ABSTRACT
The accumulation of mutations and single nucleotide polymorphisms (SNPs) in the displacement loop (D-loop) of mitochondrial DNA (mtDNA) is related to gastric carcinogenesis. To evaluate the potential relationships between DNA damage and D-loop SNPs, we measured the 8-hydroxy-2'-deoxyguanosine (8-OHdG) levels in the gastric cancer tissue by immmunostaining. Our data showed that the gastric cancer susceptibility SNP of 16519C was associated with higher 8-OHdG levels. Taken together, the cancer-risk associated D-loop SNPs might initiate carcinogenesis through increasing oxidative damage.
KEYWORDS:
Introduction
Gastric cancer is one of the most common malignancies and third leading cause of cancer-related mortality worldwide [Citation1]. Both environmental factors and genetic factors contribute to the etiology of this multi-factorial disease, and the prognosis of gastric cancer remains poor due to the delayed diagnosis [Citation2]. Several factors, including reactive oxygen species (ROS), are involved in severe damage to the gastric epithelium, thereby contributing to induction of carcinogenesis [Citation3].
Human mtDNA is a circular double-stranded DNA molecule approximately 16 kb in length, and is particularly susceptible to mutations compared to nuclear DNA due to the high rate of generation of ROS, lack of protective histones and the relatively inefficient DNA repair system in mitochondria [Citation4]. The D-loop is the only non-coding mtDNA region which contains crucial elements for replication and transcription, so accumulation of mutations and SNPs in this region may contribute to altered replication or transcription properties [Citation5]. For individuals harbouring mtDNA variants that partially inhibit the electron transport chain, excessive caloric load will increase ROS production, thus increasing cancer risk [Citation6].
Oxidative stress induced by either environmental agents or endogenous biochemical reactions has been implicated in the pathogenesis of a variety of diseases such as aging, inflammatory, degenerative diseases and carcinomas. 8-Hydroxy-2'-deoxyguanosine (8-OHdG) is one of the most commonly occurring DNA modifications and is frequently used as a specific marker of oxidative DNA damage [Citation7]. Earlier studies suggested that a number of cancer tissues, including lung cancer, breast cancer, hepatocellular carcinoma and gastric cancer, displayed higher 8-OHdG levels compared to non-tumour tissues, but the functional significance of persistent oxidative stress in cancer tissues remain unknown [Citation3,Citation8–10].
We sequenced a region of about 1 kb franking nearly all the D-loop and identified five SNPs associated with gastric cancer risk, including alleles 73, 309, 523-524, 16362 and 16519, in our previous study [Citation11]. In this study, we analysed the 8-OHdG levels in gastric cancer tissues to evaluate the relationships between these SNPs and the oxidative DNA damage.
Materials and methods
Tissue collection
Tissue samples of 106 gastric cancer patients were obtained after surgery in the Department of General Surgery at the Fourth Hospital of Hebei Medical University during the period 2007–2008, according to the guidelines of the Human Tissue Research Committee at the Hospital. Informed consent forms were obtained from all participants prior to enrollment. Gastric tissues were fixed in formalin (10%), dehydrated in absolute ethanol and embedded in paraffin immediately after resection. Serial sections (5 μm) were prepared for immunohistochemical analysis.
Measurement of 8-OHdG in gastric cancer tissues
The 8-OHdG levels were analysed using immunohistochemical staining. In brief, tissue sections were incubated with a 1:100 primary antibody dilution against 8-OHdG (Abcam, Cambridge, UK) for two days at 4 ˚C and then allowed to react with biotinylated secondary anti-mouse IgG antibody (4A Biotech Co., Ltd, Beijing, China) for 1 h at room temperature. Streptavidin was added and the colour was developed with 3,3'-diaminobenzidine (DAB).
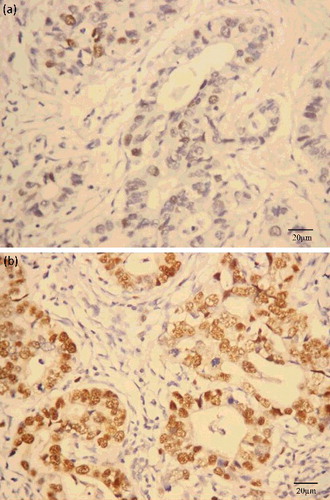
Two pathologists counted the number of 8-OHdG-stained cells in 10 random fields (IX71, Olympus Optical Co., Ltd, Tokyo, Japan) in tissue sections of gastric cancer. The percentage of positively stained cells in each field, which was termed the 8-OHdG label index (LI), were graded as follows: low, LI <50%, and high, LI >50% ().
Statistical analysis
The distribution frequency of each SNP between the high and low groups was assessed using the Chi-square test. Analysis of the clinical characteristics and their potential association with 8-OHdG levels in gastric cancer patients was also performed. The results for gastric patients with postoperative survival data were used for Kaplan–Meier analysis. Multivariate survival analysis was performed using a Cox proportional hazards model. All of the statistical analysis was conducted using SPSS 17.0 software package (SPSS Inc., Chicago, IL, USA). A P-value of P < 0.05 was considered to indicate a statistically significant difference.
Results and discussion
A total of 106 gastric cancer patients were enrolled in this study. The immunoreactivity of 8-OHdG observed in gastric cancer tissues occurred mostly in the nuclei (). The results from the Chi-square test did not reveal association of 8-OHdG levels with the clinical characteristics, including gender, age, pathological types, Her-2 status, TNM stage and intravascular tumour thrombus (). Further analysis showed that the 8-OHdG levels were associated with neither the clinical characteristics, nor the outcome of gastric cancer ().
Table 1. Clinical characteristics and their association with 8-OHdG in gastric cancer patients.
Table 2. Correlation between 8-OHdG and outcome of gastric cancer.
The potential correlation of 8-OHdG levels and gastric cancer associated D-loop SNPs including alleles 73, 309, 523–524, 16,362 and 16,519, was evaluated using the Chi-square test. As shown in , allele 16519C, which was associated with increased risk of gastric cancer, was associated with higher 8-OHdG levels in gastric cancer patients. These data demonstrated that the cancer susceptibility SNP 16519C of the mtDNA D-loop was associated with increased oxidative DNA damage.
Table 3. Correlation between 8-OHdG and gastric cancer risk associated SNPs.
Although a relationship between mitochondria, metabolism and cancer was originally proposed nearly a century ago, interest in the field has grown rapidly in recent years. Mitochondria are semi-autonomous organelles containing their own DNA, and play important roles in a variety of cellular functions, including metabolism, ROS generation and signalling, apoptosis and calcium homeostasis [Citation12]. Although several sources of ROS exist in cells, mitochondria are one of the main contributors to the oxidative status of the cell. Most ROS in mitochondria are produced by reduction of O2 to superoxide anion O2− by complexes I and III [Citation13].
The replication and expression of the mitochondrial genome are regulated by the mitochondrial D-loop, since it contains DNA-binding sites. Some severe alterations in this region might inhibit the electron transport chain, resulting in the release of high ROS levels, and the increased production of mitochondrial ROS has been proposed as a pathological mechanism in some degenerative diseases and many kinds of cancers [Citation14].
Mutations and polymorphisms in the D-loop have been associated with a number of cancers; however; only a few SNPs have been considered for prediction of cancer risk and outcome, and that with a still subtle predictive value [Citation11,Citation15,Citation16]. We postulated that these SNPs in the D-loop might change the expression of mtDNA, thereby increasing the mitochondrial ROS levels, which, in turn, would be responsible for carcinogenesis of gastric cancer. We verify our hypothesis by using 8-OHdG as an oxidative damage maker to evaluate their relationships with D-loop SNPs in the gastric cancer patients. We found that the gastric cancer susceptibility SNP of 16519C was associated with higher 8-OHdG levels. To the best of our knowledge, this is the first study to investigate the association between DNA damage and D-loop SNPs that provides major new insights into the etiology of gastric cancer. Furthermore, our observation that the 8-OHdG levels were not associated with either the clinical characteristics or the outcome of gastric cancer demonstrated that a biomarker for cancer risk is not always a biomarker for cancer outcome.
Conclusions
In summary, our results implied that the cancer risk associated D-loop SNPs might initiate carcinogenesis through increasing oxidative damage. Analysis of the correlation between the genetic polymorphisms in the D-Loop and 8-OHdG levels provides major new insights into the etiology of gastric cancer.
Acknowledgements
We would like to express our sincere thanks to all those who have helped me make this thesis better.
Disclosure statement
No potential conflict of interest was declared by all authors.
Additional information
Funding
References
- WHO. Cancer Fact sheet N°297. Geneva: World Health Organization; c2016 [ updated 2015 Feb; cited 2016 Dec 4]. Available from: http://www.who.int/mediacentre/factsheets/fs297/en/
- Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354–362.
- Raza Y, Khan A, Farooqui A, et al. Oxidative DNA damage as a potential early biomarker of Helicobacter pylori associated carcinogenesis. Pathol Oncol Res. 2014;20:839–846.
- Cruz-Bermúdez A, Vicente-Blanco RJ, Gonzalez-Vioque E, et al. Spotlight on the relevance of mtDNA in cancer. Clin Transl Oncol. 2016:1–10. DOI:10.1007/s12094-016-1561-6
- Lalmuanpuii R, Ghatak S, Pautu JL, et al. Mutation profiling in mitochondrial D-loop associated with stomach cancer and tobacco consumers. J Clin Med Genom. 2015;3:1–5.
- Gisbergen MWV, Voets AM, Starmans MHW, et al. How do changes in the mtDNA and mitochondrial dysfunction influence cancer and cancer therapy? Challenges, opportunities and models. Mutat Res. 2015;290:16–30.
- Valavanidis A, Vlachogianni T, Fiotakis C. 8-hydroxy-2'-deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009;27:120–139.
- Gackowski D, Speina E, Zielinska M, et al. Products of oxidative DNA damage and repair as possible biomarkers of susceptibility to lung cancer. Cancer Res. 2003;63:4899–4902.
- Jakovcevic D, Dedic-Plavetic N, Vrbanec D, et al. Breast cancer molecular subtypes and oxidative DNA damage. Appl Immunohistochem Mol Morphol. 2015;23:696–703.
- Li S, Wang X, Wu Y, et al. 8-Hydroxy-2'-deoxyguanosine expression predicts hepatocellular carcinoma outcome. Oncol Lett. 2012;3:338–342.
- Wang H, Wang Y, Zhao Q, et al. Identification of sequence polymorphisms in the D-loop region of mitochondrial DNA as a risk factor for gastric cancer. Mitochondrial DNA A DNA Mapp Seq Anal. 2016;27:1045–1047.
- Nunnari J, Suomalainen A. Mitochondria: in sickness and in health. Cell. 2012;148:1145–1159.
- Bleier L, Wittig I, Heide H, et al. Generator-specific targets of mitochondrial reactive oxygen species. Free Radic Biol Med. 2015;78:1–10.
- Pastukh VM, Gorodnya OM, Gillespie MN, et al. Regulation of mitochondrial genome replication by hypoxia: the role of DNA oxidation in d-loop region. Free Radic Biol Med. 2016;96:78–88.
- Wang C, Wang Y, Wang H, et al. Mitochondrial DNA haplogroup N is associated good outcome of gastric cancer. Tumour Biol. 2014;35:12555–12559.
- Guo Z, Zhao S, Fan H, et al. Identification of sequence polymorphisms in the D-Loop region of mitochondrial DNA as a risk factor for colon cancer. Mitochondrial DNA A DNA Mapp Seq Anal. 2016;27:4244–4245.