ABSTRACT
Although DNA elimination, gene silencing and duplication and pseudogenization are associated with allopolyploidization in wheat, the effect of allopolyploidy on the ecogeographical expansion and domestication of this species remains unknown. In the present study, we used 1862 mapped loci to investigate genetic changes associated with allopolyploidization by comparing bands between synthetic wheat SHW-L1 and its parental lines Aegilops tauschii ssp. tauschii AS60 (DD) and Triticum turgidum ssp. turgidum AS2255 (AABB). A total of 338 (18.15%) loci from AS60 or AS2255 were found to have been eliminated from SHW-L1. Of these loci, 56 were present only in AS2255; 212 ones were found only in AS60 and 70 ones were detected in both AS2255 and AS60. The different numbers of eliminated loci originating from AS2255 and AS60 suggest that the D genome of SHW-L1 suffered more disruption during the allopolyploidization process than the A and B genomes. In addition, 82 eliminated loci were tightly linked to express quantitative trait loci, with 53 of these loci containing open reading frame sequences predicted to encode proteins related to disease resistance, environmental adaption, nitrogen fixation, nitrogenase reductase and other processes. Further investigation of changes in these gene sequences should help elucidate the genetic mechanisms underlying the increased fitness, adaptability and competitiveness that have accompanied wheat during domestication.
Introduction
In plants, allopolyploidization is an important process arising from interspecific or inter-generic hybridization followed by chromosome doubling [Citation1]. Common wheat (Triticum aestivum L.) is an allopolyploid (2n = 6x = 42) consisting of three different genomes (AABBDD) [Citation2]. Because the diploid progenitors of wheat carry only a few traits for adaptation, their remaining populations are globally restricted to small geographical areas [Citation3,Citation4]. Diverse varieties in common wheat have been released to facilitate adaptation to various environments and management systems and to meet various human dietary requirements, ranging from Norway and Russia at 65°N to Argentina at 45°S [Citation5,Citation6].
Allopolyploidization exerts considerable stress on a newly emerged species that is reproductively isolated from its progenitors. Several mechanisms to resolve this stress have been uncovered in previous studies, such as DNA elimination, gene silencing and duplication and pseudogenization [Citation7–10]. Extensive pericentric rearrangements might also contribute to structural differences among homeologous chromosomes [Citation11]. In addition, Ph1 on wheat chromosome 5B functions to suppress inter-genomic pairing and to allow intra-genomic pairing [Citation12,Citation13]. All these mechanisms can promote the compatibility of genomes within a nucleus and make reproduction possible. Nevertheless, the immediate effect of allopolyploidization on agronomic traits is unknown, especially with respect to changes in the expression of genes associated with important agronomic traits. This fascinating issue is particularly of interest in regard to wheat domestication.
We recently constructed a high-density linkage map based on a population of 171 F8 recombinant inbred lines derived from a cross between wheat cultivars SHW-L1 and Chuanmai32 [Citation14]. The male parental line of SHW-L1 is a synthetic hexaploid wheat originating from Triticum turgidum ssp. turgidum and Aegilops tauschii ssp. tauschii lines [Citation15]. Quantitative trait loci (QTLs) for 36 traits were also identified in this population. In the present study, we used all mapped loci to clarify how chromosomal regions from the parental lines were transmitted to SHW-L1, with a special focus on loci associated with agronomic traits. The objectives of this study were to dissect genetic changes from a genome-wide perspective and to identify loci that showed expression activation associated with agronomic traits due to allopolyploidization.
Materials and methods
Plant materials
Three genotypes were used as plant materials in this study: SHW-L1, AS60 and AS2255 (…). SHW-L1 is a synthetic hexaploid wheat derived from T. turgidum ssp. turgidum AS2255 (AABB) and A. tauschii ssp. tauschii AS60 (DD) [Citation15,Citation16]. All materials were provided by the Triticeae Institute of Sichuan Agricultural University, China.
Locus elimination in synthetic hexaploid wheat SHW-L1
A total of 1862 loci, consisting of 1794 diversity arrays technology (DArT) markers and 68 simple sequence repeats (SSRs), were used for genotyping of SHW-L1, AS60 and AS2255. All loci were mapped onto a genetic linkage map covering 3766.9 cM, with an average distance of 2.0 cM per marker. The linkage map was constructed based on a SHW-L1 × Chuanmai32 (SC) population [Citation17]. The DArT array was obtained from DArT P/L (http://www.tritcarte.com.au). Hybridization of genomic DNA to the DArT array, image analysis and polymorphism scoring was as described by Akbari et al. [Citation18,Citation19]. The SSR analysis was performed as described by Yu et al. [Citation17].
Results and discussion
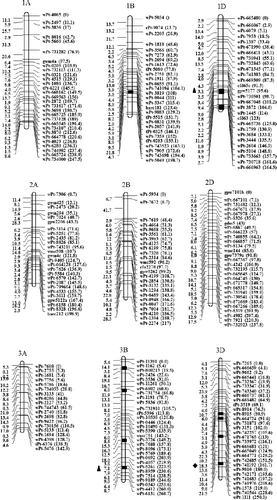
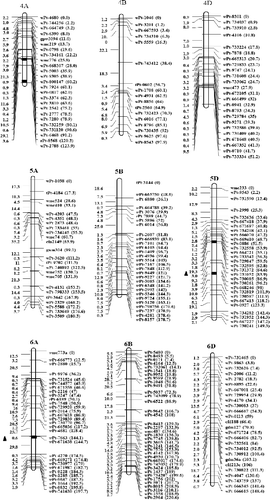
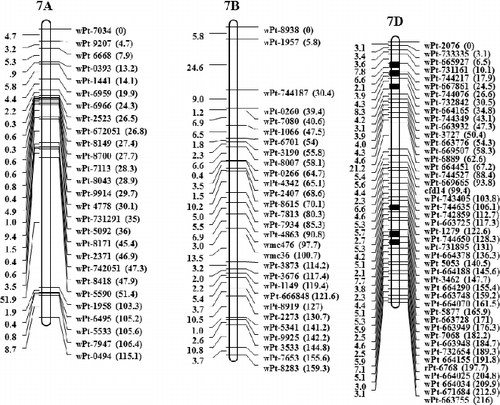
Of the 1862 loci used in this study, 522 were polymorphic between AS60 and AS2255, and 951 were genome-specific. The linkage map of SHW-L1 can be found in Yu et al. [Citation17]. Loci eliminated from the genome of SHW-L1 fell into two categories: (1) individual loci donated by either AS60 or AS2255 and (2) homologous loci donated by both AS60 and AS2255. A total of 338 loci (331 DArT markers and 7 SSRs) were found to have been eliminated in this study ().
Table 1. Elimination of alleles on 21 chromosomes induced by allopolyploidization in synthetic wheat ‘SHW-L1’.
Early hybrid generations from tetraploid wheat and Aegilops genera contain three incompatible genomes, which exert considerable stress on reproduction because of the high degree of synteny among homeologous chromosomes driving inter-genomic pairing. Genomic restructuring events and cytosine methylation can suppress this pairing and lead to diploidization [Citation9,Citation10,Citation20]. Loss and duplication of DNA sequences and pericentric rearrangements may contribute to such genomic restructuring [Citation11,Citation21]. Genotyping of SHW-L1, AS60 and AS2255 entailed the digestion of genomic DNA samples with frequently cutting restriction enzymes, including MseI, RsaI, TaqI and BstNI, followed by amplification and labelling of these digested fragments [Citation18]. Because eliminated loci were detected using each enzyme, and all loci followed Mendelian inheritance during genetic linkage map construction, we conclude that the disappearance of loci was due to DNA sequence changes rather than methylation [Citation17].
Two types of processes during hybridization may be responsible for elimination of the genotyped loci: either loss of a sequence from one or two parents, or gain of a novel sequence that is different from those of the parents. Of these eliminated loci, 56 and 212 were donated solely by AS2255 and AS60, respectively, and 70 were donated by both AS2255 and AS60. Of the 362 loci located on the A genome in the SC map, 52 (14.36%) were eliminated from SHW-L1. Eleven eliminated loci were donated by both AS60 and AS2255, nine ones were only donated by AS2255 and 32 ones were only donated by AS60. Of the 539 loci located on the B genome, 86 ones (15.96%) were absent in SHW-L1: 22 ones only from AS2255, 36 ones solely from AS60 and 28 ones donated by both AS2255 and AS60. Of the 961 loci located on the D genome, 200 (20.81%) were not present in SHW-L1. Of these eliminated loci, 10 and 49 were donated solely by AS2255 and AS60, respectively, with 13 ones donated by both AS2255 and AS60. In total, 282 loci contributed by AS60 were absent from SHW-L1. Fewer loci from AS2255 were lost, namely, 126 ones. The larger number of eliminated loci donated by AS60 indicates that the D genome was more heavily disrupted than the AB genomes during allopolyploidization. This difference may be due to the fact that allotetraploid wheat has already suffered genomic shock [Citation22]. Similar non-random DNA reduction between D and AB genomes has also been reported in previous studies [Citation21,Citation23,Citation24].
In the present study, 68 loci eliminated from A or B genomes were donated by AS60, whereas the 10 eliminated loci uniquely donated by AS2255 were located on the D genome. Using the eliminated loci from the D genome as an example, there are two plausible explanations for this finding. First, two homologous loci were present on the D genome and on the A (or B) genome as a consequence of the homology of all three wheat genomes. The loci on the D genome were eliminated in both SHW-L1 and Chuanmai32 (the other parental genotype of the mapping population), while the same locus on the A (or B) genome was present in Chuanmai32 but eliminated in SHW-L1. The second explanation could be that the locus from the D genome may have spread throughout the other genomes through the actions of retrotransposons. Indeed, a large number of ancestral transposable elements have been reported in Gramineae genomes [Citation9,Citation25]. Transposable elements make up a very large fraction of Aegilops allopolyploids genomes, with a majority of long terminal repeat retrotransposons [Citation26,Citation27]. They may mutate genes, alter gene regulation and generate new genes through transposition, proliferation, deletion, ectopic recombination and epigenetic re-patterning. Therefore, transposable elements activity is expectedly central to genome evolution [Citation28–30].
Of the 338 eliminated loci, 129 ones were clustered on regions of individual chromosomes that were subsequently lost (; ). A total of 30 eliminated regions were identified on parental chromosomes 1B, 1D, 3B, 3D, 4A, 5D, 6A and 7D. These regions consisted of 3–12 loci, with a genetic distance of 0.0–8.8 cM; 18 of these chromosomal regions were shorter than 1.0 cM and 5 ones covered regions longer than 5.0 cM. A total of eight eliminated regions uniquely donated by AS60 were identified on chromosomes 1D (wPt-732625–wPt-1445 and wPt-4180–wPt-665736), 3B (wPt-7514–wPt-9189), 5D (wPt-3825–wPt-671930), and 7D (wPt-9091–wPt-732842, wPt-744366–wPt-742914, wPt-743857–wPt-742859 and wPt-664508–wPt-734176). Only one eliminated region was donated by AS2255, wPt-8658–wPt-1573 located on 3D.
Table 2. Eliminated chromosomal regions in SHW-L1 from AS2255 and AS60.
Figure 1. Linkage map of SHW-L1 and chromosomal regions from AS60 or AS2255 eliminated in SHW-L1.
Previous studies have revealed that allopolyploidization causes immediate non-random elimination of specific noncoding sequences in wheat and related species [Citation10,Citation31,Citation32]. We previously performed QTL mapping for 36 traits in the SC population. A total of 136 genetic regions were identified, with each region containing 1–18 QTLs [Citation14,Citation15,Citation17,Citation33]. In the present study, 82 of the 338 eliminated loci were shown to be located in genetic regions containing expressed QTLs. We only detected 61 locus sequences showing linkage relationships with expressed QTLs. A total of 53 locus sequences contained open reading frames (ORFs). Six ORFs (those of wPt-9664, wPt-3037, rPt-8568, wPt-743361, wPt-742918 and wPt-744183) were predicted to encode proteins related to disease resistance and environmental adaption, with one (wPt-665342) encoding a nitrogen fixation protein and one (wPt-2523) encoding nitrogenase reductase. These sequence changes might have improved the adaptability of the newly formed allopolyploids and facilitated their fast colonization of new ecological niches, but also may reduce the resistance to some diseases in synthetic wheat in comparison to wild species due to elimination of some important chromosomal regions really presented in the Aegilops species. Such as the eliminated locus of wPt-2607, which was tightly linked with a QTL for waterlogging tolerance [Citation19]. The negative effect of the SHW-L1 allele on this eliminated locus might explain why AS60 and AS2255 showed waterlogging tolerance but SHW-L1 showed intolerance to waterlogging.
Expression changes triggered by retrotransposons have always been associated with the activation or silencing of adjacent genes during allopolyploidization [Citation21]. We identified two retrotransposons in our study corresponding to the ORFs of wPt-734079 and wPt-671684. Recent studies indicated that transposable element families may show distinct evolutionary dynamics [Citation26]. However, these processes underlying the particular trajectories of transposable elements remain unclear [Citation27]. Thus, more sequence analysis and functional validation are needed to assess the impact of transposable elements on the evolutionary trajectories of the wheat group.
Conclusions
The results from this study showed that the eliminated loci tightly linked with expressed QTLs are very important to alterations of adaption and yield during wheat evolution. That is why identification of the variation in these gene sequences and monitoring of changes in the expression of these loci between parents and progenies during allopolyploidization will be very important to uncover the genetic mechanisms underlying the evolution and adaptation in wheat.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ramsey J, Schemske D. Pathways, mechanisms, and rates of polyploid formation in flowering plants. Annu Rev Ecol Evol Syst. 1998;29:467–501.
- Huang S, Sirikhachornkit A, Su X, et al. Genes encoding plastid acetyl-CoA carboxylase and 3-phosphoglycerate kinase of the Triticum/Aegilops complex and the evolutionary history of polyploid wheat. Proc Natl Acad Sci USA. 2002;99:8133–8138.
- Kimber G, Feldman M. Wild wheats: an introduction. Columbia (MO): College of Agriculture; 1987. p. 1–142. (Special Report; no. 353).
- Van Slageren MW. Wild wheats: a monograph of Aegilops L. and Amblyopyrum (Jaub. & Spach) Eig (Poaceae). Vol. 94–97, Wageningen Agricultural University Papers. Wageningen (Netherlands): ICARDA/Agricultural University; 1994.
- Snape JW, Butterworth K, Whitechurch E, et al. Waiting for fine times: genetics of flowering time in wheat. Euphytica. 2001;119:185–190.
- Dubcovsky J, Dvorak J. Genome plasticity a key factor in the success of polyploidy wheat under domestication. Science. 2007;316:1862–1866.
- Levy AA, Feldman M. Genetic and epigenetic reprogramming of the wheat genome upon allopolyploidization. Biol J Linn Soc. 2004;82:607–613.
- Feldman M, Levy AA. Allopolyploidy – a shaping force in the evolution of wheat genomes. Cytogenet Genome Res. 2005;109:250–258.
- Feldman M, Levy AA. Genome evolution in allopolyploid wheat – a revolutionary reprogramming followed by gradual changes. J Genet Genomics. 2009;36:511–518.
- Feldman M, Levy AA. Genome evolution due to allopolyploidization in wheat. Genetics. 2012;192:763–774.
- Ma J, Stiller J, Wei YM, et al. Extensive pericentric rearrangements in the bread wheat (Triticum aestivum L.) genotype “Chinese Spring” revealed from chromosome shotgun sequence data. Genome Biol Evol. 2014;6(11):3039–3048.
- Griffiths S, Sharp R, Foote TN, et al. Molecular characterization of Ph1 as a major chromosome pairing locus in polyploid wheat. Nature. 2006;439:749–752.
- Yousafzai FK, Al-Kaff N, Moore G. Structural and functional relationship between the Ph1 locus protein 5B2 in wheat and CDK2 in mammals. Funct Integr Genomic. 2010;10:157–166.
- Yu M, Chen GY, Zhang LQ, et al. QTL mapping for important agronomic traits in synthetic hexaploid wheat derived from Aegiliops tauschii ssp. Tauschii. J Integr Agric. 2014;13(9):1835–1844
- Yu M, Chen GY, Pu ZE, et al. Quantitative trait locus mapping for growth duration and its timing components in wheat. Mol Breeding. 2015;35(44):1–11.
- Zhang LQ, Liu DC, Yan ZH, et al. Rapid changes of microsatellite flanking sequence in the allopolyploidization of new synthesized hexaploid wheat. Sci China Ser C. 2004;47:553–561.
- Yu M, Mao SL, Chen GY, et al. QTLs for uppermost internode and spike length: whether they affect wheat height at an individual QTL level in two RIL populations? Euphytica. 2014;200(1):95–108.
- Akbari M, Wenzl P, Caig V, et al. Diversity arrays technology (DArT) for high-throughput profiling of the hexaploid wheat genome. Theor Appl Genet. 2006;113:1409–1420.
- Yu M, Chen Gy. Conditional QTL mapping for waterlogging tolerance in two RILs populations of wheat. SpringerPlus [Internet]. 2013 [cited 2016 Jul 21];2:1–7. Available from: https://springerplus.springeropen.com/articles/10.1186/2193-1801-2-245
- Shaked H, Kashkush K, Ozkan H, et al. Sequence elimination and cytosine methylation are rapid and reproducible responses of the genome to wide hybridization and allopolyploidy in wheat. Plant Cell. 2001;13:1749–1759.
- Bento M, Gustafson JP, Viegas W, et al. Size matters in Triticeae polyploids: larger genomes have higher remodeling. Genome. 2011;54:175–183.
- Kashkush K, Feldman M, Levy AA. Gene loss, silencing and activation in a newly synthesized wheat allotetraploid. Genetics. 2002;160:1651–1659.
- Eilam T, Anikster Y, Millet E, et al. Nuclear DNA amount and genome downsizing in natural and synthetic allopolyploids of the genera Aegilops and Triticum. Genome. 2008;51:616–627.
- Eilam T, Anikster Y, Millet E, et al. Genome size in diploids, allopolyploids, and autopolyploids of mediterranean triticeae. Genome. 2010;52(3):275–285.
- Pestsova E, Ganal MW, Röder MS. Isolation and mapping of microsatellite markers specific for the D genome of bread wheat. Genome. 2000;43:689–697.
- Senerchia N, Wicker T, Felber F, et al. Evolutionary dynamics of retrotransposons assessed by high throughput sequencing in wild relatives of wheat. Genome Biol Evol. 2013;5:1010–1020.
- Senerchia N, Felber F, Parisod C. Contrasting evolutionary trajectories of multiple retrotransposons following independent allopolyploidy in wild wheats. New Phytol. 2014;202(3):975–985.
- Charles M, Belcram H, Just J, et al. Dynamics and differential proliferation of transposable elements during the evolution of the B and A genomes of wheat. Genetics. 2008;180:1071–1086.
- Fedoroff NV. Transposable elements, epigenetics, and genome evolution. Science. 2012;338:758–767.
- Feldman M, Levy AA. Origin and evolution of wheat and related triticeae species. In: Molnár-Láng M, Ceoloni C, Doležel J, editors. Alien introgression in wheat. Cytogenetics, molecular biology, and genomics. New York (NY): Springer; 2015. p. 21–76.
- Ozkan H, Levy AA, Feldman M. Allopolyploidy-induced rapid genome evolution in the wheat (Aegilops–Triticum) group. Plant Cell. 2001;13(8):1735–1747.
- Salina EA, Numerova OM, Ozkan H, et al. Alterations in subtelomeric tandem repeats during early stages of allopolyploidy in wheat. Genome. 2004;47:860–867.
- Pu ZE, Yu M, He QY, et al. Quantitative trait loci associated with micronutrient concentrations in two recombinant inbred wheat lines. J Integr Agric. 2014;13(11):2322–2329.