ABSTRACT
Extraction of RNA of high quality and integrity is essential for gene expression studies and all downstream RNA-based techniques. The leaves of 16 merit Malaysian rice varieties were used to isolate total RNA using five different methods. The quantity, quality and integrity of extracted RNA were confirmed using three different means. The ratios of A260/280 ranged from 2.12 to 2.20. Electrophoresis (1.5% agarose gel) was performed, illustrating intact and sharp bands representing the 28S, 18S, 5.8S and 5S ribosomal subunits of RNA, presenting intact RNA. RNA quality was verified using semi-quantitative polymerase chain reaction (sqPCR). The objective of this study was to identify different genes involved in the resistance of rice plants using high-quality RNA extracted 31 h after inoculation of Magnaporthe oryzae pathotype P7.2. The expression levels of eight blast resistance genes, Pikh, Pib, Pita, Pi21, Pi9, Os11gRGA8, OsWRKY22 and OsWRKY45, were evaluated by real-time PCR (RT-PCR). Real-time PCR was performed to identify candidate genes using RNA extracted by the TRIzol method, which showed the highest score compared with other methods in terms of RNA quantity, purity and integrity. In addition, the results of real-time PCR confirmed that the up-regulation of seven blast resistance genes may confer stronger resistance for the MR 276 variety against M. oryzae pathotype P7.2.
Introduction
An efficient and rapid method of isolating RNA of high integrity and purity is necessary for all RNA-based downstream techniques [Citation1,2]. The initial step in gene quantification is the preparation of cellular RNA or pure messenger RNA (mRNA) [Citation3]. Satisfactory and reliable results from real-time polymerase chain reaction (PCR) and reverse transcriptase PCR depend on high quality, DNA-free and intact RNA samples [Citation4]. RNA is degraded by RNases, heat and ultraviolet radiation at some stages of tissue sampling and RNA purification and storage [Citation5]. RNA may include inhibitory enzymes from tissue samples, causing a reduction in the efficiency of both the reverse transcriptase and the real-time PCR (RT-PCR) reactions, yielding unreliable quantification results [Citation6]. Most extracted RNAs are contaminated with small amounts of DNA and protein. Although these contaminants are not important for some applications, in specific cases, it may be necessary to treat the extracted RNA with RNase-free DNase to remove any traces of residual DNA [Citation7,8]. A variety of methods have been developed to evaluate RNA integrity, and measuring the size of ribosomal RNA (rRNA) subunit molecules is the most popular of these methods. mRNA is the main target of real-time PCR analysis; hence, it would be more accurate to consider the quality of mRNA directly. Moreover, the evaluation the quality of the extracted RNA used for real-time PCR may be more relevant using PCR-based methods [Citation9]. The selection of an individual RNA extraction technique can work more successfully with one specific tissue and can also affect the total RNA yield up to 10-fold [Citation10].
Rice production is decreased by approximately 10%–30% annually because of rice blast disease caused by Magnaporthe oryzae [Citation11]. The most destructive rice pathogen, M. oryzae, injures rice plants throughout their growth and development, resulting in a loss of rice yield [Citation12]. Multiple layers of defence are involved in the resistance of plants such as rice against a variety of pathogens [Citation13]. Whereas the first layer comprises passive structural barriers, the other layers are protein based. Transmembrane pattern recognition receptors (PRRs) included in the second layer act in response to microbial- or pathogen-associated molecular patterns [Citation14,15]. Within the third defence layer, avirulence effectors produced by pathogens activate particular resistance genes (R genes) in host plants [Citation13]. One of the efficient strategies for managing rice blast disease is the development of durable resistant varieties by R gene transformation [Citation16]. Developing varieties with durable resistance to blast disease is essential for improving rice production in the modernized agricultural system [Citation17]. In recent years, few blast resistance genes have been identified and characterized using specific races of pathogen [Citation11]. This study aimed to select the best validated method of RNA extraction from rice leaves, yielding RNA of the highest integrity and purity. Intact RNA of high integrity was used for evaluation of the expression levels of eight blast resistance genes against M. oryzae pathotype P7.2 via reverse transcriptase and real-time PCR.
Materials and methods
Plant materials
Sixteen merit Malaysian rice varieties listed in and M. oryzae pathotype P7.2 were provided by the Malaysian Agricultural Research and Development Institute. Rice seeds were soaked using sterile water for three days and germinated on moist Whatman filter paper (Sigma-Aldrich, St. Louis, Missouri, United States) inside Petri dishes. Subsequently, the germinated seeds were planted in pots filled with autoclaved potting soil and transferred to a greenhouse at 25 °C–30 °C for three weeks. Then, the rice plants were inoculated with a spore suspension containing 3 × 105 spores/mL of M. oryzae pathotype P7.2. Finally, the leaves of treated and mock inoculated (water inoculated) or control plants were collected separately 31 h after inoculation and frozen immediately in liquid nitrogen to facilitate nucleic acid extraction.
Table 1. Yield and susceptibility of 16 Malaysian rice varieties to blight disease, brown planthopper and sheath blight disease.
RNA extraction methods
Modified method 1 (CTAB method)
One hundred milligrams of each frozen sample were ground in liquid nitrogen to make a fine powder, using a pre-cooled (overnight at −20 °C) mortar and pestle, followed by transfer to a 2 mL Eppendorf tube. One millilitre of pre-warmed (65 °C) extraction buffer 1 [cetyl trimethylammonium bromide (2% w/v CTAB), Tris-base (0.1 mol/L, pH 8.0), ethylenediaminetetraacetic acid (25 mmol/L EDTA), polyvinylpyrrolidone (2% w/v PVP), NaCl (1.4 mol/L) and 100 mL of diethylpyrocarbonate (DEPC) treated water] was added to each tube, and the sample mixture was vortexed for a short time, followed by extraction using 500 µL of chloroform:isoamyl alcohol (CI) (24:1). The mixture was vortexed (high speed) at room temperature (25 °C) for 10 min and centrifuged at 13,000 g for 5 min at 4 °C. Next, the upper phase of each tube was transferred to another Eppendorf tube, and 250 µL of CI was added. The mixture was briefly vortexed and centrifuged at 13,000 g for 5 min at 4 °C. The aqueous phase was transferred to a new 2 mL Eppendorf tube containing 2 volumes of cold isopropanol (4 °C), and then each tube was incubated on ice for 5 min and centrifuged at 13,000 g for 10 min at 4 °C, followed by important modification stages: a short spin for 2 min at 14.1 g. A white liquid phase was subsequently observed. The supernatant was removed; the same volume of cold isopropanol (4 °C) as the last step was added; and the mixture was centrifuged for 5 min (13,000 g, 4 °C). Finally, the obtained white pellets were washed using 70% cold ethanol, air-dried for 20–30 min and re-suspended in 30 µL of DEPC-treated autoclaved water [Citation18].
Modified method 2 (SDS method)
Fresh three-week-old rice leaves (approximately 0.1 g) were frozen using liquid nitrogen, ground into a fine powder using a pre-chilled mortar and pestle and transferred into a 1.5 mL tube. Seven hundred microlitres of extraction buffer 2 [sodium dodecyl sulphate (1% w/v SDS), Tris-base (50 mmol/L, pH 8.0), EDTA (25 mol/L), PVP (4% w/v), NaCl (0.25 mol/L) and 100 mL of DEPC treated water] was added to each tube, mixed with 700 µL of CI (24:1) and vigorously vortexed to thoroughly resuspend the samples. The mixture in each tube was centrifuged at 12,860 g for 5 min at room temperature (25 °C). The aqueous phase was transferred to a new Eppendorf tube, and then an equal volume of phenol:chloroform:isoamyl alcohol (25:24:1) was added, and the mixture was centrifuged at 12,860 g for 10 min at room temperature (25 °C). The upper phase was transferred to a new Eppendorf tube, and then 1/10 volume of 3 mol/L sodium acetate (pH 5.2) and 2.5 volumes of cold absolute ethanol were added, mixed well and incubated at 4 °C for 30 min. The solution was centrifuged at 12,860 g for 20 min at 4 °C. The extraction process was stopped at the end of this step. Lastly, the pellet was rinsed with 70% cold ethanol, air-dried for 15 min and dissolved in 30 µL of DEPC-treated water [Citation19].
Modified method 3 (TRIzol method)
Approximately 0.1 g of fresh rice leaves was frozen and ground in a pre-cooled mortar and pestle using liquid nitrogen to obtain a soft powder of sample and then transferred into a 2 mL tube. The tissue powder sample was lysed in 1.5 mL of TRIzol Reagent (Invitrogen, Carlsbad, California, United States) by pipetting up and down. After incubation of the sample for 5 min at room temperature or 60 °C to permit the complete separation of nucleoprotein compounds, 0.2 mL of chloroform (1/5 volume of TRIzol) was supplemented per 1 mL of TRIzol Reagent. The sample was shaken vigorously for 15 s and incubated for 5 min at room temperature. The mixture was centrifuged at 12,000 g for 15 min at 4 °C to achieve a biphasic solution containing the lower phenol–chloroform phase with red colour and the upper colourless aqueous phase. The aqueous phase was transferred into a new Eppendorf tube, and 70% of the aqueous phase (1/2 TRIzol volume) of isopropanol was added to precipitate the RNA from the aqueous phase. The sample was kept at room temperature (25 °C) for 10 min and centrifuged at 18,000 g for 10 min at 4 °C. Subsequently, the supernatant was discarded, and 70% ethanol was added to the white RNA pellet and centrifuged at 13,000 g for 5 min at 4 °C. Lastly, the pellet was air dried for 10 min and dissolved in 30 μL of DEPC-treated water [Citation20].
Method 4 (Invitrogen method)
Approximately 0.1 g of fresh rice leaves was frozen and ground using liquid nitrogen in a pre-chilled (overnight at −20 °C) mortar and pestle to obtain a fine powder and subsequently transferred to a 2 mL Eppendorf tube. Five hundred microlitres of extraction buffer 2 [SDS (1% w/v), EDTA (25 mol/L), NaCl (0.25 mol/L), Tris-base (50 mmol/L, pH 8.0), PVP (4% w/v) and 100 mL of DEPC treated water] was added to each tube, and the mixture was vortexed for 30 s. Then, 250 µL of chloroform–isoamyl alcohol was added to each tube, and the mixture was vortexed. Next, the mixture was centrifuged at 13,000 g for 5 min at room temperature. The upper phase was transferred to a new centrifuge tube. Then, 0.1 mL of 5 mol/L NaCl was added, and the tube was tapped to mix the sample well. At the next step, 0.3 mL of chloroform was added, and the mixture was mixed thoroughly by inversion. The sample was centrifuged at 12,000 g for 10 min at 4 °C. Subsequently, the top aqueous phase was transferred to an RNase-free tube, and then an equal volume of isopropyl alcohol was added. The mixture was mixed and allowed to sit at room temperature for 10 min, followed by centrifugation at 12,000 g for 10 min at 4 °C. The supernatant was poured off, and 1 mL of 75% ethanol was added to the pellet, followed by centrifugation at 12,000 g for 5 min at 4 °C. Finally, the pellet was air dried and dissolved in 30 µL of DEPC-treated autoclaved water [Citation21].
Method 5(RNeasy plant mini kit) (Qiagen, Hilden, North Rhine-Westphalia, Germany)
One hundred micrograms of rice leaves were frozen and ground thoroughly using a pre-cooled mortar and pestle with liquid nitrogen to obtain a fine powder and then transferred into a 2 mL Eppendorf tube. Next, 450 μL of the lysis buffer RLC was added and vortexed vigorously, and then the lysate was transferred to a QIAshredder spin column using a 2 mL collection tube. The samples were centrifuged for 2 min at 14,000 g, and then the supernatant of the flow-through was transferred to a new 1.5 mL tube. Next, 0.5 volumes of ethanol (96%–100%) was added to each tube and immediately mixed by pipetting. Subsequently, the sample was transferred to an RNeasy spin column (pink), placed in a 2 mL collection tube, and centrifuged for 15 s at 10,000 g. The flow-through was discarded, and then 700 μL of Buffer RW1 was added to the RNeasy spin column and centrifuged for 15 s at 10,000 g, and then the emerged flow-through at this step was discarded. Next, 500 μL of Buffer RPE was added to the RNeasy spin column and centrifuged for 15 s at 10,000 g. The flow-through was discarded, and 500 μL of Buffer RPE was added to the RNeasy spin column and centrifuged for 2 min at 10,000 g. The RNeasy spin column was placed in a new 1.5 mL collection tube, and 30 μL of RNase-free water was added directly to the membrane of a spin column and centrifuged for 1 min at 10,000 g to elute the RNA.
Quality and integrity of extracted RNA
Analysis of ribonucleic acid integrity
Protein and phenol/carbohydrate contaminations were considered based on the A260/280 and A260/230 records, respectively, using a Nanodrop ND-1000 spectrophotometer (ImplenNanoPhotometer, Munich, Bavaria, Germany). Three millilitre aliquots of total RNA were also loaded onto 1.5% ethidium-bromide-stained agarose gel.
Semi-quantitative PCR (sqPCR) analysis
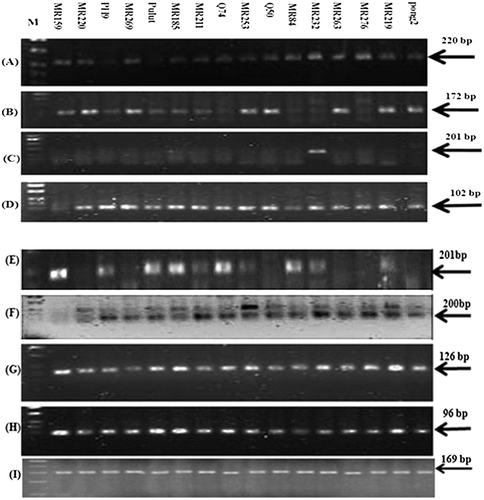
Reverse transcriptase PCR was performed to assess the quality of total RNA before further processing. Total cellular RNA was extracted from the leaves of the 16 varieties of rice previously treated with a spore suspension of M. oryzae for 31 h and untreated plants. A concentration of 1 µg/µL total RNA was transcribed into first-strand cDNA fragments using Super Script III (Invitrogen, Waltham, Massachusetts, USA). The reactions were incubated at 50 °C for 60 min and heated for inactivation at 70 °C for 15 min. Then, the template cDNAs for both controls (untreated) and treated samples were amplified using eight different primers designed according to their cDNA sequence homologies using the Primer Premier 5.0 software (PREMIER Biosoft, Palo Alto, California, USA) ().
Table 2. List of primers used for semi-quantitative and real-time PCR amplification of 10 different genes.
The following PCR (Taq DNA Polymerase, Vivantis, Oceanside, California, USA) programme was used: 94 °C for 2 min and 35 cycles of 94 °C for 30 s, followed by 55.2 °C, 60 °C, 54 °C, 60 °C, 60 °C, 52.3 °C, 62 °C, 62 °C and 52 °C, respectively, for Pikh, Pi9, Pib, OsWRKY22, Pia, Pita, Pi21, OsWRKY45 and 18S rRNA for 30 s and 72 °C for 60 s. The PCR programme was concluded with a final extension at 72 °C for 7 min. The 18S rRNA was amplified using the same cDNA templates as a reference gene. The PCR products were then run, separated on a 1.5% agarose gel and stained with ethidium bromide.
Real-time PCR analysis
The expression levels of eight blast resistant genes, Pikh, Pib, Pita, Pi21, Pi9, Os11gRGA8, OsWRKY22 and OsWRKY45, were evaluated by real-time PCR 31 h post inoculation, the time required for the blast fungus to ingress through the cell wall and enter epidermal cells [Citation22]. The α-tubulin and rRNA genes were amplified as two internal control genes, using specific primers (). A 1 µL aliquot of RNA from each sample was used to prepare 20 µL reaction volumes (based on the KAPA SYBER FAST One-Step RT-quantitative PCR, KAPA BIOSYSTEMS, Wilmington, Delaware, USA). The cycling conditions included a primary incubation step at 42 °C for 5 min and reverse transcriptase inactivation at 95 °C for 5 min and 40 cycles at 95 °C for 3 s, followed by 55.2 °C, 60 °C, 54 °C, 60 °C, 60 °C, 52.3 °C, 62 °C, 62 °C, 57.6 °C and 52 °C, respectively, for Pikh, Pi9, Pib, OsWRKY22, Os11gRGA8, Pita, Pi21, OsWRKY45, α-tubulin and 18S rRNA for 30 s and 72 °C for 3 s. For these analyses, all data were obtained from three independent biological replicates. Amplicon specificity was assessed by melting curve analysis after 40 cycles of increasing temperature from 60 °C to 95 °C (CFX96 Touch™ real-time PCR detection system, Biorad, Hercules, California, USA).
Data analysis
The collected data were standardized and statistically analysed using SAS software, version 9.3 (SAS Institute Inc., Cary, North Carolina, USA). The means comparison was performed by the Duncan test. The data obtained from real-time PCR were analysed by the Relative Expression Software Tool (Qiagen, Hilden, North Rhine-Westphalia, Germany).
Results and discussion
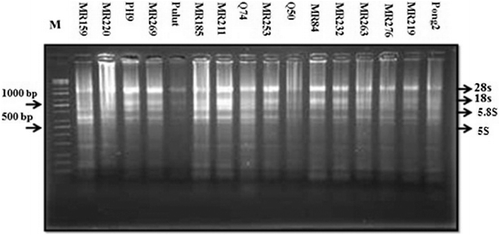
The quantity, quality and integrity of extracted RNA were examined using three different methods. First, the ratios of A260/280 for five methods ranged from 2.12 to 2.20. Second, agarose gel electrophoresis illustrated intact and sharp bands representing the 28S, 18S, 5.8S and 5S ribosomal subunits of RNA, presenting non-degraded RNA (). Finally, the quality and quantity of RNA were verified using semi-quantitative PCR (sqPCR). This analysis revealed that the extraction of RNA from the leaves of rice differs amongst the five methods ( and ).
Figure 1. Separation of ribonucleic acids, RNA, extracted from rice leaves using five methods. Lane 1: method 1 (CTAB); lane 2: method 2 (SDS); lane 3: method 3 (TRIzol); lane 4: method 4 (Invitrogen); and lane 5: method 5 (Qiagen kit) with 1.5% agarose gel electrophoresis and ethidium bromide staining (2 µg/mL for 10 min). Marker: O'GeneRuler TM DNA Ladder Mix (Fermentas, Waltham, Massachusetts, USA).
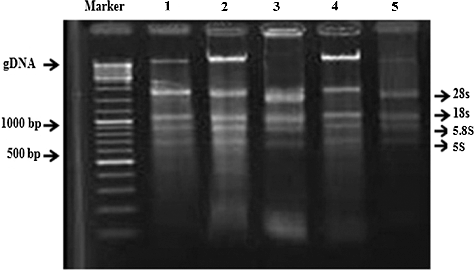
Table 3. ANOVA of concentration and purity of extracted total RNA from leaves of rice using five different protocols.
Table 4. Integrity and average yields of extracted RNA from rice leaves using five different extraction protocols and their mean comparison.
Comparison of different methods for RNA extraction from rice leaves
The RNeasy plant mini kit (method 5) did not produce high quality or quantity total RNA due to considerable DNA contamination. Two modified procedures, methods 1 (CTAB) and 2 (SDS), and the RNA extraction method 4 (Invitrogen) were tested practically to examine the efficiency of these methods over RNA extraction from rice leaves. The average concentrations of the RNA extracted from the leaves of rice using method 1 to 5 were 412.4, 668.2, 1781, 726.2 and 343.2 ng µL−1, respectively ( and ). The highest A260/280 ratios were scored using method 3 (TRIzol) (2.20 ng/µL) and method 1 (CTAB) (2.18 ng/µL).
The integrity and yield of extracted ribonucleic acids depend on several chemical substances, including isopropanol, β-mercaptoethanol and acid guanidinium thiocyanate, or even the replication of some essential steps involved in these extraction methods, which can differ in diverse tissues [Citation23–25]. The integrity of extracted RNA from the rice leaves increased significantly using method 3 (TRIzol), and the highest RNA yield was obtained by this method. TRIzol is an acid guanidinium thiocyanate that eliminates protein and DNA contamination [Citation24,26]. To enhance the RNA concentration using method 1 (CTAB), upon addition of extraction buffer at the second step, β-mercaptoethanol (2%) was added to the mixture, followed by the remaining modified steps [Citation27]. The SDS increased the concentration of RNA extracted using method 2 (SDS) and the Invitrogen kit (method 4). Both modified methods (CTAB and SDS) and the Invitrogen kit were fast and reliable for the extraction of intact RNA from the rice leaves for additional molecular analyses, such as real-time PCR and sqPCR. However, there were also some limitations to completely avoiding genomic DNA contamination. RNA samples should be treated with DNase prior to use in subsequent analyses. Moreover, treatment of RNA with DNase, reduces the RNA yield. The sodium acetate used in method 2 (SDS method) removed the enlacing polysaccharides of nucleic acids during RNA precipitation, whereas β-mercaptoethanol inhibited the oxidation reactions, thereby obtaining a high quantity of extracted RNA of high integrity [Citation27].
TRIzol (guanidium thiocyanate–phenol–chloroform) is able to maintain the RNA integrity during cell disruption and homogenization [Citation28]. In method 3 (TRIzol), the addition of chloroform to the sample following centrifugation results in its separation into aqueous and organic phases. RNA remaining in the aqueous phase could subsequently be recovered using isopropanol. To assess the purity of the RNA, spectrophotometric readings at 280 and 260 nm are commonly used for estimation of RNA integrity [Citation29].
Amplification of eight blast resistance genes amongst 16 varieties of rice using sqPCR
The quality of the extracted RNA was also evaluated using sqPCR. Using the TRIzol-extracted total RNA () as the initial nucleic acid template for RT-PCR, the expected amplicon was observed (), showing that the RNA was not contaminated with any trace of genomic DNA; moreover, the absence of the other PCR amplicon using the same and specific primers demonstrated the presence of intact and pure RNA. The results of reverse transcriptase PCR also confirmed that the extracted RNA was of satisfactory quality for use in reverse transcription to generate cDNA in subsequent experiments. Diverse expression patterns of blast resistance genes are illustrated in . sqPCR is sensitive and specific for the detection of rare mRNA and the evaluation of the transcripts obtained from a small amount of the initial sample [Citation30]. Generally, qualitative analysis of RNA alone is not sufficient to assess RNA integrity. Therefore, the quantification of particular RNA transcripts and the detection of any alterations in their expression profiling under various stresses are required for analysis of the molecular mechanisms underlying plant stress responses [Citation31].
Expression study of rice blast resistance genes
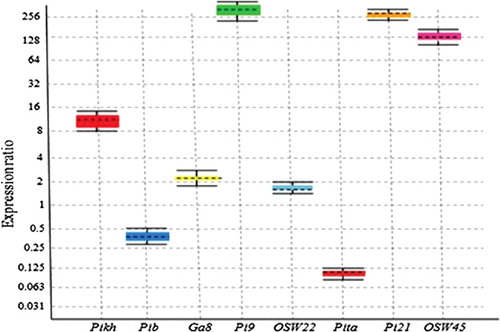
Gene expression profiling of eight blast resistance genes was performed to identify up-regulated genes in the MR276 rice variety infected by M. oryzae. RT-qPCR was used to compare the variance in gene expression between control and M. oryzae-infected rice plants. Various expression patterns of the Pikh, Pib, Os11gRGA8, OsWRKY22 and OsWRKY45 genes were detected 31 h after the inoculation of rice plants (), when the blast fungus emerged through the cell wall and entered into the epidermal cell [Citation22]. The results of this study showed that the Pikh, Pib, GA8, Pi9, Osw22, Pita, Pi21 and Osw45 genes were up-regulated significantly in MR276, whereas the Pita gene was down-regulated in the infected plants compared with the control plants (). Gene expression analysis has been used broadly to discover biological responses against various stressors such as expression analysis of receptor genes conferring blast resistance in rice [Citation32]. The RNA extracted from rice leaves using method 3 (TRIzol) showed good quality, leading to the precise differential expression of eight blast resistance genes and 18S rRNA/tubulin genes in real-time PCR. The evaluation of RNA integrity is an essential and initial step in accessing accurate gene expression data [Citation1]. Therefore, to achieve the predicted results, high quality initial resources should be used.
Table 5. Relative expression patterns of eight genes and 18SrRNA/tubulin reference genes in MR276 rice variety.
Figure 4. Relative expression levels of Pikh, Pib, Os11gRGA8 (GA8), OsWRKY22 (Osw22), Pita, Pi21 and OsWRKY45 (Osw45) genes calibrated using 18S rRNA/tubulin reference genes in infected and control MR276 plants by relative quantitative real-time PCR.
In the rice blast gene-for-gene system, R family genes encode nucleotide-binding site (NBS) proteins with leucine rich repeat (LRR) domains, except Pid2 and Pi21, which encode a receptor-like kinase [Citation33] and a proline-rich protein [Citation34], respectively. To date, only 20 out of 85 identified blast resistance genes have been cloned [Citation13]. Pikh, Pib and Pita genes are major blast resistance genes involved in the immunity of Indica rice varieties against M. oryzae in Malaysia [Citation35,36]. Induced blast resistance has been observed in Indica rice lines containing Pi9 and Os11gRGA8 genes that encode NBS-LRR protein [Citation37]. In addition, on the rice chromosome 11, Os11gRGA8 is closely linked to Pia, another major blast resistance gene [Citation18]. Various transcription factors are involved in rice resistance to blast disease, such as WRKY family proteins, which bind the promoter of W-box defence genes to control their expression [Citation38,39]. A wide range of WRKY genes are expressed in response to biotic stresses, such as blast fungi and bacterial blight diseases [Citation40–43]. An up-regulation of Pikh, Pi9, Pi21, and Osw45 genes has also been reported earlier under M. oryzae pathotype P7.2 infection in the leaves of Malaysian rice at 31 h after inoculation [Citation44]. According to a previous morphological study, the MR276 Indica rice variety is highly resistant to M. oryzae pathotype P7.2 in Malaysia [Citation45]. Thus, it can be assumed that the simultaneous activation and high expression of these network genes induced the resistance of the MR276 rice variety against M. oryzae pathotype P7.2 by preventing either the growth or the penetration of fungal populations in rice plants.
Conclusions
Overall, the results from this study indicate that the TRIzol method is the best and most suitable protocol for isolating intact and high-yield RNA from rice leaves without any DNA contamination. Although the total RNA extracted by the other methods also showed high yield, these methods did not avoid DNA contamination. RNA extracted by these methods should be treated with DNase, which reduces the RNA concentration significantly. Finally, three approaches were used to verify the quantity, quality and integrity of the RNA, confirming the suitability of the TRIzol method for the extraction of RNA from rice leaves. In addition, the results of real-time PCR confirmed that the simultaneous up-regulation of seven blast resistance genes (Pikh, Pib, Pi21, Pi9, Os11gRGA8, OsWRKY22 and OsWRKY45) may be due to resistance of the MR 276 variety against M. oryzae pathotype P7.2 31 h post-inoculation.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Fleige S, Pfaffl MW. RNA integrity and the effect on the real-time qRT-PCR performance. Mol Aspects Med. 2006;27:126–139.
- Imbeaud S, Graudens E, Boulanger V, et al. Towards standardization of RNA quality assessment using user-independent classifiers of microcapillary electrophoresis traces. Nucl Acids Res [Internet]. 2005. [cited 2016 Apr 26];33:e56. Available from: http://nar.oxfordjournals.org/content/33/6/e56.long
- Zhu QZ, Li F, Guo XQ, et al. Application of a novel fluorescence probe in the determination of nucleic acids. Analyst. 1997;122:937–940.
- Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol. 2000;25:169–193.
- Nolan T, Hands RE, Bustin SA. Quantification of mRNA using real-time RT-PCR. Nat Protoc. 2006;1:1559–1582.
- Bustin SA, Nolan T. Pitfalls of quantitative real-time reverse-transcription polymerase chain reaction. J Biomol Tech. 2004;15:155–166.
- Mannhalter C, Koizar D, Mitterbauer G. Evaluation of RNA isolation methods and reference genes for RT-PCR analyses of rare target RNA. Clin Chem Lab Med. 2000;38:171–177.
- Swift GH, Peyton MJ, MacDonald RJ. Assessment of RNA quality by semi-quantitative RT-PCR of multiple regions of a long ubiquitous mRNA. Biotechniques. 2000;28:524–531.
- Vermeulen J, De Preter K, Lefever S, et al. Measurable impact of RNA quality on gene expression results from quantitative PCR. Nucl Acids Res [Internet]. 2011 [cited 2016 Feb 14];39:e63. Available from: http://nar.oxfordjournals.org/content/39/9/e63.long
- Pfaffl MW. Quantification strategies in real-time PCR. In: Bustin SA, editor. AZ of quantitative PCR. La Jolla (CA): International University Line (IUL); 2004. p. 87–112.
- Imam J, Alam S, Mandal NP, et al. Molecular screening for identification of blast resistance genes in North East and Eastern Indian rice germplasm (Oryza sativa L.) with PCR based makers. Euphytica. 2013;196:199–211.
- Dean RA, Talbot NJ, Ebbole DG, et al. The genome sequence of the rice blast fungus Magnaporthe grisea. Nature. 2005;434:980–986.
- Azizi P, Rafii MY, Abdullah SNA, et al. Toward understanding of rice innate immunity against Magnaporthe oryzae. Crit Rev Biotechnol. 2016;36:165–174.
- Garcia AV, Hirt H. Salmonella enterica induces and subverts the plant immune system. Front Microbiol [Internet]. 2014 [cited 2016 Feb 14];5:1–6. Available from: http://journal.frontiersin.org/article/10.3389/fmicb.2014.00141/full
- Sahebi M, Hanafi MM, Wong MY, et al. Towards immunity of oil palm against Ganoderma fungus infection. Acta Physiol Plant. 2015;37:1–16.
- Dangl JL, Jones JD. Plant pathogens and integrated defence responses to infection. Nature. 2001;411:826–833.
- Jeung J, Kim BR, Cho YC, et al. A novel gene, Pi40 (t), linked to the DNA markers derived from NBS-LRR motifs confers broad spectrum of blast resistance in rice. Theor Appl Genet. 2007;115:1163–1177.
- Kiefer E, Heller W, Ernst D. A simple and efficient protocol for isolation of functional RNA from plant tissues rich in secondary metabolites. Plant Mol Biol Rep. 2000;18:33–39.
- Chan KL, Ho CL, Namasivayam P, et al. A simple and rapid method for RNA isolation from plant tissues with high phenolic compounds and polysaccharides. Protoc Exchange [Internet]. 2007 [cited 2016 Feb 14];184:1–15. Available from: http://www.nature.com/protocolexchange/protocols/208
- Simms D, Cizdziel PE, Chomczynski P. TRIzol: a new reagent for optimal single-step isolation of RNA. Focus. 1993;15:532–535.
- Rubio-Pina JA, Zapata-Perez O. Isolation of total RNA from tissues rich in polyphenols and polysaccharides of mangrove plants. Electron J Biotechnol [Internet]. 2011 [cited 2016 Feb 14];14:1–8.. Available from: http://www.ejbiotechnology.info/index.php/ejbiotechnology/article/view/778
- Parker D, Beckmann M, Enot DP, et al. Rice blast infection of Brachypodium distachyon as a model system to study dynamic host/pathogen interactions. Nat Protoc. 2008;3: 435–445.
- Blin N, Stafford DW. A general method for isolation of high molecular weight DNA from eukaryotes. Nucl Acids Res. 1976;3:2303–2308.
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159.
- Smale G, Sasse J. RNA isolation from cartilage using density gradient centrifugation in cesium trifluoroacetate: an RNA preparation technique effective in the presence of high proteoglycan content. Anal Biochem. 1992;203:352–356.
- Meng L, Feldman L. A rapid TRIzol-based two-step method for DNA-free RNA extraction from Arabidopsis siliques and dry seeds. Biotechnol J. 2010;5:183–186.
- Sahebi M, Hanafi MM, Siti Nor AA, et al. Extraction of total RNA from mangrove plants to identify different genes involved in its adaptability to the variety of stresses. Pak J Agric Sci. 2013;50:529–536.
- Xiang X, Qiu D, Hegele RD, et al. Comparison of different methods of total RNA extraction for viral detection in sputum. J Virol Methods. 2001;94:129–135.
- Chomczynski P, Sacchi N. The single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction: twenty-something years on. Nat Protoc. 2006;1:581–585.
- Carding SR, Lu D, Bottomly K. A polymerase chain reaction assay for the detection and quantitation of cytokine gene expression in small numbers of cells. J Immunol Methods. 1992;151:277–287.
- Marone M, Mozzetti S, De Ritis D, et al. Semiquantitative RT-PCR analysis to assess the expression levels of multiple transcripts from the same sample. Biological Proced Online. 2001;3:19–25.
- Heid CA, Stevens J, Livak KJ, et al. Real time quantitative PCR. Gen Res. 1996;6:986–994.
- Chen X, Shang J, Chen D, et al. AB-lectin receptor kinase gene conferring rice blast resistance. Plant J. 2006;46:794–804.
- Fukuoka S, Saka N, Koga H, et al. Loss of function of a proline-containing protein confers durable disease resistance in rice. Science. 2009;325:998–1001.
- Tanweer FA, Rafii MY, Sijam K, et al. Cloning and characterization of two major blast resistance genes Pi-b and Pi-kh from Malaysian rice variety Pongsu Seribu 2. Plant Omics J. 2015;8:257–263.
- Azizi P, Rafii MY, Abdullah SNA, et al. Over-expression of the Pikh gene with a CaMV 35S promoter leads to improved blast disease (Magnaporthe oryzae) tolerance in rice. Front Plant Sci [Internet]. 2016 [cited 2016 Oct 30];7:1–14. Available from: http://journal.frontiersin.org/article/10.3389/fpls.2016.00773/full
- Liu G, Lu G, Zeng L, et al. Two broad-spectrum blast resistance genes, Pi9 (t) and Pi2 (t), are physically linked on rice chromosome 6. Mol Genet Gen. 2002;267:472–480.
- Rushton PJ, Torres JT., Parniske M, et al. Interaction of elicitor-induced DNA-binding proteins with elicitor response elements in the promoters of parsley PR1 genes. EMBO J. 1996;15:5690–5700.
- Maleck K, Levine A, Eulgem T, et al. The transcriptome of Arabidopsis thaliana during systemic acquired resistance. Nat Genet. 2000; 26:403–410.
- Ryu HS, Han M, Lee SK, et al. A comprehensive expression analysis of the WRKY gene superfamily in rice plants during defense response. Plant Cell Rep. 2006;25:836–847.
- Wen N, Chu Z, Wang S. Three types of defense-responsive genes are involved in resistance to bacterial blight and fungal blast diseases in rice. Mol Genet Gen. 2003;269:331–339.
- Shimono M, Koga H, Akagi AYA, et al. Rice WRKY45 plays important roles in fungal and bacterial disease resistance. Mol Plant Pathol. 2012;13:83–94.
- Liu Y, Qi X, Young ND, et al. Characterization of resistance genes to rice blast fungus Magnaporthe oryzae in a "Green Revolution" rice variety. Mol Breed. 2015;35:1–8.
- Azizi P, Rafii MY, Mahmood M, et al. Differential gene expression reflects morphological characteristics and physiological processes in rice immunity against blast pathogen Magnaporthe oryzae. PloS one [Internet]. 2015 [cited 2016 Oct 30];10:e0126188. Available from: http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0126188
- Azizi P. Development of transgenic rice variety MR219 resistant to blast disease through transformation with Pikh gene [dissertation]. Serdang: Institute Tropical Agriculture, Universiti Putra Malaysia; 2015.