ABSTRACT
Genetic homogeneity is known to be the most important prerequisite in the micropropagation of Jatropha curcas L. to produce true-to-type plants. The detection of genetic homogeneity in clonal micropropagation for elite plants at an early stage is required, to avoid any increase in variation in the next stage of micropropagation. The genetic homogeneity was assessed during shoot bud formation from petiole explants of J.curcas (P1 × P3) hybrid with different concentrations of thidiazuron (TDZ) in a range of 0.5–4.0 mg/L using inter simple sequence repeat (ISSR) markers. Out of 23 ISSR primers, 16 primers produced clear, distinct and reproducible bands. A total of 96 bands, ranging in size from 100 to 1013 bp were generated. Based on the band data, a total of 94 bands were monomorphic (98%) and two bands were polymorphic (2%). All banding patterns from the shoot buds induced by 0.5, 1.0 and 2.0 mg/L TDZ were monomorphic, but 4.0 mg/L gave 2% polymorphism. These findings indicated that concentrations of 0.5, 1.0 and 2.0 mg/L TDZ did not trigger any somaclonal variation and could, therefore, be considered suitable for application in clonal micropropagation of J. curcas hybrid using petioles as explant material.
Introduction
Jatropha curcas L. has many common names in different countries worldwide, where it is known as purging or physic nut (English), Jarak pagar (Malaysian and Indonesian) and Ratan-jayot (Bengali). J. curcas is significantly important for its value in alternative medicine, and as a renewable energy source, due to its superior performance and environmental features Citation[1]. Recently, Jatropha oil has been used as an aviation biofuel due to its high stability even at low temperatures Citation[2]. Carbon dioxide and smoke emissions from Jatropha oil are also lower than from petroleum diesel, thus making Jatropha oil an eco-friendly source Citation[3]. Several therapeutic activities have also been reported for Jatropha, since it contains many phenolic compounds, which can have antiviral Citation[4], anticancer, anti-inflammatory and antioxidant properties Citation[5].
Major limitations exist for the large-scale cultivation of J. curcas as an energy crop, particularly due to the unavailability of seeds. This might be due to problems during the establishment of nurseries and to inconsistent seed yield because of the heterogeneous nature of the plant material Citation[6]. Micropropagation is the best technique to produce a large amount planting material with desired traits in a short time and small space. The micropropagation of J. curcas has been reported from various explants, such as nodes Citation[7], shoot tips Citation[8], petioles Citation[9,10], leaves Citation[11], hypocotyls Citation[12] and embryos Citation[13]. The establishment of Jatropha plants from various explants will enhance the production of plant material from the elite mother plant. In such cases, Jatropha seeds would not be used as planting material, since these are used for oil production.
The in vitro propagation of J. curcas using thidiazuron (TDZ) as the basic plant growth regulator has been reported by some researchers Citation[14–16]. TDZ is a substituted phenylurea compound that has been shown to possess both auxin- and cytokinin-like effects, although it is structurally different from either auxins or purine-based cytokinins Citation[17].
Genetic stability is the most crucial aspect to be considered when generating planting material via micropropagation, to maintain the superior quality of the donor plants. According to Pierik Citation[18], the petiole is genetically stable to be used as explant material, as it originates from somatic cells. Several studies on genetic stability following regeneration from petioles in various plant species have shown a high degree of monomorphism Citation[19,20]. To the best of our knowledge, no report exists concerning the genetic stability of plants derived from petioles of J. curcas (P1 × P3) hybrid. According to Islam Citation[21], the J. curcas (P1 × P3) hybrid offers advantages over other Jatropha plants in terms of early fruiting and a high seed yield per plant. Therefore, in this study, the clonal micropropagation for this hybrid needs to be established for large-scale production. During the clonal production of elite plants, somaclonal variation is known to reduce some of their commercial value. Thus, it is important to determine any variation that occurs at an early stage of in vitro development Citation[22].
Some molecular methods have been previously applied for genetic stability studies in J. curcas, such as the use of amplified fragment length polymorphisms (AFLPs) Citation[23], random amplified polymorphic DNA (RAPD) Citation[24] and flow cytometry Citation[25]. The use of inter simple sequence repeats (ISSRs) offers unique advantages over other molecular markers, since their application does not require any genomic information of the target species, it only requires a small amount of template DNA, is rapidly performed Citation[26] and is highly efficient in detecting highly polymorphic DNA among Jatropha genotypes Citation[27,Citation28]. An ISSR marker system has been successfully used to detect somaclonal variation in J. curcas Citation[25,29,30].
This study was conducted to determine the genetic stability of in vitro-raised shoot buds of petiole explants of J. curcas hybrid (P1 × P3) induced using various concentrations of TDZ (0.5, 1.0, 2.0 and 4.0 mg/L).
Materials and methods
Plant material and explant preparation
Petioles from mature plants were collected from a three-year-old J.curcas hybrid (P1 x P3) in Living Lab Energy and Future Crops (UKM Kuala Pilah). The petioles were washed under running tap water for 15 min and were rinsed with two drops of teepol. The petioles then were soaked in 3 mL systemic fungicide Myzim in 500 mL of sterile distilled water for 30 min. The petioles were rinsed three times in sterile distilled water containing one drop of Tween-20 and were then surface-sterilized with 70% ethanol for 1 min, rinsed three times and treated 5 min with 0.1% (w/v) mercuric chloride (HgCl2) followed by five rinses with sterile distilled water. The petioles then were cut into 0.5–1.0 cm sections and the edges were removed for shoot induction.
Induction of shoots and culture conditions
The petiole explants were placed horizontally on Murashige and Skoog (MS) medium Citation[31]. Shoot buds were induced using different concentrations of TDZ at 0.5, 1.0, 2.0 or 4.0 mg/L on MS medium. All the samples were incubated at 25 ± 2 °C with a 16 h light/8 h dark photoperiod at a light intensity of 35–40 µmol m−2 s−1 (cool white fluorescent tubes) Citation[16]. The formation of shoot buds was observed within 7–8 weeks of culture. Thirty explants were used for each concentration and the experiments were performed in triplicate.
DNA extraction and DNA quantification
Genomic DNA was extracted from leaves of hybrid mother plants (P1 × P3) and regenerated shoot buds using the InnuPREP plant DNA kit supplied by Analytic Jena (Jena, Germany). The plant materials were ground in liquid nitrogen, which helped to freeze the plant material to increase the surface area for DNA extraction. DNA was quantified using a Nanodrop Spectrophotometer (Thermo Scientific, Waltham, MA, United States) at 260 nm.
Primers
A total of 23 ISSR primers (13 from the UBC primer set no. 9, University of British Columbia, Canada, and 10 primers from Sigma were tested on DNA of J. curcas (P1 × P3) hybrid mother plants and shoot buds induced in vitro ().
Table 1. ISSR primers of the UBC set and primers from SIGMA used in this study.
Polymerase chain reaction (PCR) amplification conditions
The PCR amplification was performed in a total reaction volume of 50 µL which contained 25 µL master mix (Bioline, London, UK), 2 µL primers (1 µmol/L), 3 µL DNA (200 µg/µL) and 20 µL Nanopure water. The DNA amplification was performed at 95 °C for 3 min for initial denaturation, followed by 34 cycles at 95 °C for 3 s, 50 °C for 1 min and 72 °C for 1 min for extension, followed by a final step extension at 72 °C for 10 min. All reactions were carried out in a PCR thermocycler (Eppendorf Mastercycler DNA engine, Hamburg, Germany). After amplification, each PCR reaction was analysed by electrophoresis in a 1% Tris-acetate-EDTA (ethylenediaminetetraacetic acid) buffer agarose gel, stained using Gelred and visualized by a Gel Documentation System (Bio-Rad).
Data scoring and analysis
Only distinct, reproducible and well-resolved fragments ranging from 100 to 1000 bp were considered in the analysis. These bands were scored either as present (1) or absent (0) for each of the ISSR markers within the shoot buds induced by different concentrations of TDZ and in the mother plants. The presence of DNA bands at a low intensity that could not be readily distinguished as present or absent was considered ambiguous markers and were not scored. The size of each band was estimated using a DNA ladder (HyperLadder 100 bp Plus, Bioline, London, UK). Data are presented as mean values with standard deviation (±SD) of 30 explants per treatment in three independent experiments.
Results and discussion
This study was conducted to screen for genetic variation at an early stage of shoot bud formation from tissue culture of J. curcas that used matured petioles as a starting material. According to Liu et al. Citation[10], petioles that are too young or too old are not suitable as starting material, since they have low regeneration frequency. Furthermore, petioles that are too young are susceptible to sterilization agents and lead to the death of the tissue. Therefore, mature petioles were selected as the best explant material for the study. The genetic variation was determined by employing the ISSR–PCR assay. Shoot buds were induced by all concentrations of TDZ tested, ranging from 0.5 to 4.0 mg/L (). A concentration of 1.0 mg/L TDZ gave the highest number of shoot buds (p < 0.01), compared to other concentrations of TDZ. These results are in agreement with the study by Zhang et al. Citation[15], where the same concentration of 1.0 mg/L of TDZ is also reported to give highest shoot bud formation ().
Figure 1. Three-year-old (P1 × P3) hybrid plants (A) grown in Living Lab Energy and Future Crops (UKM Kuala Pilah); mature petiole explants (B) collected from the field; callus-mediated shoot buds (C) induced from MS supplemented with 1.0 mg/L TDZ; shoot buds at the elongation stage (D).
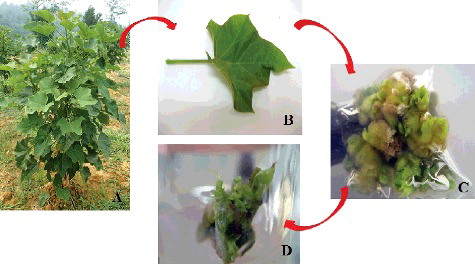
Table 2. Effect of different concentrations of TDZ on the percentage of shoot bud induction.
All the primers () applied in this study were selected according to Grativol et al. Citation[28] and Kumar et al. Citation[29]. In total, 23 ISSR primers were screened for amplified bands, but 16 primers were chosen for further analysis, as they generated 96 clear and reproducible amplification products. Thus, on average, six bands were amplified per primer. The primer UBC 834 amplified the maximum number of 14 bands, whereas UBC 811 amplified the lowest number of three bands (). All the primers amplified scorable bands that ranged in size between 100 bp and 1013 kb.
Table 3. Performance of different ISSR primers for the detection of genetic stability in sample shoot buds of J. curcas (P1 × P3) hybrid.
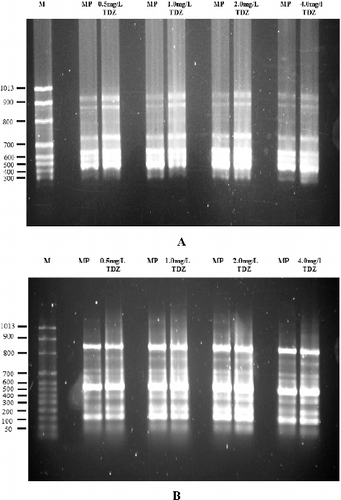
In this study, 94 bands were monomorphic (98%) and two bands were polymorphic (2%), giving a total of 96 bands. The amplification of monomorphic bands from shoot buds of J. curcas was obtained using the primers I5 and UBC 889 and representative gel profiles are shown in . Fifteen out of 16 primers produced monomorphic bands, i.e. ones that are present in all individuals and are inherited by the subsequent generation, confirming the genetic stability of the shoot buds produced by TDZ in vitro. The total number of 96 marker bands amplified during the assay in this study is sufficient to detect any somaclonal variation. This is comparable to the 40, 45 and 51 bands amplified in other previous studies for various plant species employing ISSR-based markers to evaluate genetic stability Citation[32–34]. The ISSR analysis in this study showed that the shoot buds generated from tissue culture were completely uniform, suggesting a high level of genetic homogeneity among them. This indicated that petioles represent an explant source that can produce true-to-type plants in J. curcas micropropagation, instead of shoot tips and nodal explants. According to Pierik Citation[18], plants raised from petioles are more resistant to genetic variation because they originate from somatic tissue. For example, the use of petioles of broccoli as explant material gives a high degree of monomorphism Citation[20]. In J. curcas, a few studies have used petioles as explant material for micropropagation Citation[9,10,16]. However, to the best of our knowledge, there are no reports on the genetic stability in petiole explants of J. curcas. In this study, genetic variation was detected in the shoot buds at the induction stage, because during this early stage, the cells are in the adaptation period. During this period, the plant material experiences a high level of stress, and variation might be induced to occur. No detected variation during the early stage of shoot bud formation indicates a reduced probability of somaclonal variation. According to Sharma et al. Citation[6], the screening of somaclonal variation in micropropagated plantlets at an early stage of development is necessary to avoid an increase in variation in the future.
Figure 2. Monomorphic bands of ISSR products amplified from in vitro-raised shoot buds treated with different concentrations of TDZ (0.5, 1.0, 2.0 and 4.0 mg/L) and from mother plants of J. curcas using ISSR 5 (A) and UBC 889 (B) primers. Lane M, molecular size marker; MP, mother plant.
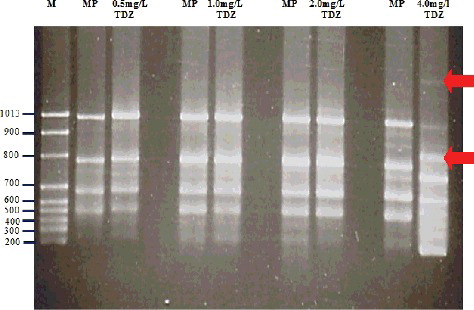
The role of growth hormones is one factor that can cause genetic changes in vitro, and when applied at higher concentrations, hormones can cause genetic instability, or so-called spontaneous variation Citation[35]. For example, a high concentration of cytokinin (6 mg/L BAP) applied to the initial culture can lead to somaclonal variation in J. curcas using axillary buds as explant material Citation[6]. Some studies have reported that the application of TDZ at high concentrations leads to phenotypic abnormalities and to the inhibition of shoot regeneration and root development in J. curcas Citation[15], as well as in other plants Citation[36]. This is probably related to the somaclonal variation (genetic instability) that was observed following exposure to a high TDZ concentration of 4.0 mg/L, at which genetic variation occurred in this study (). In this study, lower TDZ concentrations (0.5, 1.0 and 2.0 mg/L) did not induce somaclonal variation. This is consistent with the results for two in vitro-derived orchid species that gave 100% monomorphism Citation[37] and 97% genetic fidelity at low concentrations of TDZ Citation[36]. Overall, the results from the present study indicated that concentrations of TDZ in the range of 0.5–2.0 mg/L did not trigger any somaclonal variation and could, therefore, be considered suitable for application in clonal micropropagation of J. curcas hybrid using petioles as explant material.
Figure 3. Polymorphic bands of ISSR products amplified from in vitro-raised shoot buds treated with different concentrations of TDZ (0.5, 1.0, 2.0 and 4.0 mg/L) and from mother plants of J. curcas using UBC 856 ISSR primers. Lane M, molecular size marker; MP, mother plant. Note: Polymorphic bands are shown by an arrow at 4 mg/L TDZ.
Conclusions
This study demonstrated that the use of TDZ at concentrations of 0.5, 1.0 and 2.0 mg/L is suitable for the induction of shoot buds from petioles of J. curcas and no genetic variation was induced. However, the application of 4.0 mg/L can produce a small amount of genetic variation (2%). This is of practical importance, since the early detection of genetic variation is useful and important to generate true-to-type plants. The early detection of somaclonal variation using ISSR analysis is effective for micropropagation techniques in any plant species.
Acknowledgments
The authors gratefully acknowledge Faculty of Engineering and Built Environment, Universiti Kebangsaan Malaysia for the financial support. The authors want to thank Department of Biotechnology, Kulliyyah of Science IIUM for the workplace provided in molecular laboratory. The authors also wish to thank all staffs and students in Living Lab Energy and Future Crops, Kuala Pilah.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Dahake R, Roy S, Patil D, et al. Potential anti-HIV activity of Jatropha curcas L. leaf extracts. J Antivir Antiretrovir. 2013;5:160–165.
- Baroutian S, Aroua MK, Raman AAA, et al. Blended aviation biofuel from esterified Jatropha curcas and waste vegetable oils. J Taiwan Inst Chem Eng. 2013;44:1–6.
- Achten WMJ, Verchot L, Franken YJ, et al. Jatropha bio-diesel production and use. Biomass Bioenergy. 2008;32:1063–1084.
- Patil D, Roy S, Dahake R, et al. Evaluation of Jatropha curcas Linn. leaf extracts for its cytotoxicity and potential to inhibit hemagglutinin protein of influenza virus. Ind J Virol. 2013;24:220–226.
- Oskoueian E, Abdullah N, Saad WZ, et al. Antioxidant, anti-inflammatory and anticancer activities of methanolic extracts from Jatropha curcas Linn. J Med Plants Res. 2011;5:49–57.
- Sharma S, Pamidimarri DVNS, Anand KGV, et al. Assessment of genetic stability in micropropagules of Jatropha curcas genotypes by RAPD and AFLP analysis. Ind Crops Prod. 2011;34:1003–1009.
- Datta MM, Mukherjee P, Ghosh B, et al. In vitro clonal propagation of biodiesel plant (Jatropha curcas L.). Current Sci. 2007;93(10):6–10.
- Rajore S, Batra A. Efficient plant regeneration via shoot tip explant in Jatropha curcas L. J Plant Biochem Biot. 2005;14:73–75.
- Kumar N, Vijay Anand KG, Reddy MP. In vitro regeneration from petiole explants of non-toxic Jatropha curcas. Ind Crops Prod. 2011;33:146–151.
- Liu Y, Tong X, Hui W, et al. Efficient culture protocol for plant regeneration from petiole explants of physiologically mature trees of Jatropha curcas L. Biotechnol Biotechnol Equip. 2015;29:479–488.
- Khurana-Kaul V, Kachhwaha S, Kothari SL. Direct shoot regeneration from leaf explants of Jatropha curcas in response to thidiazuron and high copper contents in the medium. Biol Plant. 2010;54:369–372.
- Kaewpoo M, Te-Chato S. Influence of explant types and plant growth regulators on multiple shoot formation from Jatropha curcas. Sci Asia. 2009;35:353–357.
- Mohan N, Nikdad S, Singh G. Studies on seed germination and embryo culture of Jatropha curcas L. under in vitro conditions. Biotechnol Bioinf Bioeng. 2011;1:187–194.
- Nasir NANM, Anuar N, Yaakob Z. Induction of multiple shoot bud formation from J. curcas L. J Appl Sci Agric. 2014;9:63–69.
- Zhang C, Fu S, Tang G, et al. Factor influencing direct shoot regeneration from mature leaves of Jatropha curcas, an important biofuel plant. In vitro Cell Dev Biol Plant. 2013;49:529–540.5
- Kumar N, Reddy MP. Thidiazuron (TDZ) induced plant regeneration from cotyledonary petiole explants of elite genotypes of Jatropha curcas: a candidate biodiesel plant. Ind Crops Prod. 2012;39:62–68.
- Guo B, Abbasi BH, Zeb A, et al. Thidiazuron : a multi-dimensional plant growth regulator. Afr J Biotechnol. 2011;10:8984–9000.
- Pierik R. Commercial aspects of micropropagation. In: Prakash J, Pierik RLM, editors. Horticulture – new technologies and applications. Dordrecht: Kluwer Academic; 1991; p. 141–153.
- Chalageri G, Babu UV. In vitro plant regeneration via petiole callus of Viola patrinii and genetic fidelity assessment using RAPD markers. Turk J Bot. 2012;36:358–368.
- Kumar P, Gambhir G. Molecular analysis of genetic stability in in vitro regenerated plants of broccoli (Brassica oleracea L. var. italica). Current Sci. 2015;109:1470–1475.
- Islam AKM. Improvement of biodiesel production through genetic studies of J. curcas L [dissertation]. Bangi: National University Malaysia (UKM); 2011.
- Nimisha JJK, Anu MA, Nambisan P. Evaluation of somaclonal variation in callus cultures of Jatropha curcas maintained on different hormonal combinations using RAPD markers. World J Agri Sci. 2012;8:616–623.
- Santos CAF, Drumond MA, Rodrigues MA, et al. Genetic similarity of Jatropha curcas accessions based on AFLP markers. Crop Breed Appl Biot. 2010;10:364–369.
- Leela T, Naresh B, Reddy MS, et al. Morphological, physico-chemical and micropropagation studies in Jatropha curcas L and RAPD analysis of the regenerants. Appl Energy. 2011;88:2071–2079.
- Rathore MS, Yadav P, Mastan SG, et al. Evaluation of genetic homogeneity in tissue culture regenerates of Jatropha curcas L. using flow cytometer and DNA-based molecular markers. Appl Biochem Biotech. 2014;172:298–310.
- Cai Y, Sun D, Wu G, et al. ISSR-based genetic diversity of Jatropha curcas germplasm in China. Biomass Bioenergy. 2010;34:1739–1750.
- Umamaheswari D, Paramathma M, Manivannan N. Molecular genetic diversity analysis in seed sources of Jatropha (Jatropha curcas L.) using ISSR Markers. Electronic J Plant Breed. 2010;1:268–278.
- Grativol C, da Fonseca Lira-Medeiros C, Silva Hemerly A, et al. High Efficiency and reliability of inter-simple sequence repeats (ISSR) markers for evaluation of genetic diversity in brazilian cultivated Jatropha curcas L. accessions. Mol Biol Rep. 2011;38:4245–4256.
- Kumar RS, Parthiban KT, Govinda Rao M. Molecular characterization Jatropha genetic resources through inter-simple sequence repeat (ISSR) markers. Mol Biol Rep. 2009;36:1951–1956.
- Siju S, Ismanizam I, Wickneswari R. Genetic homogeneity in Jatropha curcas L individuals as revealed by microsatellite markers: implication to breeding strategies. Braz J Bot. 2016;36:861–868.
- Murashige T, Skoog F. A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol Plant. 1962;15:473–497.
- Lata H, Chandra S, Techen N, et al. Molecular analysis of genetic fidelity in Cannabis sativa L. plants grown from synthetic (encapsulated) seeds following in vitro storage. Biotechnol Lett. 2011;33:2503–2508.
- Lata H, Chandra S, Techen N, et al. Molecular analysis of genetic fidelity in micropropagated plants of Stevia rebaudiana Bert. using ISSR marker. Am J Plant Sci. 2013;4:964–971.
- Chandrika M, Rai VR, Kini KR. Assessment of genetic stability of in vitro grown Dictyospermum ovalifolium. Biol Plantarum. 2008;52:735–739.
- van Harten AM. Mutation breeding: theory and practical application. Cambridge (UK): Cambridge University Press; 1998. Chapter 5.5, Somaclonal variation; p. 167–181.
- Paromik B, Kumaria S, Diengdoh R, Tandon P. Genetic stability and phytochemical analysis of the in vitro regenerated plants of Dendrobium Nobile Lindl., an endangered medicinal orchid. Meta Gene Biol Plantarum. 2014;2:489–504.
- Ferreira WDM, Kerbauy GB, Costa APP. Micropropagation and genetic stability of a Dendrobium hybrid (Orchidaceae). In Vitro Cell Dev Biol Plant. 2006;42:568–571.
