ABSTRACT
The aim of the present study was to assess the systemic effect of thymoquinone (TQ) on bone healing by starting TQ administration, either 40 days before, or on the day of the surgical procedure and continuing during the healing period of 28 days. Eighteen experimental rats were divided into three groups and defects were created in their tibias. The following procedures were performed for each group: Control group (C): No systemic drug administration (n = 6); Test group 1 (T1): Systemic TQ was administered daily starting 40 days before creation of the defect and additionally during the post-operative healing period of 28 days (n = 6); Test group 2 (T2): Systemic TQ was administered daily after creation of the defect and during the healing period of 28 days (n = 6). Quantitative measurement for new bone formation, osteoblast lining and semi-quantitative measurement of capillary intensities were examined and statistically analysed. There was a significant increase in the ratio of new bone per total defect area and new bone trabeculae lined by active osteoblasts in both test groups (T1 and T2) compared to control group (p < 0.05). However the difference between T1 and T2 was not statistically significant. TQ-administered groups also showed an increase in capillary intensity in the defect area compared to the control group (p < 0.05). Systemic administration of TQ either starting 40 days before or on the day of surgery accelerated new bone formation in a rat model and can be advocated as an adjunct to expedite bone healing.
Introduction
Rehabilitation of the bone defects is a common procedure in oral surgery. The rapid recovery after surgical procedures is crucial in order to regain normal functioning of the bone and surrounding structures. There have been an increasing number of studies on this subject [Citation1]. Several approaches, such as the local or systemic administration of growth factors [Citation2,3] or mesenchymal stem cells [Citation4–6], can represent advantageous alternatives to bone graft. Also, the use of natural products as an alternative to conventional treatment in healing and treatment on various diseases, as well as bone defects have been on the rise in the last few decades [Citation7,8].
Defects of a size that does not allow spontaneous bone regeneration during the lifetime of the animal are defined as critical size defects [Citation9]. The exact diameter of critical and non-critical size defects is still being debated [Citation10].
Considering the detrimental effect of oxidants, various host-modulatory agents such as antioxidants have been widely investigated for their ability to cope with the oxidant-related breakdown of hard tissues and for their possible role in the promotion of bone healing [Citation11,12]. It has also been demonstrated that antioxidants may accelerate new bone formation [Citation7,8]. For this reason, antioxidant agents continue to generate interest particularly in regard to their effect on new bone formation.
Thymoquinone (TQ) is known as the bioactive constituent of the volatile black seed (Nigella Sativa (NS)) oil. TQ exhibits many pharmacological properties, which include antibacterial, antihistaminic, anti-inflammatory and hypoglycemic actions [Citation7,13,Citation14,15,16,17]. TQ also displays an antioxidant response through its potent superoxide-scavenging ability [Citation13]. Superoxides play a role as intermediate molecules in the activation of osteoclasts, which is especially important in the area of bone resorption [Citation11]. TQ reduces bone resorption and the level of IL-1α and −6 and TNF-α known as pro-inflammatory cytokines. Therefore osteoclastic activity, which causes bone resorption is prevented [Citation12]. Considering these antioxidant properties of TQ, its important role in the acceleration of bone formation is proven [Citation7]. Recently, Kara et al. [Citation7] determined that the systemic use of TQ might have a positive effect in accelerating new bone formation in the rapid mid-palatal expansion procedure, thus it was thought that systemic use of TQ may have a potential therapeutic effect with regard to accelerating bone formation in the hard tissue defects. However, to the best of the authors' knowledge, there is not yet any study, which investigates the systemic administration of TQ for the treatment of bone defects. Therefore, the aim of the present study was to assess the systemic effect of TQ on bone healing by starting TQ administration, either 40 days before or on the day of the surgical procedure, and continuing during the healing period of 28 days.
Material and methods
The experimental procedures were approved by the Yeditepe University Animal Care and Ethics Committee (392/2014 YÜDHEK, Istanbul, Turkey). An animal cohort comprised three-month-old male rats (Rattus norvegicus Albinus, Wistar, Philadelphia, PA, USA), weighing between 200 and 250 g were used. All animals were kept according to the guidelines for laboratory animal treatment and care. Eighteen rats were randomly allocated into three groups:
Control group (C): The defect was left to heal without any intervention (n = 6).
Test group 1 (T1): Systemic TQ was administered daily by oro-gastric way starting 40 days before the creation of the defect and continued during the healing period of 28 days (n = 6) [Citation7].
Test group 2 (T2): Systemic TQ was administered daily by oro-gastric way after the creation of the defect (starting the day of the surgery) and continued during the healing period of 28 days (n = 6).
In the TQ groups, TQ was administered systemically at a rate of 10 mg/kg/day [Citation7].
NS seeds were purchased from a local herb store. The seeds of NS were powdered in a mixer. Then, 20 g of the powdered seeds were added to 400 mL distilled water and the extraction was carried out by steam distillation. The process of distillation continued until about 200 mL of the distillate were collected. The distillate was extracted three times with chloroform. Moisture was removed by anhydrous sodium sulphate and the resultant extract was evaporated using a water bath (40 °C); this led to the appearance of the volatile oil. Then 1 mL NS oil was mixed with 100 mL phosphate buffered solution (pH = 7.4) to obtain the TQ solution for oro-gastric administration [Citation15].
Surgical procedure
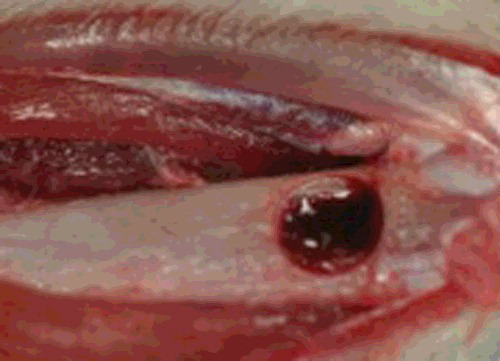
The same operator performed all surgical procedures under strict aseptic conditions. Eighteen rats were weighed before subcutaneous injection. The rats were anesthetized using ketamine (20 mg/kg) (Ketasol %10, Richter Pharma Ag, Wels, Austria) and xylazine (10 mg/kg) (Rompun %2, Bayer AG, Istanbul, Turkey) combination. The operation site was then shaved and disinfected. A linear 15 mm incision was made on the right tibiae and full-thickness skin-muscle periosteal flaps were reflected. After flap elevation, a hole with a diameter of 3 mm was drilled on the cortex of the medial side of the bone (). The drill was introduced into the bone with a depth of 3 mm [Citation18]. The soft tissue was sutured subcutaneously with polivycril 5.0 suture (Ethicon, Sao Paulo, Brazil) and superficially with silk 3.0 sutures (Silk 3.0, Dogsan Medical Supplies Industry, Trabzon, Turkey).
Sacrification procedure
Twenty-eight days after surgery, all animals were sacrificed on the same day with an intracardiac, anesthetic ketamine (20 mg/kg) (Ketasol %10, Richter Pharma Ag) and xylazine (10 mg/kg) (Rompun %2, Bayer AG) injection.
Clinical observation
The rats were also observed for signs of weight loss. Neither of the animals was lost during surgical procedure nor in the post-operative period (28 days). During dissection for bone harvesting, the surgical area, the soft tissue and longitudinal section of the tibiae were assessed for pus and/or abscess formation.
Histology and histomorphometry
The following test methodology was applied as described in previous reports [Citation19,20]. The test animals were sacrificed, bone tissue specimens were removed and placed in 10% neutral buffered formalin (pH 7.0) at room temperature for fixation. After decalcification in De Castro solution (chloral hydrate, nitric acid, distilled water), all samples were dehydrated in a graded series of ethanol for embedding in paraffin by using an automated tissue processor (Thermo Scientific Spin Tissue Processor, STP-120, Waldorf, Germany). Approximately 4–5 μm thick sections were cut with a sliding-microtome (Thermo Fisher Scientific, HM 430, Waldorf, Germany). The sections were stained with hematoxylin and eosin (HE) and Masson's trichrome (MT) and evaluated for bone defect healing. MT was preferred because it produces high contrast images with red bone, green osteoid-cartilage and purple cell cytoplasm. Two independent investigators evaluated the bone defect area using a light microscope (BX61, Olympus, Tokyo, Japan) attached with a computerized digital camera (DP72, Olympus).
Quantitative analysis of newly formed bone and osteoblast lining
Bright-field images were analysed quantitatively by image-processing program (DP2-BSW, Olympus). Number of pixels corresponding to new trabecular bone area in each image were quantified, divided by the total number of pixels corresponding to total defect area and converted to μm2 in each specimen.
Osteoblasts were quantified based on their morphology on HE-stained sections for length of their linear apposition along new bone surfaces relative to total new bone surface length for three randomly selected high power fields (200×) and were reported as a fraction (%) average for each sample [Citation21].
Semi-quantitative analysis of capillary intensity
Capillary intensities of the connective tissue surrounding the bone trabeculae were rated as mild (+), moderate (++) or strong (+++).
Statistical analysis
Histological parameters were analysed statistically. Analysis of new bone area, osteoblast lining and number of capillaries were normally distributed as determined by the Shapiro–Wilk test; therefore they were analysed with one-way ANOVA followed by post hoc Holm–Sidak testing. Statistical calculations were performed using Sigma Plot for Windows, version 12.0 (Systat Software Inc., San Jose, CA, USA); p < 0.05 was considered to be significant.
Results and discussion
Improving and accelerating bone healing after surgical procedures is one of the most challenging procedures in oral surgery. The present study investigated the systemic effect of TQ on bone healing in surgically created critical size rat tibia defects.
Regenerative techniques for bone are generally based on models, which are similar to human anatomy and biology. Although rats are currently the most commonly used animals for in vivo research there is no simple preclinical model to allow the evaluation of these regenerative strategies [Citation22]. Different sites are used to evaluate the bone healing of defects created in rats, such as tibia [Citation23,24], calvaria [Citation25] and jaws [Citation26]. Each site has advantages and disadvantages [Citation23,24]. The challenge in using calvarial defect model is the small size of the rat. The tibia and femur are also used to create segmental or cylindrical bone defects. The tibial proximal epiphysis has a medial face that is suitable for inducing bone defects, as it has a wide and slightly convex surface that is devoid of muscle insertion [Citation27]. Defects of a size that does not allow spontaneous bone regeneration during the lifetime of the animal is defined as critical size defects [Citation9]. However, because most studies are of limited duration and do not extend over the entire life of the animal, the critical size defect in animal research refers to the size of a defect that will not heal over the duration of the study [Citation9,10]. The exact diameter of critical and non-critical size defects is still being debated. In the present study, a diameter of 3 mm trephine bur was used to create circular defects surgically. Defects created in the control group were not completely healed, whereas defects treated by TQ were almost partially healed after 28 days.
Many studies have demonstrated that there is a correlation between oxidative stress and bone metabolism. Oxidative stress caused by excessive generation of intracellular reactive oxygen species (ROS) can exert adverse biological effects on bone through inhibiting bone cell differentiation in the preosteoblastic cell line and in the marrow of the stromal cell line. ROS can also directly promote osteoclast formation and activity [Citation28,Citation29,30]. Thus, considering the detrimental effect of oxidants, various host-modulatory agents such as antioxidants have been widely investigated for their ability to cope with the oxidant-related breakdown of hard tissues and for their possible role in the promotion of bone healing. It has also been demonstrated that antioxidants may accelerate new bone formation. For this reason, antioxidant agents continue to generate interest particularly in regard to their effect on new bone formation. TQ has been used for many centuries in folk medicine for the promotion of good health and for the treatment of many acute, as well as, chronic conditions. Several beneficial pharmacological effects have been attributed to TQ. Amongst these, the antioxidant effect is the most prominent. The antioxidant response of TQ originates from its ability to scavenge free radicals; its scavenging power is as effective as that of superoxide dismutase in its ability to neutralize superoxide anions [Citation10]. Considering these antioxidant properties of TQ, its important role for preventing periodontitis and diminishing alveolar bone resorption was shown in a rat model [Citation31].
In addition to antioxidant properties, TQ has anti-inflammatory, antimicrobial, antitumour, immunomodulatory, bronchodilatory, hypotensive, antidiabetic, hepatoprotective, gastroprotective, antihistaminic and neuroprotective effect [Citation13–Citation17,32,33]. As a result of its antioxidant and anti-inflammatory properties, TQ has cytoprotective effects [Citation32]. Tekeoglu et al. [Citation34] examined the anti-inflammatory effects of TQ on arthritis in rats and stated that TQ suppressed adjuvant-induced arthritis in rats. Houghton et al. [Citation35] explored the mechanisms of the anti-inflammatory effects of TQ and determined that TQ prevented thromboxane and leukotriene B4 synthesis from eicosanoids by inhibiting the cyclooxygenase and lipooxygenase enzymes. Vaillancourt et al. [Citation36] evaluated the in vitro and in vivo effects of TQ on rheumatoid arthritis and determined that TQ was effective in decreasing the arthritis score, osteoclastogenic activity and bone resorption.
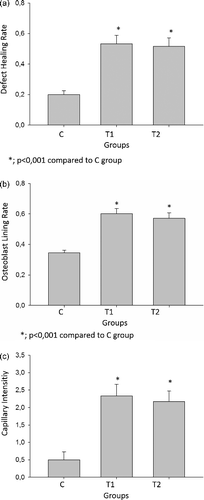
Descriptive statistical data of the present study are given in . Histological data () demonstrated that the ratio of new bone per total defect area was significantly higher in both T1 and T2 groups compared to that of the control group (P < 0.001). However, in the present study, no statistically significant difference was observed between the experimental groups ((A)) regarding new bone per total defect area ratio and osteoblast lining of new bone trabeculae. New bone trabeculae lined by active osteoblasts were significantly more in T1 and T2 groups when compared to control group (P < 0.001). No significant differences were found between T1 and T2 groups regarding osteoblastic lining of the new bone trabeculae ((B)). Connective tissue surrounding new bone trabeculae was more vascular in the TQ-treated groups when compared to control group (P < 0.05); thus the TQ-administered groups also showed an increase in capillaries compared to control group ((C)). All these histologic processes are indicative that early administration might have no additional advantage related to bone healing of the critical size defects and administration can be initiated on the day of surgery, expecting similar healing outcome.
Table 1. Descriptive statistical data are presented for the bone defect healing scores, osteoblast lining scores and capillary intensity scores.
Figure 2. Representative photomicrographs of the defect area in each group (A and B: control group, C and D: test 1 group, E and F: test 2 group). Defect area consists of a fibrous callus and new bone trabeculae in all groups. Note: The high amount of new bone trabeculae and the active osteoblasts lining of these trabeculae in TQ groups when compared to control group. Arrows show the border of the defect. CB: compact bone, NB: new bone, CT: connective tissue, HE: haematoxylin & eosin, MT: Masson's trichrome.
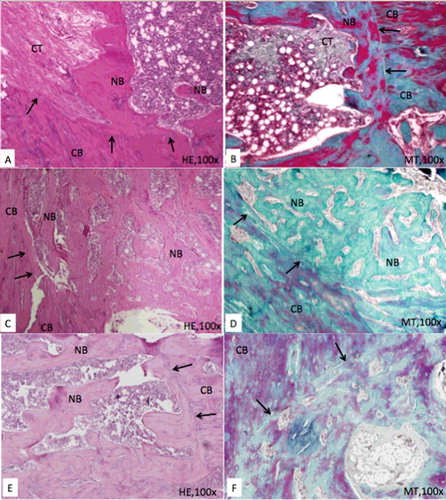
Figure 3. (a) Effect of TQ on defect healing. Statistically significant values are indicated with a (*) (p < 0.01 vs. control and TQ groups) and (**) (p < 0.05 vs. control & TQ groups). (b) Effect of TQ on osteoblastic lining. Statistically significant values are indicated with a (*) (p < 0.01 vs. control & TQ groups) and (**) (p < 0.05 vs. control and TQ groups). (c) Effect of TQ on capillary intensity. Statistically significant values are indicated with a (*) (p < 0.01 vs. control &TQ groups) and (**) (p < 0.05 vs. control & TQ groups)
In this study, the new bone formation in the cortical region of the surgical defect was evaluated both histologically and histomorphometrically. New bone formation was observed along the borders of the surgical defect both in the control and experimental groups. The ossification process started from the edges of the cortical bone and progressed towards the centre of the defect. Connective tissue callus in the defect area consisted of osteoprogenitor cells, fibres and new blood vessels and guided the formation of new bone trabeculae by intramembranous ossification. Active osteoblasts synthesizing new bone matrix were numerous at new bone sites, lining the edges of the trabeculae. Hematopoietic precursor cells in the marrow cavity were detected in the slides. The histological results demonstrated that systemic administration of 10 mg/kg of TQ could enhance the repair of critical sized defects mainly by accelerating vascularization. The results also showed that the use of systemic TQ did not cause any undesirable reaction and all animals recovered without any complication.
Kirui et al. [Citation28] examined the physiological responses associated with sustained delivery of TQ in the femoral defect animal model (bone healing). According to their results, TQ increased bone healing and little or no side effects were observed. Kara et al. [Citation7] investigated the systemic TQ administration for accelerating the formation of bone in the mid-palatal suture in rapid maxillary expansion and reported that systemic effect of TQ on bone healing starting 40 days before the surgical procedure has positive effect in accelerating new bone formation in the rapid mid-palatal expansion procedure. However, the results of this study showed that early administration might have no additional advantage related to bone healing.
The limitations of the study can be summarized as follows: The evaluations were performed at a single time period of 28 days. Additional subgroups may be added to evaluate early bone healing at days 7 and 14. Another limitation is the use of a same dose of TQ. Although administration of TQ was proposed daily at a rate of 10 mg/kg/day [Citation7], a different dose of TQ can also be investigated. Furthermore, rat model supplies a small animal model with small bone defects in comparison to the size of the large animals or human bone defects. Although the present model is still small, the agents or techniques used to stimulate or impair bone healing in rats may produce different results in humans; however, this model can serve as an excellent reliable first step in such evaluations.
Conclusion
Within the limits of this study, the results suggest that there is no significant difference between systemic daily administration of TQ starting 40 days before surgery or starting the day of surgery and continuing during the healing period of 28 days daily and they both have a positive effect on bone healing. Systemic TQ administration may shorten the period of healing and therefore have positive effect on healing. Since the defects are common problems in the oral rehabilitation, the use of systemic TQ will appear to be a favourable alternative therapeutic tool for guided bone regeneration procedures. Future studies are needed to evaluate different doses of systemic TQ and local application of TQ on bone defects either alone or with systemic administrations. Another area of research can be the assessment of the effect of TQ in potentially impaired healing conditions such as diabetic rats to further consolidate the positive results obtained in the present study.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Anitua E, Sánchez M, Orive G, et al. The potential impact of the preparation rich in growth factors (PRGF) in different medical fields. Biomaterials. 2007;28:4551–4560.
- Lutolf MP, Weber FE, Schmoekel HG, et al. Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nat Biotechnol. 2003;21:513–518.
- Holstein JH, Orth M, Scheuer C, et al. Erythropoietin stimulates bone formation, cell proliferation, and angiogenesis in a femoral segmental defect model in mice. Bone. 2011;49:1037–1045.
- Bruder SP, Kurth AA, Shea M, et al. Bone regeneration by implantation of purified, culture‐expanded human mesenchymal stem cells. J Orthop Res. 1998;16:155–162.
- Cancedda R, Mastrogiacomo M, Bianchi G, et al. Bone marrow stromal cells and their use in regenerating bone. Novartis Found Symp. 2003;249:133–147.
- Yang Y, Hallgrimsson B, Putnins EE. Craniofacial defect regeneration using engineered bone marrow mesenchymal stromal cells. J Biomed Mater Res A. 2011;99:74–85.
- Kara MI, Erciyas K, Altan AB, et al. Thymoquinone accelerates new bone formation in the rapid maxillary expansion procedure. Arch Oral Biol. 2012;57:357–363.
- Altan BA, Kara IM, Nalcaci R, et al. Systemic propolis stimulates new bone formation at the expanded suture: a histomorphometric study. Angle Orthod. 2012:83:286–291.
- Schmitz JP, Hollinger JO. The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin Orthop Relat Res. 1986;205:299–308.
- Poser L, Matthys R, Schawalder P, et al. A standardized critical size defect model in normal and osteoporotic rats to evaluate bone tissue engineered constructs. BioMed Research Int. 2014;2014:34865.
- Garrett IR, Boyce BF, Oreffo RO, et al. Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. J Clin Invest. 1990;85:632–639.
- Houghton PJ, Zarka R, Heras BDL, et al. Fixed oil of Nigella sativa and derived thymoquinone inhibit eicosanoid generation in leukocytes and membrane lipid peroxidation. Planta Med. 1995;61:33–36.
- Nader MA, El-Agamy DS, Suddek GM. Protective effects of propolis and thymoquinone on development of atherosclerosis in cholesterol-fed rabbits. Arch Pharm Res. 2010;33:637–643.
- Morsi NN. Antibacterial effect of crude extracts of Nigella sativa on multiple antibiotics-resistant bacteria. Acta Microbiol Pol. 2000;49:63–74.
- Kanter M, Coskun O, Uysal H. The antioxidative and antihistaminic effect of Nigella sativa and its major constituent, thymoquinone on ethanol-induced gastric mucosal damage. Arch Toxicol. 2006;80(4):217–24.
- Taka E, Mazzio EA, Goodman CB, et al. Anti-inflammatory effects of thymoquinone in activated BV-2 microglial cells. J Neuroimmunol. 2015;286:5–12.
- Al-Hader A, Aqel M, Hasan Z. Hypoglysemic effect of volatile oil of Nigella sativa seeds. Int J Pharmacog. 1993;31:96–100.
- Bastos MF, Brilhante FV, Bezerra JP, et al. Trabecular bone area and bone healing in spontaneously hypertensive rats: a histometric study. Braz Oral Res. 2012;24(2):170–176.
- Bölgen N, Korkusuz P, Vargel I, et al. Stem cell suspension injected HEMA-lactate-dextran cryogels for regeneration of critical sized bone defects. Artif Cells Nanomed Biotechnol. 2014;42:70–77.
- Aydin HM, Korkusuz P, Vargel I, et al. A 6-month in vivo study of polymer/mesenchymal stem cell constructs for cranial defects. J Bioact Compat Polym. 2011;26:207–221.
- Uğraş A, Güzel E, Korkusuz P, et al. Glucosamine-sulfate on fracture healing. Ulus Travma Acil Cerrahi Derg. 2013;19:8–12.
- Neyt JG, Buckwalter JA, Carroll NC. Use of animal models in musculoskeletal research. Iowa Orthop J. 1998;18:118–123.
- Kütan E, Duygu-Çapa G, Özçakir-Tomruk C, et al. Efficacy of doxycycline release collagen membrane on surgically created and contaminated defects in rat tibiae: a histopathological and microbiological study. Arch Oral Biol. 2016;63:15–21.
- Ribeiro LL, Bosco, AF, Nagata MJ, et al. Influence of bioactive glass and/or acellular dermal matrix on bone healing of surgically created defects in rat tibiae: a histological and histometric study. Int J Oral Maxillofac Implants. 2008;23:811–817.
- Nagata MJ, Furlaneto FA, Moretti AJ, et al. Bone healing in critical-size defects treated with new bioactive glass/calciumsulfate: a histologic and histometric study in rat calvaria. J Biomed Mater Res B. 2010;95:269–275.
- Schliephake H, Zghoul N, Jäger V, et al. N. Bone formation in trabecular bone cell seeded scaffolds used for reconstruction of the rat mandible. Int J Oral Maxillofac Surg. 2009;38:166–172.
- Le Guehennec L, Goyenvalle E, Aguado E, et al. Small-animal models for testing macroporous ceramic bone substitutes. J Biomed Mater Res B. 2005;72:69–78.
- Kirui PK, Cameron J, Benghuzzi HA, et al. Effects of sustained delivery of thymoquinone on bone healing of male rats. Biomed Sci Instrum. 2004;40:111–116.
- Galli C, Passeri G, Macaluso GM. FoxOs, Wnts and oxidative stress-induced bone loss: new players in the periodontitis arena? J Periodontal Res. 2011;46:397–406.
- Bai XC, Lu D, Bai J, et al. Oxidative stress inhibits osteoblastic differentiation of bone cells by ERK and NF-kappaB. Biochem Biophys Res Commun. 2004;314:197–207.
- Ozdemir H, Isa MI, Erciyas K, et al. Preventive effects of thymoquinone in a rat periodontitis model: a morphometric and histopathological study. J Periodontol Res. 2012;47:74–80.
- Chehl N, Chipitsyna G, Gong Q, et al. Anti-inflammatory effects of the Nigella sativa seed extract, thymoquinone, in pancreatic cancer cells. HPB. 2009;11:373–381.
- Katter M. Protective effects of Nigella sativa on the neuronal injury in frontal cortex and brain stem after chronic toluene exposure. Neurochem Res. 2008;11:2241–2249.
- Tekeoglu I, Dogan A, Ediz L, et al. Effects of thymoquinone (volatile oil of black cumin) on rheumatoid arthritis in rat models. Phytother Res. 2007;21:895–897.
- Houghton PJ, Zarka R, Heras BDL, et al. Fixed oil of Nigella sativa and derived thymoquinone inhibit eicosanoid generation in leukocytes and membrane lipid peroxidation. Planta Med. 1995;61:33–36.
- Vaillancourt F, Silva P, Shi Q, et al. Elucidation of molecular mechanisms underlying the protective effects of thymoquinone against rheumatoid arthritis. J Cell Biochem. 2011;112:107–117.