbit IgG antibodies (Promega, Madison, WI, USA) for 2 h at room temperature. Membranes were washed with TBS-T followed by three washes with TBS; signals were detected by the addition of 5-bromo-4-chloro-3-238 indolylphosphate/nitroblue tetrazolium solution (Sigma), then quantified and digitally analyzed using the image J program. The intensity of each band was measured and subtracted from the background. The expression ratio of target proteins was normalized against β-actin [Citation15]. All chemicals used in this study were of analytical grade.
Statistical analysis
All statistical analyses were performed using SPSS 17.0 software. Group comparisons were performed using the one-way analysis of variance to test homogeneity of variances via Levene's test and followed with Tukey's multiple comparison test. Values in the same row with different superscripts are significant (P < 0.05), while values with same superscripts are not significantly different (P > 0.05).
Results and discussion
UII/GPR14 signal has been identified to mediate pro-inflammatory responses via regulating cytokines expression [Citation4,Citation8,Citation16–Citation18]. In this study, urantide, a special antagonist of GPR14, was used to investigate the effect of GPR14 signal in liver inflammatory injury. The present data suggested that inhibition of GPR14 alleviated CCl4-induced liver injury in mice evidenced by the influenced liver function, inflammatory response and antioxidant ability, indicating a protective role of GPR14 inactivation in liver injury.
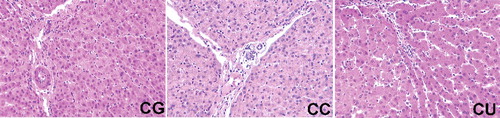
Injected CCl4 has been demonstrated to generate trichloromethyl radical, which can cause severe liver damage with necrotic and apoptotic hepatocellular injury [Citation19]. Blood ALT and AST activities have been widely used to serve as the biomarkers of liver injury in various liver diseases, such as chronic hepatitis C [Citation20], alcoholic liver disease [Citation21] and cirrhosis [Citation22]. In this study, ALT and AST activities in the control group were 52.37 ± 3.53 and 126.17 ± 4.26 U/L, respectively. While CCl4 injection significantly increased serum ALT and AST activities (P < 0.05), indicating a liver injury model. Compared with CC group, urantide treatment markedly alleviated CCl4-induced liver injury evidenced by the decreased ALT activity (P < 0.05) (). Meanwhile, massive cell necrosis and loss of hepatocyte architecture around the blood vessels were observed in CC group and urantide treatment extensively attenuated CCl4-induced hepatic histopathological damage (). Similar to previous reports [Citation23,Citation24], the present results demonstrated that CCl4 injection significantly enhanced serum ALT and AST activities, suggesting a liver injury model. Furthermore, the effect of UII/GPR14 signal has been investigated on CCl4-induced liver injury and the results demonstrated that UII and GPR14 were upregulated in CCl4-induced liver injury. Liang et al. reported that inhibition of UII/GPR14 alleviated acute liver failure in mice [Citation11]. In this study, we also found that inhibition of GPR14 via intravenous injection of urantide alleviated CCl4-induced liver injury.
Table 1. Serum ALT and AST activities of mice (n = 10) U/L. Data are presented as mean ± SEM. The values having different superscript letters were significantly different (P < 0.05).
Figure 1. Effects of urantide injection on histological structure via HE staining (×100). (A) CG group; (B) CC group; and (C) CU: group. Massive cell necrosis and loss of hepatocyte architecture around the blood vessels were observed in CC group and urantide treatment extensively attenuated CCl4-induced hepatic histopathological damage.
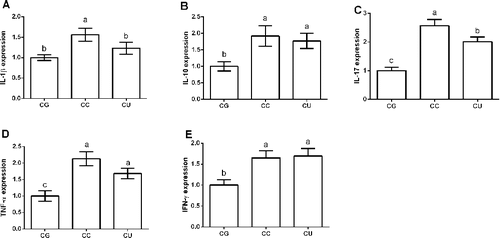
Inflammatory response in the liver plays a vital role in the progress of liver diseases [Citation9,Citation25,Citation26]. In this study, we found marked increases of pro-inflammatory cytokines (i.e. IL-1β, IL-10, IL-17, TNF-α and IFN-γ) expression in CCl4-challenged mice (P < 0.05) (). Inhibition of UII/GPR14 signal using urantide significantly reduced IL-1β and IL-17 expression (P < 0.05), suggesting that the upregulation of these cytokines may be a consequence of UII/UTR (UII receptor) system activation induced by CCl4 exposure. Similarly, urantide has also been demonstrated to prevent the increases of pro-inflammatory cytokines such as IL-1β, TNF-a and IFN-γ in lipopolysaccharide/D-galactosamine-induced liver inflammation [Citation4]. Thus, we speculated that UII/GPR14 signal might be involved in CCl4-induced inflammation and inhibition of UII/GPR14 might exhibit an anti-inflammatory function in vivo and in vitro models.
Figure 2. Effects of urantide injection on liver cytokines expression. (A) IL-1β expression; (B) IL-10 expression; (C) IL-17 expression; (D) TNF-α expression; and (E) IFN-γ expression. Data are presented as mean ± SEM. The values having different superscript letters were significantly different (P < 0.05; n = 6 or 8).
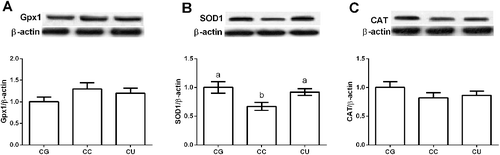
In the CCl4-induced hepatotoxicity, over-production of free radical species and dysfunction of antioxidant ability have been demonstrated to be involved in liver injury [Citation27,Citation28]. In this study, liver oxidative stress was evaluated after CCl4 injection and the results exhibited that CCl4-induced liver injury further caused oxidative stress through enhancing MDA production and inhibiting SOD activity (P < 0.05) (). Although urantide failed to affect liver MDA level (P > 0.05), SOD activity was significantly increased in the CU group compared with CC group (P < 0.05). Furthermore, the western blot results showed that CCl4 injection downregulated liver SOD1 expression (P < 0.05) (), which is similar with its activity in the liver after CCl4 exposure. Meanwhile, urantide markedly enhanced liver SOD1 expression (P < 0.05) compared with the CC group. Increased antioxidant function has been suggested to play a protective role in CCl4-induced liver injury [Citation29].
Table 2. Liver oxidative stress parameters of mice (n = 10). Data are presented as mean ± SEM. The values having different superscript letters were significantly different (P < 0.05; n = 4).
Figure 3. Effects of urantide injection on liver Gpx1 (A), SOD1 (B), and CAT (C) expression. Data are presented as mean ± SEM. The values having different superscript letters were significantly different (P < 0.05; n = 6).
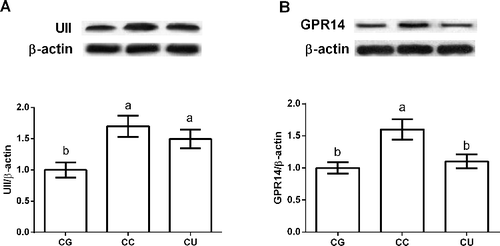
Urantide has been used to inhibit UII/GPR14 signal in this study. Thus, we further investigated UII and GPR14 signal in CCl4-induced liver injury (). The results showed that CCl4 injection activated UII/GPR14 via upregulation of UII and GPR14 expression in the liver (P < 0.05). Urantide, a special antagonist of GPR14, significantly reduced liver GPR14 expression (P < 0.05) compared with CC group.
Conclusion
UII/GPR14 signal involves in CCl4-induced liver injury. Inhibition of UII/GPR14 via urantide injection alleviated CCl4-induced liver injury via regulating inflammation and oxidative stress. Thus, UII/GPR14 may serve as a potential target for therapeutic intervention in liver injury.
Disclosure statement
The authors declared that they have no competing interests.
References
- Ross B, McKendy K, Giaid A. Role of urotensin II in health and disease. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1156–1172.
- Ong KL, Lam KS, Cheung BM. Urotensin II: its function in health and its role in disease. Cardiovasc Drugs Ther. 2005;19:65–75.
- McDonald J, Batuwangala M, Lambert DG. Role of urotensin II and its receptor in health and disease. J Anesth. 2007;21:378–389.
- Liang DY, Liu LM, Ye CG, et al. Inhibition of UII/UTR system relieves acute inflammation of liver through preventing activation of NF-kappa B pathway in ALF mice. Plos One. 2013;8.
- Zhang JY, Chen ZW, Yao H. Protective effect of urantide against ischemia-reperfusion injury via protein kinase C and phosphtidylinositol 3 '-kinase - Akt pathway. Can J Physiol Pharm. 2012;90:637–645.
- Zhao J, Yu QX, Kong W, et al. The urotensin II receptor antagonist, urantide, protects against atherosclerosis in rats. Exp Ther Med. 2013;5:1765–1769.
- Mei YF, Jin H, Tian W, et al. Urantide alleviates monocrotaline induced pulmonary arterial hypertension in Wistar rats. Pulm Pharmacol Ther. 2011;24:386–393.
- Liu LM, Liang DY, Ye CG, et al. The UII/UT system mediates upregulation of proinflammatory cytokines through p38 MAPK and NF-kappa B pathways in LPS-stimulated Kupffer cells. Plos One. 2015;e0121383.
- Hirai F, Matsui T. Status of food intake and elemental nutrition in patients with Crohn's disease. Integr Food Nutr Metab. 2015;2:148–150.
- Li MY, Ryan P, Batey RG. Traditional Chinese medicine prevents inflammation in CCl4-related liver injury in mice. Am J Chin Med. 2003;31:119–127.
- Liang DY, Liu LM, Ye CG, et al. Inhibition of UII/UTR system relieves acute inflammation of liver through preventing activation of NF-kappaB pathway in ALF mice. PLoS One. 2014;8:e64895.
- Rong Y, Lu Z, Zhang H, et al. Effects of casein glycomacropeptide supplementation on growth performance, intestinal morphology, intestinal barrier permeability and inflammatory responses in Escherichia coli K88 challenged piglets. Anim Nutr. 2015;2:54–59.
- Wu Y, Jiang Z, Zheng C, et al. Effects of protein sources and levels in antibiotic-free diets on diarrhea, intestinal morphology, and expression of tight junctions in weaned piglets. Anim Nutr. 2015;3:170–176.
- Zuo J, Ling B, Long L, et al. Effect of dietary supplementation with protease on growth performance, nutrient digestibility, intestinal morphology, digestive enzymes and gene expression of weaned piglets. Anim Nutr. 2015;4:278–282.
- Nakamura K, Matsuoka H, Nakashima S, et al. Oral administration of apple condensed tannins delays rheumatoid arthritis development in mice via downregulation of T helper 17 (Th17) cell responses. Mol Nutr Food Res. 2015;7:1406–1410
- Shori AB, Baba AS. Fermented milk derives bioactive peptides with antihypertensive effects. Integr Food Nutr Metab. 2015;2:178–181.
- Zaki SA, Amin WSM, Nagi HM. The functional role of tomato and carrot on histopathological lesions of brain, small intestine and prostate in mice treated with acrylamide. Integr Food Nutr Metab. 2014;1:1–6.
- Hendy HA-RE, Gemeai AROA-. Effect of broccoli intake on antioxidant in the liver and kidney tissues of hyperglycemic rats. Integr Food Nutr Metab. 2014;1:1–6.
- Recknagel RO, Glende EA, Jr., Dolak JA, et al. Mechanisms of carbon tetrachloride toxicity. Pharmacol Ther. 1989;43:139–154.
- Park G, Jones DB, Katelaris P. Value of AST/ALT ratio as fibrotic predictor in chronic hepatitis C. Am J Gastroenterol. 2005;100:1623–1624.
- Nyblom H, Berggren U, Balldin J, et al. High AST/ALT ratio may indicate advanced alcoholic liver disease rather than heavy drinking. Alcohol Alcohol. 2004;39:336–339.
- Nyblom H, Bjornsson E, Simren M, et al. The AST/ALT ratio as an indicator of cirrhosis in patients with PBC. Liver Int. 2006;26:840–845.
- Chen X, Meng Q, Wang C, et al. Protective effects of calycosin against CCl4-induced liver injury with activation of FXR and STAT3 in mice. Pharm Res. 2015;32:538–548.
- Cui Y, Yang X, Lu X, et al. Protective effects of polyphenols-enriched extract from Huangshan Maofeng green tea against CCl4-induced liver injury in mice. Chem Biol Interact. 2014;220:75–83.
- Syn WK. Repair-associated inflammation in nonalcoholic fatty liver disease. Clin Med (Lond). 2013;13(Suppl 6):s15–19.
- Yilmaz Y, Ozturk O, Alahdab YO, et al. Serum osteopontin levels as a predictor of portal inflammation in patients with nonalcoholic fatty liver disease. Dig Liver Dis. 2013;45:58–62.
- Wang J, Tang L, White J, et al. Inhibitory effect of gallic acid on CCl4-mediated liver fibrosis in mice. Cell Biochem Biophys. 2014;69:21–26.
- McCann MJ, Dalziel JE, Bibiloni R, et al. An integrated approach to assessing the bio-activity of nutrients in vitro: the anti-oxidant effects of catechin and chlorogenic acid as an example. Integr Food Nutr Metab. 2015;2:197–204.
- Zhu L, Sun Y, Zhang G, et al. Radical-scavenging and anti-oxidative activities of TBN in cell-free system and murine H9c2 cardiomyoblast cells. J Antioxid Activ. 2015;1:55–68.