ABSTRACT
Cisplatin is widely used for the treatment of a variety of cancers but with a high incidence of hepatotoxicity. Baicalein is originally isolated from the root of Scutellaria baicalensis Georgi with broad bioactivities. The present study aims to investigate the protective effect of baicalein against cisplatin-induced acute liver injury and the underlying mechanism of this protective effect. Administration of cisplatin (40 mg/kg) for 24 h increased the serum alanine and aspartate aminotransferases and alkaline phosphatase levels, while baicalein could reverse all those changes induced by cisplatin. Liver histological analysis further evidenced the protection of baicalein against cisplatin-induced liver injury. Baicalein counteracted the increased liver malondialdehyde (amount induced by cisplatin, while baicalein could further increase the cisplatin-induced elevation of the amount of reduced glutathione in the liver. Further results showed that baicalein reversed the cisplatin-induced decrease in the activities of superoxide dismutase, catalase, glutathione peroxidase, glutathione-S transferase and glutathione reductase. On the other hand, baicalein alleviated the increase in the serum levels of tumour necrosis factor alpha and interleukin 6 induced by cisplatin. Taken together, our results demonstrate that baicalein can inhibit cisplatin-induced hepatic oxidative stress and inflammation, which contributes greatly to the amelioration of cisplatin-induced liver injury.
Introduction
Cisplatin is a potent chemotherapeutic drug that is widely used to treat patients with multiple solid tumours, including ovarian, testicular and head and neck cancers [Citation1,Citation2]. In spite of its considerable clinical benefit, cisplatin therapy is accompanied with undesirable side effects, which has been an increasing concern to clinical doctors. Cisplatin has been documented to cause toxicity in animals and humans [Citation3,Citation4]. It has been elucidated that one of the main target organs of cisplatin toxicity is the liver [Citation5], and that cisplatin intake could cause severe liver injury, including hepatocytic vacuolation, centrilobular necrosis, lymphocyte infiltration and so on [Citation6,Citation7]. Despite being the focus of intense research in recent years, obtaining an effective therapy against cisplatin-induced hepatotoxicity still remains a great challenge lying ahead.
Extensive attention worldwide has been directed at flavonoids, a large class of phytochemicals with variable phenolic structures, owning to their considerable health benefits [Citation8,Citation9]. Baicalein is a flavonoid originally isolated from the roots of Scutellaria baicalensis Georgi, a plant widely used in China. Baicalein has been demonstrated to possess potent antioxidant and anti-inflammatory properties [Citation10,Citation11], and has a wide range of pharmacological effects, including antiviral, anti-tumour, neuroprotective and nephroprotective activity [Citation12–15], etc. Besides, baicalein is liver-protective and alleviates hepatotoxin-induced liver injury [Citation16,Citation17]. It is reported that baicalein increases the cytotoxicity of cisplatin [Citation18]. It has also been demonstrated that baicalein prevents cisplatin-induced renal damage in mice [Citation19]. However, to the best of our knowledge, the effect of baicalein in hepatoprotection during cisplatin administration has not been reported so far. Therefore, the present study was undertaken to investigate the effects of baicalein on cisplatin-induced liver injury and the underlying mechanisms involved.
Materials and methods
Drugs and reagents
Cisplatin was purchased from Sigma Chemical Co. (St. Louis, MO, USA). Baicalein was purchased from Nanjing Goren Biotech Co., Ltd. (Nanjing, China). Alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), malondialdehyde (MDA), reduced glutathione (GSH), superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione reductase (GR) and glutathione-S transferase (GST) kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Enzyme-linked immunosorbent assay (ELISA) kits for tumour necrosis factor alpha (TNF-α) and interleukin 6 (IL-6) were purchased from R&D System (Minneapolis, MN, USA).
Animals and treatments
Male ICR (Institute for Cancer Research) mice weighing 18–22 g were purchased from Yangzhou University (Yangzhou, China). The mice were fed with a standard laboratory diet and water ad libitum. All animal procedures were performed in accordance with the institutional animal care guidelines approved by Huai'an Institute for Food and Drug Control.
After acclimatization to standard laboratory conditions, mice were divided into five groups: (1) vehicle control (n = 10), (2) cisplatin (40 mg/kg) (n = 11), (3) cisplatin (40 mg/kg) + baicalein (30 mg/kg) (n = 10), (4) cisplatin (40 mg/kg) + baicalein (60 mg/kg) (n = 11) and (5) baicalein (60 mg/kg) (n = 10). The mice were pretreated orally with baicalein (at doses of 30 mg/kg and 60 mg/kg, respectively) for six consecutive days, and cisplatin (40 mg/kg, dissolved in 0.9% normal saline) was injected intraperitoneally (i.p.) one hour after the last baicalein treatment. Twenty-four hours later, blood samples were obtained by extirpating the eyeball and mice were sacrificed by cervical dislocation, and then livers from all mice were removed for further research.
Serum biomarkers for liver injury
The blood samples from all mice were allowed to coagulate for 2 h at room temperature. Serum was then isolated following centrifugation at 3500 r/min for 10 min (Heraeus Fresco 21, Thermo Scientific, Waltham, MA, USA). Serum alanine/aspartate aminotransferase (ALT/AST) and ALP activities were measured with kits according to the manufacturer's instructions.
Histological examination
The liver samples were fixed with 15% formalin, and embedded in paraffin for histological assessment of liver damage. Samples were sectioned (5 μm), stained with haematoxylin and eosin (H&E) and examined under a light microscope (E200MV, Nanjing Nikon Jiangnan Optical Instrument Co., Ltd., Nanjing, China) for liver damage.
Measurement of lipid peroxidation (LPO) in liver
MDA was assayed according to the manufacturer's instructions. The tissue protein concentrations were determined according to the Bradford method. The liver MDA level was obtained based on protein concentrations of samples and expressed as nmol/mg protein.
Measurement of GSH content
Liver GSH content was determined using a GSH detection kit, and the content was assessed according to the absorbance measured at 420 nm and normalized by protein concentration of the same sample.
Enzymatic assays
Liver tissue was homogenized in ice-cold 0.9% normal saline. After centrifugation at 3500 r/min for 15 min at 4 °C (Heraeus Fresco 21, Thermo scientific, Waltham, MA, USA), the supernatant was transferred to new tubes for further analysis. The activities of SOD, CAT, GPx, GR and GST in tissues were determined according to the manufacturer's instructions, respectively, and the results were calculated based on the corresponding protein concentration in the samples determined by the Bradford method.
ELISA analysis
TNF-α and IL-6 levels were assessed with mouse ELISA kits commercially available according to the manufacturer's instructions. The results were expressed as pictogram of cytokine per millilitre.
Statistical analysis
All experimental data were expressed as mean values with standard error of the means (±SEM). Significant differences were determined by one-way analysis of variance, using Statistical Package for Social Sciences software (SPSS, Inc., Chicago, IL, USA) version 16.0 for Windows. P < 0.05 was considered as statistically significant difference.
Results and discussion
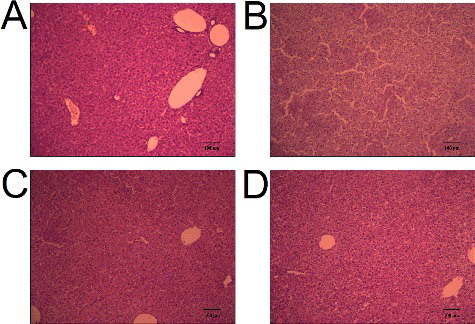
The elevation of ALT, AST and ALP activities in serum is used as a diagnostic indicator for acute liver injury [Citation20]. As shown in , a single administration of cisplatin (40 mg/kg) for 24 h markedly increased the serum activities of ALT, AST and ALP as compared to those in the control (P < 0.001), indicating that cisplatin successfully induces liver injury. At the same time, pretreatment with baicalein (60 mg/kg) for six days could clearly reverse this cisplatin-induced increase (P < 0.01, P < 0.05, P < 0.01), suggesting that baicalein (60 mg/kg) could protect against cisplatin-induced liver injury. Histological analysis () showed that the mice treated with cisplatin exhibited severe liver damage with visible destruction of liver structure and hepatocellular necrosis ((B)). Interestingly, baicalein (60 mg/kg) pretreatment strongly inhibited the development of hepatic lesions induced by cisplatin ((C)). These results further confirmed the protective action of baicalein against cisplatin-induced liver injury. There are some reports about the protective effect of baicalein against liver injury [Citation16,Citation17]. The present results demonstrated that baicalein prevented liver injury induced by cisplatin.
Figure 1. Serum enzymes in cisplatin-treated mice with or without administration of baicalein. Serum ALT and AST activities (A); serum ALP activity (B).
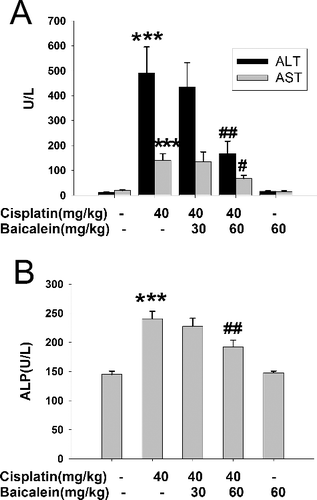
Figure 2. Liver histological analysis of cisplatin-treated mice with or without administration of baicalein: control group (A), cisplatin only group (B), cisplatin + baicalein (60 mg/kg) group (C) and baicalein (60 mg/kg) only group (D).
Oxidative stress causes oxidative damage and has been involved in the pathogenesis of many hepatotoxin-induced liver injuries [Citation21–23]. Under normal conditions, the body maintains a dynamic equilibrium between the production and detoxification of free radicals via the antioxidant defence system in the body. However, hepatic oxidative stress often takes place in drug-induced liver injury and the balance is disturbed. With the increase in free radicals and the decrease in hepatic antioxidant defence, LPO occurs, which is a free radical-related process [Citation24]. As the main end product, MDA is a highly toxic molecule and its interaction with DNA and proteins has often been referred to as potentially mutagenic and atherogenic as well as being considered a marker of lipid peroxidation (reviewed in [Citation25]). As shown in (A), intraperitoneal injection of cisplatin alone dramatically increased the liver MDA amount (P < 0.01), while pretreatment with baicalein (60 mg/kg) reversed this effect (P<0.01). However, baicalein (30 mg/kg) had no effect on the liver MDA amount. These results indicate that baicalein (60 mg/kg) could protect against liver LPO injury induced by cisplatin.
Figure 3. The levels of liver MDA (A) and GSH (B) in cisplatin-treated mice with or without administration of baicalein.
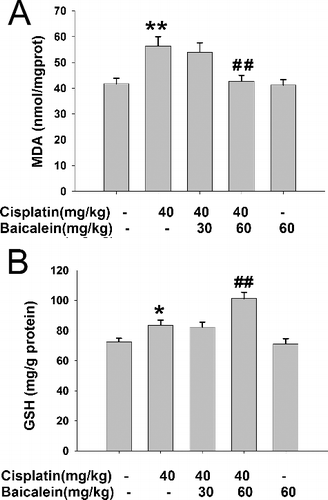
GSH is a tripeptide non-enzymatic antioxidant and the central redox regulator that plays an important role in the detoxification and elimination of drugs and in the protection of cells against exogenous toxins [Citation26]. In our study, the GSH content was measured to assess the protective effect of baicalein against cisplatin-induced oxidative stress injury. The results presented in (B) showed that administration of cisplatin significantly increased the liver GSH level (P < 0.05), suggesting that cisplatin may cause oxidative stress injury and the body, in turn, induces such an increase in the GSH level to cope with this harmful effect. Interestingly, pretreatment with baicalein (60 mg/kg) further increased the liver GSH level (P < 0.01), whereas baicalein (30 mg/kg) had no effect on the increased GSH level induced by cisplatin. These results suggest that baicalein (60 mg/kg) could promote the production of GSH to protect hepatocytes against cisplatin-induced liver oxidative stress injury.
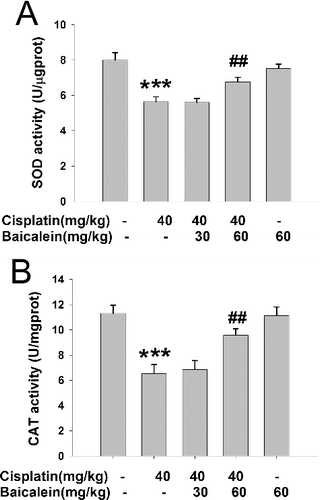
Antioxidant enzymes constitute the first line of defence in organisms against free radicals and toxic substances by catalysing their conversion into harmless products [Citation27]. SOD and CAT are antioxidant enzymes that function mutually to eliminate free radicals produced in the process of oxidative stress. As a metalloenzyme, SOD catalyses the dismutation of superoxide generated during oxidative stress into oxygen and hydrogen peroxide [Citation28], while CAT can further detoxify hydrogen peroxide into water and oxygen without producing toxic free radicals [Citation29]. As shown in , cisplatin caused a decrease in the liver activities of SOD and CAT (P < 0.001), whereas baicalein (60 mg/kg) prevented such a decrease (P < 0.01). Baicalein (30 mg/kg) had no effect on the cisplatin-induced decrease in the SOD and CAT levels. These results suggest that intracellular antioxidant enzymes may also be involved in the mechanism underlying the protective effect of baicalein against cisplatin-induced liver oxidative stress injury.
Figure 4. The activities of liver SOD (A) and CAT (B) in cisplatin-treated mice with or without administration of baicalein.
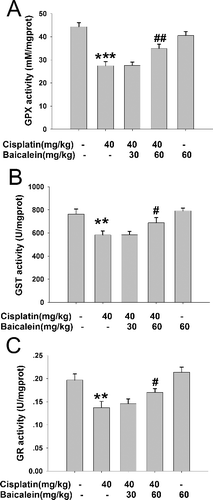
GPx, GST and GR are all glutathione-related enzymes. Among them, GST is an important phase II enzyme which catalyses the conjugation of reactive metabolites with GSH [Citation30]. GPx catalyses the GSH-dependent reduction of H2O2 and hydroperoxides to non-toxic products and glutathione disulfide (GSSG), while GSSG can then be converted back to GSH by GR using NADPH (nicotinamide adenine dinucleotide phosphate) [Citation31,Citation32]. In the present study (), there was an obvious decrease in the liver activities of GPx, GST and GR in cisplatin-induced mice compared to the controls (P < 0.001, P < 0.01, P < 0.01), whereas the activities of GPx, GST and GR significantly increased with baicalein (60 mg/kg) pretreatment (P < 0.01, P < 0.05, P < 0.05). However, there were no significant changes in the activities of these enzymes in the baicalein (30 mg/kg) group. These results suggest that glutathione-related enzymes may also participate in the protective action of baicalein against cisplatin-induced liver oxidative stress injury. The results further confirm that cisplatin causes an imbalance between cellular oxidants and antioxidants, while baicalein could reverse such a condition.
Figure 5. The activities of liver GPx (A), GST (B) and GR (C) in cisplatin-treated mice with or without administration of baicalein.
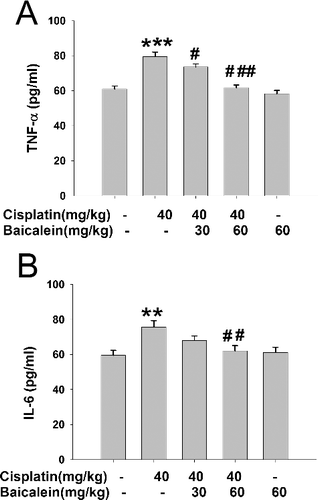
TNF-α and IL-6 are pleiotropic cytokines that are associated with various physiological and pathological conditions [Citation33]. They are rapidly produced by macrophages in response to tissue damage and contribute to liver damage [Citation34]. As shown in , cisplatin increased the serum levels of TNF-α and IL-6 (P < 0.001, P < 0.01), whereas baicalein (60 mg/kg) prevented such increase(P < 0.001, P < 0.01). Baicalein (30 mg/kg) could decrease the induced levels of TNF-α (P < 0.05), but had no effect on the cisplatin-induced increase in the levels of IL-6. These results suggest that mitigated inflammation may be involved in the protective effect of baicalein against cisplatin-induced liver inflammatory injury.
Figure 6. Serum TNF-α (A) and IL-6 (B) levels in cisplatin-treated mice with or without administration of baicalein.
Baicalein is a very effective protector against liver injury. In this regard, it has been reported to ameliorate concanavalin A-induced hepatitis via the apoptosis of activated lymphocytes [Citation16]. Baicalein also has the ability to protect against acute liver failure induced by D-galactosamine/lipopolysaccharides [Citation17]. Recent results have demonstrated that baicalein pretreatment reduces liver ischemia/reperfusion injury via induction of autophagy in rats [Citation35]. Another agent, rutin, which is similar in chemical structure to baicalein, has also shown hepatoprotective activity against biliary obstruction or CCl4-induced liver injury [Citation36,Citation37]. Similarly, quercetin has been demonstrated to be liver-protective in a variety of animal models [Citation38–40]. The hepatoprotective effect of baicalein can match that of rutin and quercetin. The results from the present study confirm that baicalein acts as a protector against the liver injury induced by cisplatin in mice, indicating that baicalein could be an effective pharmacotherapy for the prevention or treatment of liver diseases.
Conclusions
In conclusion, the present study provides evidence that baicalein prevented the hepatotoxicity induced by cisplatin in mice via inhibiting liver oxidative stress injury and inflammation. Further studies are in progress in our laboratory to explore the influence of baicalein on the anti-tumour activity of cisplatin.
Disclosure statement
There is no conflict of interest to disclose.
Additional information
Funding
References
- Karadeniz A, Simsek N, Karakus E, et al. Royal jellymodulates oxidative stress and apoptosis in liver and kidneys of rats treated with cisplatin. Oxid Med Cell Longev. 2011;2011:981793–981802.
- Bagri PK, Kapoor A, Kalwar A, et al. Comparative analysis of cisplatin-induced nephrotoxicity in head and neck cancer and carcinoma cervix during concurrent chemoradiotherapy. South Asian J Cancer. 2014;3:217–220.
- Liu HE, Bai KJ, Hsieh YC, et al. Multiple analytical approaches demonstrate a complex relationship of genetic and nongenetic factors with cisplatin-and carboplatin-induced nephrotoxicity in lung cancer patients. Biomed Res Int [Internet]. 2014 [cited 2016 May 24];2014:937429. Available from: https://www.hindawi.com/journals/bmri/2014/937429/
- Palipoch S, Punsawad C. Biochemical and histological study of rat liver and kidney injury induced by cisplatin. J Toxicol Pathol. 2013;26:293–299.
- Cho YE, Singh TS, Lee HC, et al. In-depth identification of pathways related tocisplatin-induced hepatotoxicity through an integrative method based on an informatics-assisted label-free protein quantitation and microarray gene expression approach. Mol Cell Proteomics [Internet]. 2012 [cited 2016 May 24];11:M111.0108841–M111.010884-14. Available from: http://www.mcponline.org/content/11/1/M111.010884.long
- Sohn JH, Han KL, Kim JH, et al. Protective effects of macelignan on cisplatin-induced hepatotoxicity is associated with JNK activation. Biol Pharm Bull. 2008;31:273–277.
- Dkhil MA, Al-Quraishy S, Aref AM, et al. The potential role of Azadirachta indica treatment on cisplatin-induced hepatotoxicity and oxidative stress in female rats. Oxid Med Cell Longev [Internet]. 2013 [cited 2016 May 24];2013:741817. Available from: https://www.hindawi.com/journals/omcl/2013/741817/
- Lv X, Zhao S, Ning Z, et al. Citrus fruits as a treasure trove of active natural metabolites that potentially provide benefits for human health. Chem Cent J. 2015;9:68–85.
- Bladé C, Aragonès G, Arola-Arnal A, et al. Proanthocyanidins in health and disease. Biofactors. 2016;42(1):5–12.
- Kang KA, Zhang R, Piao MJ, et al. Baicalein inhibits oxidative stress-induced cellular damage via antioxidant effects. Toxicol Ind Health. 2012;28:412–421.
- Lee W, Ku SK, Bae JS. Anti-inflammatory effects of baicalin, baicalein, and wogonin in vitro and in vivo. Inflammation. 2015;38:110–125.
- Zandi K, Teoh BT, Sam SS, et al. Novel antiviral activity of baicalein against dengue virus. BMC Complement Altern Med [Internet]. 2012 [cited 2016 May 24];12:214. Available from: http://bmccomplementalternmed.biomedcentral.com/articles/10.1186/1472-6882-12-214
- Han Z, Zhu S, Han X, et al. Baicalein inhibits hepatocellular carcinoma cells through suppressing the expression of CD24. Int Immunopharmacol. 2015;29:416–422.
- Moon JH, Park SY. Baicalein prevents human prion protein-induced neuronal cell death by regulating JNK activation. Int J Mol Med. 2015;35(2):439–445.
- Wang W, Zhou PH, Xu CG, et al. Baicalein attenuates renal fibrosis by inhibiting inflammation via down-regulating NF-κB and MAPK signal pathways. J Mol Histol. 2015;46:283–290.
- Zhang Y, Shan L, Hua Y, et al. Baicalein selectively induces apoptosis in activated lymphocytes and ameliorates concanavalin a-induced hepatitis in mice. PLoS One [Internet]. 2013 [cited 2016 May 24];8:e69592. Available from: http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0069592
- Wu YL, Lian LH, Wan Y, et al. Baicalein inhibits nuclear factor-κB and apoptosis via c-FLIP and MAPK in D-GalN/LPS induced acute liver failure in murine models. Chem Biol Interact. 2010;188:526–534.
- Wang Y, Wang Q, Zhang S, et al. Baicalein increases the cytotoxicity of cisplatin by enhancing gap junction intercellular communication. Mol Med Rep. 2014;10:515–521.
- Sahu BD, Mahesh Kumar J, Sistla R. Baicalein, a bioflavonoid, prevents cisplatin-induced acute kidney injury by up-regulating antioxidant defenses and down-regulating the MAPKs and NF-κB pathways. PLoS One [Internet]. 2015 [cited 2016 May 24];10:e0134139. Available from: http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0134139
- Rajesh MG, Latha MS. Preliminary evaluation of the antihepatotoxic activity of Kamilari, a polyherbal formulation. J Ethnopharmacol. 2004;91:99–104.
- Shi Y, Jiang J, Shan Z, et al. Oxidative stress and histopathological alterations in liver of Cyprinus carpio L. induced by intraperitoneal injection of microcystin-LR. Ecotoxicology. 2015;24:511–519.
- Brown JM, Kuhlman C, Terneus MV, et al. S-adenosyl-l-methionine protection of acetaminophen mediated oxidative stress and identification of hepatic 4-hydroxynonenal protein adducts by mass spectrometry. Toxicol Appl Pharmacol. 2014;281:174–184.
- Martins NM, Santos NA, Curti C, et al. Cisplatin induces mitochondrial oxidative stress with resultant energetic metabolism impairment, membrane rigidification and apoptosis in rat liver. J Appl Toxicol. 2008;28:337–344.
- Romero FJ, Bosch-Morell F, Romero MJ, et al. Lipid peroxidation products and antioxidants in human disease. Environ Health Perspect. 1998;106(Suppl. 5):1229–1234.
- Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis. 2005;15:316–328.
- Deponte M. Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim Biophys Acta. 2013;1830:3217–3266.
- Deng R, Hua X, Li J, et al. Oxidative stress markers induced by hyperosmolarity in primary human corneal epithelial cells. PLoS One [Internet]. 2015 [cited 2016 May 24];10(5):e0126561. Available from: http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0126561
- Behndig A, Svensson B, Marklund SL, et al. Superoxide dismutase isoenzymes in the human eye. Invest Ophthalmol Vis Sci. 1998;39:471–475.
- Yamanaka K, Hasegawa A, Sawamura R, et al. Cellular response to oxidative damage in lung induced by the administration of dimethylarsinic acid, a major metabolite of inorganic arsenics, in mice. Toxicol Appl Pharmacol. 1991;108:205–213.
- Dakrory AI, Fahmy SR, Soliman AM, et al. Protective and curative effects of the sea cucumber Holothuria atra extract against DMBA-induced hepatorenal diseases in rats. Biomed Res Int [Internet]. 2015 [cited 2016 May 24];2015:563652. Available from: https://www.hindawi.com/journals/bmri/2015/563652/
- Abdel-Moneim AM, Al-Kahtani MA, El-Kersh MA, et al. Free radical-scavenging, anti-inflammatory/anti-fibrotic and hepatoprotective actions of taurine and silymarin against CCl4 induced rat liver damage. PLoS One [Internet]. 2015 [cited 2016 May 24];10(12):e0144509. Available from: http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0144509
- Kadasa NM, Abdallah H, Afifi M, et al. Hepatoprotective effects of curcumin against diethyl nitrosamine induced hepatotoxicity in albino rats. Asian Pac J Cancer Prev. 2015;16:103–108.
- Qu L, Xin H, Zheng G, et al. Hepatoprotective activity of the total saponins from Actinidia valvata Dunn root against carbon tetrachloride-induced liver damage in mice. Evid Based Complement Alternat Med [Internet]. 2012 [cited 2016 May 24];2012:216061. Available from: https://www.hindawi.com/journals/ecam/2012/216061/
- Lin X, Huang R, Zhang S, et al. Methyl helicterate protects against CCl4-induced liver injury in rats by inhibiting oxidative stress, NF-κB activation, Fas/FasL pathway and cytochrome P4502E1 level. Food Chem Toxicol. 2012;50:3413–3420.
- Liu A, Huang L, Guo E, et al. Baicalein pretreatment reduces liver ischemia/reperfusion injury via induction of autophagy in rats. Sci Rep. 2016;6:25042.
- Hafez MM, Al-Harbi NO, Al-Hoshani AR, et al. Hepato-protective effect of rutin via IL-6/STAT3 pathway in CCl4-induced hepatotoxicity in rats. Biol Res. 2015;48:30.
- Pan PH, Lin SY, Wang YY, et al. Protective effects of rutin on liver injury induced by biliary obstruction in rats. Free Radic Biol Med. 2014;73:106–116.
- Li X, Jin Q, Yao Q, et al. Quercetin attenuates the activation of hepatic stellate cells and liver fibrosis in mice through modulation of HMGB1-TLR2/4-NF-κB signaling pathways. Toxicol Lett. 2016;261:1–12.
- Yu X, Xu Y, Zhang S, et al. Quercetin attenuates chronic ethanol-induced hepatic mitochondrial damage through enhanced mitophagy. Nutrients [Internet]. 2016 [cited 2016 Oct 31];8:E27. Available from: http://www.mdpi.com/2072-6643/8/1/27
- Zou W, Liu W, Yang B, et al. Quercetin protects against perfluorooctanoic acid-induced liver injury by attenuating oxidative stress and inflammatory response in mice. Int Immunopharmacol. 2015;28:129–135.
