ABSTRACT
Intercellular adhesion molecule-1 (ICAM-1), a member of the large immunoglobulin superfamily of cell adhesion molecules, is a constituent component of the blood–testis barrier, and it plays a significant role in the homeostasis of spermatogenesis. The E469K polymorphism in the human ICAM-1 gene has been previously associated in various inflammatory/autoimmune disorders. However, the role of the ICAM-1 E469K polymorphism in spermatogenesis remains unclear. The aim of the present study is to analyse the possible association between the ICAM-1 E469K polymorphism and male infertility with non-obstructive azoospermia (NOA) patients within a group of men from Turkey. We included 111 infertile male with NOA and 114 fertile male as control subjects to the study. Genotyping was made by polymerase chain reaction–restriction fragment length polymorphism. The frequency of the genotype and the allele of ICAM-1 E469K were not significantly different between the control group and the patients (P > 0.05). This is the first study to investigate the role of the ICAM-1 gene polymorphism in male infertility with NOA. We conclude that the E469K polymorphism of ICAM-1 is not a risk factor for NOA in the Turkish population.
Introduction
Infertility is a worldwide reproductive health problem defined as the inability to conceive after one year of regular unprotected intercourse, and it affects 10%–15% of all couples worldwide. Male factors account for approximately 50% of all infertility cases, and about 60%–75% of these cases are still unexplained or idiopathic. The most common cause of idiopathic male infertility is impaired spermatogenesis, in which azoospermia is present in about 10%–15% of infertile men [Citation1,Citation2]. Azoospermia is defined as the absence of sperm in semen, and can be typically divided into two major categories: obstructive azoospermia and non-obstructive azoospermia (NOA) in which the testicle fails to produce mature sperm in the ejaculate [Citation2].
There are numerous causes of NOA, ranging from hormonal, environmental/lifestyle factors, reactive oxygen species, epigenetic abnormalities and genetic factors, such as the mutations of chromosomal, gene and mitochondrial DNA [Citation1,Citation2]. Yq microdeletions are the best-known gene mutations leading to impaired spermatogenesis, which may involve several genes within the azoospermia factor region. Moreover, a number of mutations of novel or known autosomal genes have been considered to be associated with male infertility in humans. These genes include TAF4B and ZMYND15, SYCP3, NR5A1, MTHFR, SHBG, FSHR, YBX2 and ART3 as well as TAF7 L on the X-chromosome [Citation2]. Despite the large number of genes known to contribute to impaired spermatogenesis, the total contribution of these genes to the condition is very small [Citation2].
During spermatogenesis, Sertoli–Sertoli and Sertoli–germ cell junctions have an important role in germ cell movement in the seminiferous epithelium. For spermatogenesis to reach completion, restructuring takes place at these junctions because developing germ cells are non-motile, and must move ‘up and down’ the seminiferous epithelium to accommodate the cellular events of mitosis, meiosis, spermiogenesis and spermiation [Citation3–5]. Furthermore, preleptotene spermatocytes at the basal compartment must traverse the blood–testis barrier (BTB), also known as the Sertoli cell barrier, to enter the adluminal compartment. Thus, it is indispensable that restructuring and turnover take place at the Sertoli cell–cell and Sertoli–germ cell junctions during spermatogenesis [Citation3–5]. If germ cell adhesion were to be compromised for a period of time longer than usual, or spermatocytes cannot traverse the BTB, germ cells would slough from the seminiferous epithelium, spermatogenesis would be arrested and subfertility or infertility may result [Citation5]. It has been shown that the restructuring of the Sertoli–Sertoli and Sertoli–germ cell adhesion involves some changes in cytoskeleton and cellular adhesion molecules such as cadherins, claudin and integrins to regulate the junction-restructuring events during spermatogenesis. However, recent research advances have shown that members of the ICAM family, especially ICAM-1, are also likely regulatory molecules that coordinate these events [Citation4].
ICAM-1 encodes a member of the immunoglobulin superfamily of cell adhesion molecules. ICAM-1 is located on chromosomes 19p13.3–p13.2 and includes 7 exons. Several single-nucleotide polymorphisms (SNPs) of ICAM-1 have been previously reported [Citation6]. Of these SNPs, the ICAM-1 gene 1462A>G (E469K;rs5498) polymorphism has been identified (i.e. an A to G substitution in codon 469) in Exon 6 of the ICAM-1 gene. This SNP is a non-synonymous substitution resulting in an amino acid substitution from glutamic acid (E) to lysine (K) [Citation7,Citation8]. It is reported that this SNP is common in all populations and might influence the serum level and activity of ICAM-1. This polymorphism has been shown to be associated with several syndromes, such as coronary heart disease (CHD) [Citation8], multiple sclerosis [Citation9], type 1 diabetes [Citation10], colorectal cancer [Citation11] and Alzheimer’s disease [Citation12]. However, the association between ICAM-1 SNPs and male infertility has not yet been studied previously. To the best of our knowledge, no study has been reported to investigate the association between the ICAM-1 E469K polymorphism and male infertility with NOA. Therefore, the aim of this study is to investigate the possible role of the E469K polymorphism in the ICAM-1 gene in spermatogenesis impairment. Thus, we carried out a case-control study to explore the possible association between this SNP in the ICAM-1 gene and male infertility with NOA. Understanding the molecular mechanism of abnormal spermatogenesis and the genes involved is important in developing both diagnostic tools and treatment strategies for male infertility [Citation13].
Subjects and methods
Study population
One hundred and eleven infertile men with NOA (mean age 32.4 ± 5.2 years) and 114 fertile controls of comparable age group (36.9 ± 6.7 years on average) in south-east Turkey were recruited in this study. Only idiopathic infertile men with NOA, with at least one year of infertility, were included. Individuals with known causes of infertility, including genetic factors (chromosome anomalies, azoospermia factor [AZF] microdeletions), lifestyle factors (e.g. alcoholism and occupation), clinical factors affecting the fertility (drug intake, infections, hereditary hypothalamic-pituitary abnormalities, varicocele and cryptorchidism, etc.) and men whose partner had factors involved in infertility were excluded from this study. The control group was selected from healthy men who have had fathered at least one healthy child within one year without assisted reproductive measures. Both the men participating in the infertility workup as well as the fertile controls were recruited within the same geographical region.
All studied fertile and infertile men were referred from the Urology Department of Dicle University Hospital to the Medical Biology and Genetics Department. The study was approved by the Ethics Committee of Dicle University's Faculty of Medicine and all participants gave their informed consent (87/26.02.2016).
SNPs selection and genotyping of ICAM-1 gene polymorphisms
In the present study, we selected one non-synonymous SNP, 1462A>G (E469K; rs5498) of the ICAM-1 gene for genotyping [Citation10]. This polymorphism is located in Exon 6 of the ICAM-1 gene. The SNP ID number and detailed sequence information is available in the public SNP database (http://www.ncbi.nlm.nih.gov/SNP/).
Heparinised peripheral venous blood (2 mL) was collected from each subject and stored in tubes containing ethylenediaminetetra acetic acid at −20 °C until the DNA extraction step. Genomic DNA was extracted from whole blood by salting out [Citation14].
Polymerase chain reaction–restriction fragment length polymorphism (PCR-RFLP) was used to determine the SNP with appropriate primer sets and restriction enzyme as previously described by Miller et al. [Citation15]. The primer sets and enzymes used in this study are shown in
Table 1. Primer sequences, annealing temperature, restriction enzyme and allele sizes used for the ICAM-1 gene E469K polymorphism.
The PCR reaction was carried out in a 20 μL reaction volume containing 1×PCR buffer, 2 mmol/L MgCl2, 0.2 mmol/L of each deoxynucleotide triphosphate (dNTPs; Fermentas, St. Leon-Rot, Germany), 80 ng of DNA, 0.2 μmol/L of each primer (Bio Basic Inc., Markham, Canada) and 1 unit of Taq DNA Polymerase (Fermentas). The PCR conditions were: 3 min of initial denaturation at 94 °C, followed by 30 amplification cycles. Each cycle consisted of denaturation at 94 °C for 30 s, 58 °C annealing for 30 s and extension at 72 °C for 30 s, with a final extension step of incubation at 72 °C for 5 min (SensoQuest labcycler, SensoQuest GmbH, Göttingen, Germany).
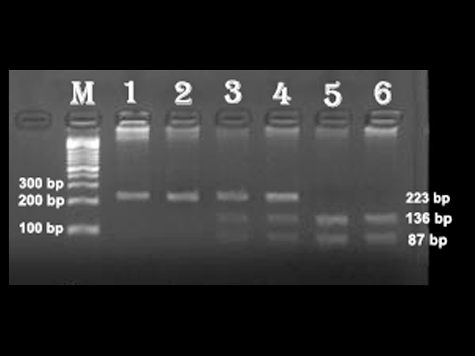
For genotyping of the ICAM-1 gene 1462A>G (E469K) polymorphism, RFLP analysis was carried out by PCR-amplified products followed by BstUI restriction enzyme (New England Biolabs, Beverly, MA, USA) digestion of the PCR-amplified products at 37 °C overnight. The digested products were separated in a 3% agarose gel along with a 100–1500 bp DNA ladder (BioBasic Inc., Markham, Canada) and stained with ethidium bromide. Ethidium bromide-stained gels were analysed using the AlphaImager Imaging System (AlphaInnotech, San Leandro, CA, USA). The homozygous AA genotype yielded one fragment of 223 bp, the polymorphic GG genotype yielded two digestion fragments of 136 and 87 bp and the heterozygous AG genotype yielded three fragments of 223, 136 and 87 bp ().
Statistical analysis
The differences in the genotype and allele frequency were analysed by the chi-squared test. Deviation from the Hardy–Weinberg equilibrium (HWE) for genotypes was analysed. Statistical analyses were performed using SPSS software (version 11.5 for Windows, SPSS Inc., Chicago, IL, USA). Fisher's exact test was used to analyse the genotype and allele frequencies of the polymorphisms. Statistical significance was defined as P ≤ 0.05 and all statistical tests were two-tailed. The results were expressed as means with standard deviation (±SD) if the variables were continuous and as percentage if the variables were categorical.
Results and discussion
Although association of the ICAM-1 E469K polymorphism with several diseases has been previously shown [Citation7], as yet, there is no final conclusion about the association of this polymorphism with diseases. For example, Jiang et al. [Citation16] explored the possible role of the ICAM-1 E469K polymorphism in the susceptibility to CHD and myocardial infarction and found that GG and AG genotypes were possibly associated with susceptibility to these conditions in Germans. Similar results have been demonstrated by several studies in the Egyptian and Chinese population [Citation17,Citation18]. In a meta-analysis study, Ji et al. [Citation8] found that the ICAM-1 E469K polymorphism is associated with CHD risk and the G allele is a more significant risk factor for developing CHD among Asian and Caucasian populations. However, Mo et al. [Citation19] found that the A allele of ICAM-1 E469K might be a genetic risk factor for CHD. In other reports, it is demonstrated that this SNP of the ICAM-1 gene is associated with Behcet's disease in Japanese patients [Citation6], type 1 diabetes in a Swedish population [Citation10] and colorectal cancer in a Chinese population [Citation11]. In contrast, there are also results that report no association between this SNP and diseases, such as ischemic heart disease in the Irish population [Citation20], acromegaly in the Turkish population [Citation7], celiac disease in the north Indian population [Citation21] and non-diabetic metabolic syndrome in the Turkish population [Citation22].
In the present study, the E469K (rs5498) polymorphism in the ICAM-1 gene was identified in the patients with NOA and the control subjects within a Turkish population. The genotype and allele frequencies of this SNPs among the infertile men and fertile controls are summarized in . To the best of our knowledge, the present study is the first to investigate the association between the ICAM-1 E469K polymorphism and the risk of developing NOA. We found no association between the ICAM-1 E469K polymorphism and NOA in the Turkish population (P > 0.05). However, further analysis needs to cover a much larger population or other ethnic groups as well.
Table 2. Genotype and allele frequencies of the ICAM-1 gene among patients with idiopathic azoospermia and fertile controls.
The heterozygosity index for rs5498 in the genotype distribution of this population was high both in the patients and the controls: 53.15% and 44.74%, respectively (). These results show similarity with our previous study on the role of this polymorphism in non-diabetic metabolic syndrome in the Turkish population, in which the results did not show any associations between this polymorphism and disease [Citation22]. In addition, the high frequency of the heterozygous genotype for ICAM-1 E469K in the present study is also in accordance with that reported in other previous studies on Turkish, Danish, Finnish, Japanese and Swedish populations [Citation10]. Although our study had a limited number of patients, we could speculate that this situation may reflect that there may be a common ancestor in these populations.
ICAMs are cell–cell and cell–extracellular matrix adhesion receptors that are known to be expressed in a number of epithelial and endothelial cells, leukocytes, platelets, erythrocytes, macrophages, dendritic cells, fibroblasts, some tumour cells, as well as testicular cells [Citation4,Citation5]. It is reported that ICAMs are crucial regulatory molecules of spermatogenesis. ICAM-1 and its biologically active soluble fragment (sICAM-1) generated from the proteolytic cleavage of cell membrane-bound ICAM-1 have antagonistic effects on the Sertoli cell tight junction-permeability barrier. While ICAM-1 maintains the barrier function, sICAM-1 may be used by preleptotene spermatocytes to serve as a likely ‘molecular switch’ to ‘open up’ the BTB [Citation5]. Furthermore, sICAM-1 may also induce the loss of cell adhesion between Sertoli and germ cells for normal spermatogenesis [Citation3–5]. In a recent study, it has been observed that a decrease in ICAM-1 coincides with disintegration of the seminiferous epithelium induced by busulfan, resulting in cessation of spermatogenesis [Citation3]. The genetic polymorphism (rs5498) resulting in the substitution of lysine to glutamate (E469K) in Exon 6 of the ICAM-1 gene is common. Genome-wide association analyses indicate that this substitution may influence ligand binding, the plasma concentration and the activity of the ICAM-1 gene [Citation7]. This substitution also has a role in the genetic regulation of sICAM-1 levels. In our study, however, there was no association between this polymorphism and NOA in the surveyed Turkish population.
Previous in vivo and in vitro studies have shown that the pro-inflammatory cytokines (such as TNF-α and interleukin-1α) and several ICAMs are significant regulators of Sertoli–Sertoli/BTB and Sertoli–germ cell junctions, and differentially expressed during spermatogenesis. Barrier restructuring correlates with ICAM-1 and its soluble forms (sICAM-1), generated from the proteolytic cleavage of the ICAM extracellular domain, suggesting that it may be critical for adhesion as non-motile germ cells traverse the ‘opened’ BTB. It is reported that these regulatory molecules (e.g. inflammatory mediators such as TNF-α) are needed to induce cleavage of the ICAM extracellular domain. Thus, the loss and/or defects of these molecules may cause infertility in animals and humans [Citation3–5,Citation23–26]. Although, the data from immunohistochemical studies demonstrated the role of the ICAM genes on spermatogenesis, there is no molecular genetic study to explain the role of ICAM-1 gene defect in spermatogenesis in both animals and humans. To our knowledge, this is the first study to investigate the association of this polymorphism with idiopathic infertility in men. Such studies will contribute substantially for the identification of genes involved in spermatogenesis. Future studies might unravel whether defects in the gene also lead to infertility in human males.
Conclusions
This study showed that the ICAM-1 E469K polymorphism was not involved in the susceptibility to NOA in the studied cohort. More comprehensive studies within larger patient groups, preferably involving multiple ethnicity and multiple centres, are required to explain any potential role of the ICAM-1 E469K polymorphism as a male infertility risk factor in NOA.
Disclosure statement
The authors declare that there are no conflicts of interest.
References
- Balkan M, Atar M, Erdal ME, et al. The possible association of polymorphisms in MTHFR, MTRR, and MTHFD1 genes with male infertility. Int Med J. 2013;20(4):404–408.
- Ayhan Ö, Balkan M, Guven A, et al. Truncating mutations in TAF4B and ZMYND15 causing recessive azoospermia. J Med Genet. 2014;51:239–244.
- Cai Y, Liu T, Fang F, et al. Involvement of ICAM-1 in impaired spermatogenesis after busulfan treatment in mice. Andrologia. 2016;48:37–44.
- Mruk DD, Xiao X, Lydka M, et al. Intercellular adhesion molecule 1: recent findings and new concepts involved in mammalian spermatogenesis. Semin Cell Dev Biol. 2014;29:43–54.
- Xiao X, Mruk DD, Cheng CY. Intercellular adhesion molecules (ICAMs) and spermatogenesis. Hum Reprod Update. 2013;19:167–186.
- Iwao M, Morisaki H, Morisakia T. Single-nucleotide polymorphism g.1548G>A (E469K) in human ICAM-1 gene affects mRNA splicing pattern and TPA-induced apoptosis. Biochem Biophys Res Commun. 2004;317:729–735.
- Oguz E, Tabur S, Akbas H, et al. The association of ICAM E469K with cardiovascular characteristics of acromegaly. Eur Rev Med Pharmacol Sci. 2015;19:1673–1679.
- Ji YN, Wang Q, Zhan P. Intercellular adhesion molecule 1 gene E469K polymorphism is associated with coronary heart disease risk: a meta-analysis involving 12 studies. Mol Biol Rep. 2012;39:6043–6048.
- Cournu-Rebeix I, G´enin E, Lesca G, et al. Intercellular adhesion molecule-1: a protective haplotype against multiple sclerosis. Genes Immun. 2003;4:518–523.
- Ma J, Zhang D, Brismar K, et al. Evaluation of the association between the common E469K polymorphism in the ICAM-1 gene and diabetic nephropathy among type 1 diabetic patients in GoKinD population. BMC Med Genet. 2008;27(9):47.
- Wang QL, Li BH, Liu B, et al. Polymorphisms of the ICAM-1 exon 6 (E469K) are associated with differentiation of colorectal cancer. J Exp Clin Cancer Res. 2009;13(28):139.
- Pola R, Flex A, Gaetani E, et al. Intercellular adhesion molecule- 1 E469K gene polymorphism and Alzheimer´s disease. Neurobiol Aging. 2003;24:385–387.
- Zhang J, Zhou DX, Wang HX, et al. An association study of SPO11 gene single nucleotide polymorphisms with idiopathic male infertility in Chinese Han population. Assist Reprod Genet. 2011;28(8):731–736.
- Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215.
- Miller MA, Kerry SM, Dong Y, et al. Circulating soluble E-selectin levels and the Ser 128Arg polymorphism in individuals from different ethnic groups. Nutr Metab Cardiovasc Dis. 2005;15:65–70.
- Jiang H, Klein RM, Niederacher D, et al. C/T polymorphism of the intercellular adhesion molecule-1 gene (exon 6, codon 469). A risk factor for coronary heart disease and myocardial infarction. Int J Cardiol. 2002;84(2–3):171–177.
- Mohamed AA, Rashed L, Amin H, et al. K469E polymorphism of the intercellular adhesion molecule-1 gene in Egyptians with coronary heart disease. Ann Saudi Med. 2010;30:432–436.
- Wen PE, Lu FH, Zhou W, et al. Study on relationship between K/E gene polymorphism and angina. Zhongguo Gong Gong Wei Sheng. 2008;24:808–809.
- Mo HW, Huang YS, Hong YD, et al. Relationship between the serum level, gene polymorphisms of intercellular adhesion molecule-1 and sputum stasis syndrome of coronary heart disease. J New Chin Med. 2009;41:25–28.
- McGlinchey PG, Spence MS, Patterson CC, et al. The intercellular adhesion molecule-1 (ICAM-1) gene E469K polymorphism is not associated with ischaemic heart disease: an investigation using family-based tests of association. Eur J Immunogenet. 2004;31:201–206.
- Kaur G, Rapthap CC, Kumar S, et al. Polymorphism in L-selectin, E-selectin and ICAM-1 genes in Asian Indian pediatric patients with celiac disease. Hum Immunol. 2006;67(8):634–638.
- Akbas H, Oguz E, Tabur S, et al. ICAM-1 E469K and E-Selectin S128R polymorphisms with non-diabetic metabolic syndrome. J Harran Uni Med Fac. 2015;12(1):58–66.
- Aivatiadou E, Mattei E, Ceriani M, et al. Impaired fertility and spermiogenetic disorders with loss of cell adhesion in male mice expressing an interfering Rap1 mutant. Mol Biol Cell. 2007;18(4):1530–1542.
- Lui WY, Sze KL, Lee WM. Nectin-2 expression in testicular cells is controlled via the functional cooperation between transcription factors of the Sp1, CREB, and AP-1 families. J Cell Physiol. 2006;207(1):144–157.
- Obholz KL, Akopyan A, Waymire KG, et al. FNDC3A is required for adhesion between spermatids and Sertoli cells. Dev Biol. 2006;298(2):498–513.
- Rivas B, Huang Z, Agoulnik AI. Normal fertility in male mice with deletion of β-catenin gene in germ cells. Genesis. 2014;52(4):328–332.