ABSTRACT
During the screening programme for antibiotic produced by actinomycetes, an antibiotic was isolated from the fermentation broth of Streptomyces anulatus NEAE-94. The ethyl acetate extract of S. anulatus NEAE-94 showed high biological activity against Staphylococcus aureus NRRL B-313, multidrug-resistant S. aureus and Bacillus subtilis NRRL B-543. Thin-layer chromatography of the ethyl acetate extract showed one yellowish orange spot with an Rf value of 0.83. The structural elucidation of the isolated compounds was carried out using ultraviolet–visible mass spectrometry (MS), Fourier transform-infrared spectroscopy (FT-IR), proton nuclear magnetic resonance (1H-NMR) and gas chromatography–mass spectrometry (GC–MS) analyses. Characterization of the ethyl acetate extract revealed the presence of 22 compounds, including an unsaturated fatty acid, saturated fatty acids, fatty acid esters, alkanes, alkenes and a triterpene. The antimicrobial efficacy of S. anulatus NEAE-94 might be due to the synergistic effects of the identified compounds.
Introduction
There has been a steady increase in antibiotic resistance during the past several decades, accounting for the long list of currently available antimicrobial agents that are now insufficient for the control of microbial infections [Citation1,Citation2]. Staphylococcus aureus is a bacterial pathogen responsible for serious infections in humans and is becoming increasingly resistant to many commonly used antibiotic treatments [Citation3]. The occurrence of multidrug-resistant S. aureus means high risks especially in surgical intensive care units. Different strains of S. aureus can cause serious infections including boils and pimples, wound infections, pneumonia, osteomyelitis, septicemia, food intoxication and toxic shock syndrome. The rising frequency of antibiotic resistance among pathogenic S. aureus has created an urgent need to discover novel antibiotics with a broad spectrum of activity against resistant organisms [Citation4].
Over two thirds of the clinically useful antibiotics of natural origin are produced by Actinomycetes [Citation5], among which the major contributor is Streptomyces [Citation6]. Most Streptomyces and other actinomycetes produce a diverse array of antibiotics including aminoglycosides (streptomycin and its relatives), anthracyclines, glycopeptides, β-lactams, macrolides (erythromycin and its relatives), nucleosides, peptides, polyenes, polyethers, tetracyclines, chloramphenicol, ivermectin, rifamycins and most other clinically-useful antibiotics [Citation7,Citation8].
According to Hanson [Citation9], the structure elucidation of natural products can be divided into four stages: (1) preliminary characterization (determination of physical constants, molecular weight and identification of functional groups), (2) structural simplification (identification of carbon skeleton), (3) relative stereochemistry (position of functional groups and their relative configuration in space), (4) absolute configuration. Numerous techniques and analytical systems like mass spectrometry (MS), Fourier transform-infrared spectrum (FT-IR), nuclear magnetic resonance (1H-NMR), elemental analysis and gas chromatography–mass spectrometry (GC–MS) analyses have been developed for the analysis and characterization of active compounds from micro-organisms. The secondary metabolites from micro-organisms have characteristic chromatographic profiles and reactivity towards staining reagents used in thin-layer chromatography (TLC) under specified reaction conditions, which allows the separation and identification of compounds in mixtures [Citation10].
This study aimed at the extraction, isolation of potent antibiotic produced by Streptomyces anulatus NEAE-94 active against multidrug-resistant strains. Furthermore, the antibiotic was characterized by ultraviolet (UV)–visible spectroscopy, MS, 1H-NMR, FT-IR and GC–MS analyses.
Materials and methods
Micro-organisms and growth conditions
Streptomyces anulatus NEAE-94 was kindly provided by the first author, Dr Noura El-Ahmady El-Naggar (Department of Bioprocess Development, Genetic Engineering and Biotechnology Research Institute, City for Scientific Research and Technological Applications, Alexandria, Egypt). This isolate was maintained on slopes containing starch-nitrate agar medium of the following composition: 20 g/L of starch, 2 g/L of KNO3, 1 g/L of K2HPO4, 0.5 g/L of MgSO4.7H2O, 0.5 g/L of NaCl, 3 g/L of CaCO3, 0.01 g/L of FeSO4.7H2O, 20 g/L agar and distilled water up to 1 L. Slopes were incubated for a period of 7 days at 30°C. The isolate was stored as spore suspensions in 20% (v/v) glycerol at −20°C [Citation11] for subsequent investigation.
Antimicrobial agent activities were tested against a group of multidrug-resistant bacteria isolated from various clinical samples and kindly provided by the Infection Control Unit, Department of Medical Microbiology and Immunology, Faculty of Medicine, Mansoura University, Mansoura, Egypt: Staphylococcus aureus A9897 (this strain is resistant to vancomycin, co-clavulanic acid-amoxicillin, gentamicin, trimethoprim-sulfamethoxazole, oxacillin, amikacin and tobramycin), Pseudomonas aeruginosa T9934 (resistant to ceftriaxone, gentamicin, cefotaxime, trimethoprim-sulfamethoxazole and co-clavulanic acid-amoxicillin) and Klebsiella pneumonia A9898 (resistant to trimethoprim-sulfamethoxazole, co-clavulanic acid-amoxicillin, gentamicin, ceftriaxone, amikacin and cefotaxime). The antimicrobial activities were also tested against a group of bacteria belonging to the Culture Collection of NRRL: Gram-positive (S. aureus NRRL B-313 6538 and Bacillus subtilis NRRL B-543), Gram-negative (Escherichia coli NRRL B-210 and Pseudomonas aeruginosa NRRL B-23) and Candida albicans NRRL Y-477. Stock cultures of the test organisms were maintained on nutrient agar slants. The inoculated agar medium was incubated for 24 h at 30°C, and then maintained at 4°C until further use.
Inoculum preparation
Erlenmeyer (250 mL) flasks containing 50 mL of yeast-malt extract broth (1% malt extract, 0.4% dextrose, 0.4% yeast extract, 2% agar; pH 7.0) were inoculated with three disks of 9 mm diameter taken from the 7-day-old stock culture grown on starch nitrate agar medium [Citation12]. The flasks were incubated for 48–72 h in a rotary incubator shaker at 30 °C and 200 r/min and were used as inoculum for subsequent experiments.
Production conditions
Fifty millilitres of fermentation medium were dispensed in 250-mL conical (Erlenmeyer) flasks, inoculated with previously prepared inoculum. The inoculated flasks were incubated on a rotatory incubator shaker at 150–250 rpm and 25–30°C. After the specified incubation time for each set of experimental trials, the mycelium of each isolate was collected by centrifugation at 5000 g (Z326K centrifuge, Hermle Labortechnik GmbH, Germany) for 15 min. The cell-free supernatant was used for antimicrobial activity assays.
Antagonistic action against microbial test strains
The well-diffusion technique was used to test the ability of the isolate to inhibit the growth of several Gram-positive, Gram-negative bacteria and C. albicans. Fifty millilitres of nutrient agar medium were poured into Petri plates. After solidifying, plates were inoculated with test strains and wells were punched out using a 9-mm cork borer. One hundred microliters of tested filtrates were transferred into each well. All plates were incubated at 30°C for 24 h. After the incubation period, the plates were observed for zone formation around the wells. The zone of inhibition was calculated by measuring the diameter of the inhibition zone around the well (in mm) including the well diameter.
Extraction and antimicrobial assay of antimicrobial metabolites extracts
The fermentation broth of S. anulatus NEAE-94 was prepared and centrifuged at 5000 g for 20 min at 4°C. The supernatant was filtered; the filtrate was subjected to solvent extraction to recover antibacterial metabolites in pure form. Organic solvents (n-butanol, chloroform, ethyl acetate, n-hexane, benzene, petroleum ether, carbon tetrachloride and diethyl ether) were used in the extraction of the antibacterial metabolites. Different solvents were added to the filtrate in a ratio of 1:1 (v/v) and were shaken vigorously for 1 h for complete extraction. As ethyl acetate was standardized as the best solvent to extract the antibiotic, the organic ethyl acetate phase was collected and concentrated by evaporation to near dryness under reduced pressure by using a rotary evaporator. The antimicrobial activity of the crude extract was tested by using the filter paper disc diffusion method assay on multidrug-resistant S. aureus. The antibiotic was precipitated by addition of petroleum ether to the concentrated ethyl acetate extract with gentle stirring.
Thin-layer chromatography
Readymade pre-coated TLC plates were used for separation of crude compounds. Using the capillary tube, a row of spots of the active fraction of ethyl acetate extract was applied 1.5 cm above the bottom of the TLC plates. The spots were left to dry. The TLC plate was placed vertically in a developing beaker containing the solvent chloroform/ethyl acetate, in a ratio of 1:9, covered with the glass in order to prevent the evaporation of the solvents. The solvent was allowed to run till it moved up to 80% of the TLC plate. After running, the sheet was kept at room temperature for the complete drying of the plate. After drying, the Rf value of the spot separated on the TLC plate was determined. Then the plates were viewed under UV light. The developed yellowish orange spot was scrapped, mixed with ethyl acetate and centrifuged at 3000 g for 15 min. The supernatant was collected and impregnated with sterile filter paper disc and was tested against multidrug-resistant S. aureus spread on the surface of the plate containing nutrient agar medium by the disc diffusion method. The plate seeded was then incubated at 30°C for 24 h. The presence of inhibition zones around the disc was determined.
FT-IR spectroscopy
The infrared (IR) spectrum of the ethyl acetate extract was measured (as KBr discs) in the range of 400–4000 cm−1 on FT-IR spectrophotometer (Shimadzu FTIR-8400 S, Kyoto, Japan). The important IR bands, such as ʋ (C–N), ʋ (O–H), ʋ (CH), ʋ (C = C), ʋ (NH), ʋ (CO) and (CH) symmetric and asymmetric stretching, and stretching frequencies were studied to determine the presence of functional groups in the ethyl acetate crude extracts.
UV spectroscopy
The UV absorption spectrum of the purified active compound (dissolved in chloroform) was scanned in a UV–visible spectrophotometer (Optizen Pop, Daejeon, South Korea). It was used to detect the presence of chromophores like dienes, aromatics, polyenes, and conjugated ketones, etc. [Citation13].
1H-NMR spectroscopy
The NMR spectrum of isolated products was obtained on a DRX-400 (1H 400 MHz) instrument from BRUKER (Karlsruhe, Germany). The internal standard was tetramethylsilane.
GC–MS spectroscopy
GC–MS analysis of the isolated products was performed using a Perkin-Elmer GC-Clarus 500 gas chromatograph (Shelton, CT, USA) and gas chromatograph interfaced to a mass spectrometer (GC–MS) equipped with an Elite-I, fused silica capillary column (30 mm × 0.25 mm 1D × 1 μm df, composed of 100% dimethylpolysiloxane). For GC–MS detection, an electronionization system with ionizing energy of 70 eV was used. Helium gas (99.999%) was used as the carrier gas at a constant flow rate of 1 mL/min and an injection volume of 2 μL was employed (split ratio of 10:1). The injector temperature was 250°C and the ion-source temperature, 280°C. The oven temperature was programmed from 110°C (isothermal for 2 min) to 200°C, with an increase of 10°C/min, then 5°C/min to 280°C, ending with a 9 min isothermal at 280°C. Mass spectra were taken at 70 eV; a scan interval of 0.5 s and fragments from 45 to 450 Da. The total GC running time was 36 min. The relative % amount of each component was calculated by comparing its average peak area to the total areas.
The software adopted to handle the mass spectrum and chromatograms was Turbo-Mass ver. 5.2.
Results and discussion
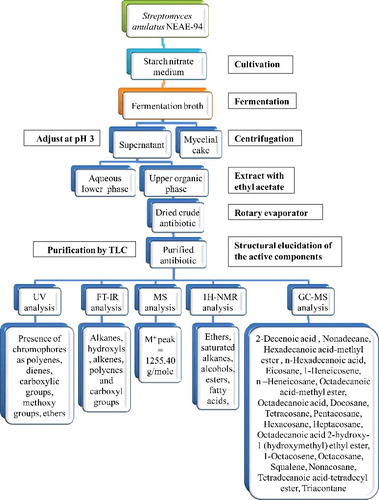
As a part of our search for more potent antibiotic, one promising isolate, S. anulatus NEAE-94, was found to produce a broad spectrum antibiotic with strong antibacterial activity against several pathogenic multidrug-resistant bacteria (Figure S1 in the Online Supplementary Appendix) and Candida albicans as described in our earlier report [Citation14]. This strain was, therefore, selected for further isolation of its bioactive metabolites and their structure elucidation. shows a schematic representation of the procedures involved in antibiotic isolation, purification, characterization and structure elucidation.
Figure 1. Schematic representation of the procedures for antibiotic isolation, purification, characterization and structure elucidation.
Actinomycetes, and especially Streptomyces species, are commonly recognized as a prolific source of secondary metabolites [Citation15]. Due to their widespread distribution and remarkable capacity to produce a wide range of bioactive compounds [Citation16], Streptomyces species have attracted considerable commercial interest. Soil streptomycetes are major producers of bioactive secondary metabolites [Citation17–20]. It has been reported that organic solvents always provide a higher efficiency in extracting compounds for antimicrobial activities compared to water-based methods [Citation21,Citation22]. In our study, the antimicrobial products were extracted using ethyl acetate; the extract had different levels of activity against the tested Gram-positive, Gram-negative bacteria and C. albicans. Our previous results on the antimicrobial activity against all tested strains, bacteria and C. albicans [Citation14], showed that the halo diameters obtained with S. aureus NRRL B-313, multidrug-resistant S. aureus and Bacillus subtilis NRRL B-543 were 27, 20 and 20 mm, respectively, whereas those obtained with E. coli NRRL B-210, P. aeruginosa NRRL B-23, multidrug-resistant P. aeruginosa T9934 and C. albicans NRRL Y-477 were 18, 17, 15 and 17 mm, respectively.
Different solvents like benzene, diethyl ether, ethyl acetate, hexane, chloroform, petroleum ether, butanol and carbon tetrachloride were used and tested for the extraction of the antibiotic. As seen in , ethyl acetate was the best solvent for maximum antibiotic extraction. No antimicrobial metabolite activity was seen with petroleum ether and solvent control wells.
Table 1. Diameter of inhibition zone (mm) formed by multidrug-resistant Staphylococcus aureus following exposure to crude extracts from the fermentation broth of Streptomyces anulatus NEAE- 94.
Thin-layer chromatography
The purification and separation of crude compounds was done using TLC [Citation23]. The Rf value was calculated to be 0.83 (). Then the plates were viewed under UV light. The spot was scrapped out and put in vial, then checked again for antimicrobial activity by the agar well diffusion method. The purified oily, yellowish orange active compound () yielded by the filtrate of S. anulatus NEAE-94 was subjected to spectroscopic analyses in an effort to elucidate its structure. The chemical structure of the antibiotic was studied on the basis of UV, FT-IR, MS, 1H-NMR and GC–MS analysis.
Table 2. Physico-chemical properties of bioactive metabolites in the ethyl acetate extract from the fermentation broth of Streptomyces anulatus NEAE-94.
UV spectroscopy
The UV absorption spectrum of the purified active compound (dissolved in chloroform) indicated the presence of R-COOH with a maximum absorption at λmax of 206 nm, C = O group with a maximum absorption at λmax of 218 nm, conjugated polyene with a maximum absorption at λmax of 225 nm, CHCl3 solvent with a maximum absorption at λmax of 237 nm (CHCl3), aromatic ring with a maximum absorption at a λmax of 256 nm, C = O group with a maximum absorption at λmax of 428 nm, (conjugated double bond system) with a maximum absorption at λmax of 434 nm and polypyrrole with a maximum absorption at λmax of 446 nm.
FT-IR spectroscopy
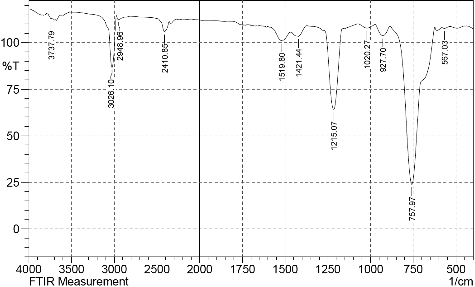
The FT-IR spectra exhibited bands at 567.03, 757.97, 927.7, 1020.27, 1215.07, 1421.44, 1519.8, 2410.85, 2948.96, 3026.1 and 3737.79 cm−1 from which the presence of alkanes, alkenes, hydroxyl and carboxyl groups was inferred [Citation24,Citation25]. The IR spectrum of the compound () included a diagnostic peak at 3737 cm−1, which is indicative of the –OH group. However, the peak at 3026 cm−1 was assigned to aromatic C–H stretching. The peak appearing at 2948 cm−1 was assigned to CH3– and –CH2– stretching (alkanes). The peak appearing at 2410 cm−1 was assigned to OH (alcohol) symmetric stretching. The peak appearing at 1519 cm−1 was assigned to C = C stretching (alkene). The peak appearing at 1421 cm−1 was assigned to COO−1 stretching (fatty acid ester). The peak appearing at 1215 cm−1 was assigned to C–O group. The peak appearing at 1020 cm−1 was assigned to ether. The peak appearing at 927 cm−1 was assigned to CH3. The peak appearing at 757 cm−1 was assigned to alkene and the peak appearing at 567 cm−1 was assigned to alkyl halide.
1H-NMR spectrum
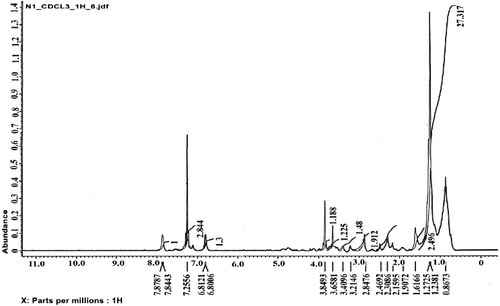
The 1H-NMR spectrum of the isolated compound ( and ) revealed RCH2R group in the region of 1.23–1.27 ppm; two solvent residual peaks at 7.2 and 0.86 ppm; a peak at 1.6 ppm, which was assigned to RCH2 CH2R (alkane); a peak at 1.9 ppm, which was assigned to R2C = CRCHR2 (alkene); a peak in the region of 2.1–2.3 ppm, which was assigned to RCOCH3; a peak at 2.5 ppm, which was assigned to COOR (fatty acid ester); a peak at 2.8 ppm, which was assigned to ArCH2R; a peak at 3.2 ppm, which was assigned to R2CHOR; a peak at 3.4 ppm, which was assigned to RCH2OH; a peak at 3.6 ppm, which was assigned to OCOR (fatty acid ester); a peak at 3.84 ppm, which was assigned to RCOOCH3 and ones in the region of 6.8–6.81 ppm and 7.84–7.87 ppm, which were attributed to aromatic H.
Table 3. 1H-NMR chemical shifts of the bioactive metabolites in the ethyl acetate extract from the fermentation broth of Streptomyces anulatus NEAE-94.
MS spectroscopy
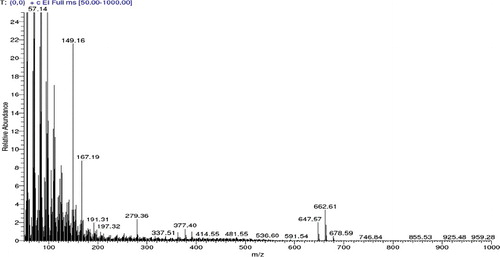
The electron impact (EI) mass spectrum confirmed that the molecular weight of the antibiotic was 1255.40 g/mole (M+ peak) ( and ).
Table 4. Bioactive constituents identified in the ethyl acetate extract from the fermentation broth of S. anulatus NEAE-94 using GC-MS analysis.
GC–MS spectroscopy
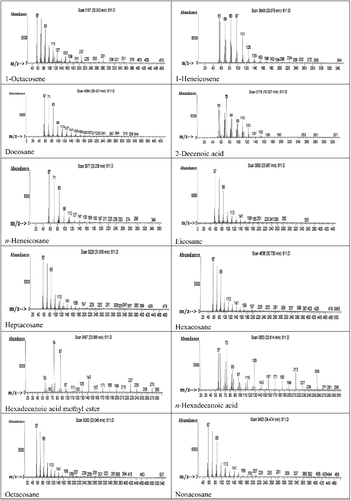
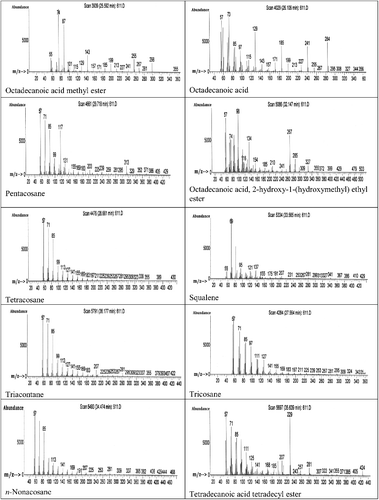
GC–MS analysis of the ethyl acetate extract was carried out and the profiles of the fractions indicated the presence of a different number of chemical compounds with different retention times and abundance. The chemical structures of the components are illustrated in . GC–MS analysis indicated presence of 22 major compounds including an unsaturated fatty acid (2-decenoic acid), saturated fatty acids (n-hexadecanoic acid and octadecanoic acid), fatty acid esters (hexadecanoic acid methyl ester, octadecanoic acid methyl ester, octadecanoic acid-2-hydroxy-1 (hydroxymethyl) ethyl ester and tetradecanoic acid tetradecyl ester), alkanes (nonadecane, docosane, n-heneicosane, tetracosane, pentacosane, hexacosane, heptacosane, octacosane, eicosane and triacontane), alkenes (1-heneicosene and 1-octacosene) and a triterpene (squalene) (). The highest peak area (%) of 9.83 was obtained for octadecanoic acid with retention time of 26.11 min and the lowest peak area (%) of 0.67 was obtained for 1-octacosene with retention time of 32.55 min (). The constituents were characterized as the follows:
2-Decenoic acid: GC-EIMS: m/z (rel. int.) = 171(3), C10 H18 O2[M+], 152(8), 137(3), 123(22), 110(45), 99(40), 84(46), 73(100), 69(58), 56(64), 55(68).
n-Nonadecane: GC-EIMS: m/z (rel. int.) = 268(20), C19H40[M+], 254(3), 239(4), 225(4), 211(6), 197(6), 183(6), 169(7), 155(7), 141(10), 127(12), 113(15), 99(18), 85(57), 71(77), 57(100).
Hexadecanoic acid methyl ester: GC-EIMS: m/z (rel. int.) = 270(15), C17H34O2[M+], 256(3), 239(12), 227(20), 213(4), 199(5), 185(5), 171(4), 157(3), 143(23), 129(12), 120(3), 111(3), 97(10), 87(73), 74(100), 65(3), 57(18), 55(22).
Hexadecanoic acid: GC-EIMS: m/z (rel. int.) = 256(45), C16H32O2[M+], 239(3), 227(15), 213(47), 199(15), 185(42), 171(42), 157(42), 143(15), 129(65), 115(41), 97(43), 85(50), 73(100), 71(56), 60(73), 57(75), 55(57).
n-Eicosane: GC-EIMS: m/z (rel. int.) = 282(12), C20H42[M+], 267(12), 256(3), 241(6), 225(5), 201(5), 185(6), 169(6), 141(7), 113(15), 85(60), 69(25), 71(81), 57(100), 55(28).
1-Heneicosene: GC-EIMS: m/z (rel. int.) = 294, C21H42[M+], 288(3), 272(3), 252(3), 238(3), 224(5), 206(3), 194(3), 182(3), 168(4), 153(4), 139(7), 125(23), 111(45), 97(93), 83(100), 69(83), 57(83), 55(90).
n-Heneicosane: GC-EIMS: m/z (rel. int.) = 296(12), C21H44[M+], 281(3), 267(3), 253(3), 239(4), 225(4), 211(4), 197(4), 183(5), 169(5), 155(6), 141(7), 127(10), 113(12), 99(21), 97(26), 85(59), 71(79), 57(100), 55(28).
Octadecanoic acid methyl ester: GC-EIMS: m/z (rel. int.) = 298(21), C19H34O2[M+], 281(3), 267(10), 255(25), 241(5), 227(3), 213(6), 199(20), 185(6), 177(3), 157(4), 143(26), 129(8), 115(4), 101(6), 87(75), 75(22), 74(100), 55(22).
Octadecanoic acid: GC-EIMS: m/z (rel. int.) = 284(52), C18H36O2[M+], 255(7), 241(50), 227(14), 213(4), 199(14), 185(50), 171(14), 157(8), 143(12), 129(66), 115(14), 97(46), 85(48), 73(100), 60(73), 57(84), 55(6).
n-Docosane: GC-EIMS: m/z (rel. int.) = 310(82), C22H46[M+], 284(9), 267(10), 241(15), 225(15), 211(15), 197(16), 183(15), 169(18), 155(17), 141(18), 127(21), 113(25), 99(32), 85(69), 71(87), 69(28), 57(100), 55(28).
Tricosane: GC-EIMS: m/z (rel. int.) = 324(9), C23H48[M+], 309(8), 295(5), 281(6), 267(6), 253(5), 239(6), 225(6), 211(6), 197(5), 183(6), 169(7), 155(7), 141(8), 127(11), 125(16), 113(15), 111(27), 99(20), 97(40), 85(54), 83(52), 71(73), 57(100), 55(85).
n-Tetracosane: GC-EIMS: m/z (rel. int.) = 338(80), C24H50[M+], 309(10), 295(12), 281(14), 267(16), 253(15), 239(15), 225(16), 211(15), 197(15), 183(16), 169(15), 155(16), 141(17), 12(18), 113(22), 99(24), 85(65), 71(82), 57(100), 55(23).
n-Pentacosane: GC-EIMS: m/z (rel. int.) = 352(75), C25H52[M+], 329(7), 313(25), 295(8), 281(13), 259(13), 239(16), 225(14), 203(15), 183(16), 169(16), 155(17), 141(20), 131(26), 117(65), 99(32), 85(66), 71(81), 57(100), 55(27).
n-Hexacosane: GC-EIMS: m/z (rel. int.) = 366(36), C26H54[M+], 337(5), 323(5), 309(7), 295(7), 281(8), 267(8), 253(8), 239(9), 225(8), 211(9), 197(9), 183(10), 169(10), 155(12), 141(13), 127(15), 113(18), 111(24), 99(26), 97(35), 85(67), 71(83), 57(100), 55(23).
n-Heptacosane: GC-EIMS: m/z (rel. int.) = 380(3), C27H56[M+], 357(5), 341(4), 325(5), 309(4), 281(6), 253(6), 225(6), 197(6), 183(6), 169(7), 155(8), 141(9), 127(12), 113(17), 111(18), 99(26), 97(27), 85(67), 71(84), 57(100), 55(25).
Octadecanoicacid 2-hydroxy-1-(hydroxymethyl)ethyl ester: GC-EIMS: m/z (rel. int.) = 358, C21H42O4[M+], 327(15), 309(3), 285(25), 267(57), 241(3), 210(10), 185(10), 154(15), 134(52), 116(48), 98(100), 84(58), 74(83), 57(84), 55(62).
1-Octacosene: GC-EIMS: m/z (rel. int.) = 392, C28H56[M+], 379(3), 351(3), 327(3), 309(3), 281(10), 253(4), 225(3), 207(20), 191(4), 169(7), 153(18), 127(30), 111(34), 85(68), 71(88), 69(58), 57(100), 55(39).
n-Octacosane: GC-EIMS: m/z (rel. int.) = 394(18), C28H58[M+], 369(6), 351(6), 323(5), 301(5), 281(6), 259(5), 239(7), 207(7), 189(6), 169(8), 155(7), 141(72), 127(12), 113(15), 111(25), 99(27), 85(66), 71(84), 57(100), 55(24).
Squalene: GC-EIMS: m/z (rel. int.) = 410(3), C30H50[M+], 386(3), 367(3), 341(3), 327(3), 313(3), 299(3), 281(4), 267(3), 253(3), 231(3), 207(5), 191(4), 175(3), 155(3), 137(17), 121(17), 95(17), 81(53), 71(19), 69(100), 57(19), 55(17).
n-Nonadecane: GC-EIMS: m/z (rel. int.) = 408(27), C29H60[M+], 382(3), 365(6), 337(4), 309(5), 281(5), 267(6), 253(6), 225(6), 207(7), 191(7), 183(7), 169(7), 155(8), 141(10), 127(13), 113(16), 99(26), 97(25), 85(66), 71(83), 69(28), 57(100).
Tetradecanoic acid tetradecylester: GC-EIMS: m/z (rel. int.) = 424(8), C28H56O2[M+], 405(3), 385(3), 371(3), 355(3), 341(3), 322(3), 307(3), 281(15), 257(10), 243(5), 229(97), 207(30), 185(10), 168(10), 141(10), 125(28), 111(40), 85(58), 71(75), 69(60), 57(100).
Triacontane: GC-EIMS: m/z (rel. int.) = 422(3), C30H62[M+], 407(3),393(3), 379(3), 355(3), 33793), 323(3), 309(3), 259(3), 281(6), 267(3), 253(3), 239(3), 225(3), 207(8), 183(4), 169(5), 155(10), 141(12), 127(20), 113(22), 99(27), 85(67), 71(85), 69(28), 57(100).
Figure 5. Chemical structure of bioactive components identified in the ethyl acetate extract from the fermentation broth of Streptomyces anulatus NEAE-94, using GC–MS analysis.
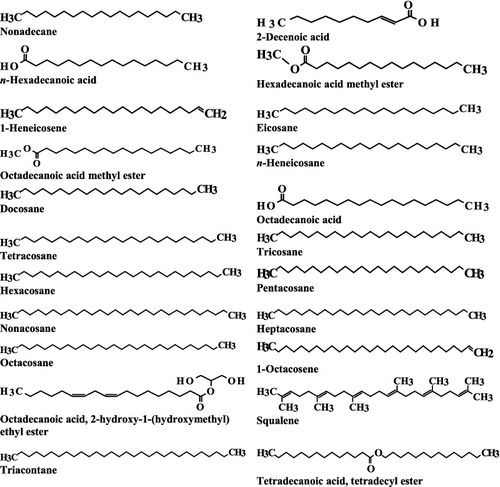
Figure 6. GC–MS chromatogram of bioactive components identified in the ethyl acetate extract from the fermentation broth of Streptomyces anulatus NEAE-94.
Some of these fatty acids have been detected from actinobacteria. Fatty acids are promising as antimicrobial agents in medicine and the food industry due to their safety, non-specific mode of action and the lack of resistance mechanisms against the actions of these fatty acids [Citation26,Citation27]. When S. aureus colonization occurs in the skin, human skin summons a battery of host-defence mechanisms responsible for natural protective immunity, which include fatty acids and antimicrobial peptides [Citation28]. The most potent antibacterial fatty acid in mice is 9-hexadecenoic acid, and in humans, its isomer 6-hexadecenoic acid [Citation29]. In humans and mice, these two 16-carbon monounsaturated fatty acids block the growth of S. aureus in the skin [Citation29–31]. It has been hypothesized that fatty acids act by destabilizing the lipid bilayers of the bacterial membrane and disrupting the membrane functions [Citation32] due to a range of effects on cellular metabolism [Citation33–38].
Cis-2-decenoic acid (C2DA) is an unsaturated fatty acid. It disperses biofilm in many strains of micro-organisms [Citation39]. C2DA has been reported to inhibit the growth of multidrug-resistant S. aureus and biofilm in vitro [Citation39]. In 2006, Karanja et al. [Citation40] identified non-adecane from Chy 4-10, a novel Streptomyces isolate from Chyulu National Park, by GC–MS. Nonadecane is a component of the bacteriocin produced by a mixed culture of Aeromonas sp. and Enterobacter sp. and is responsible for its high antibiofilm activity [Citation41]: it has cytotoxic and antimicrobial effect [Citation42]. Selvin et al. [Citation43] detected hexadecanoic acid and hexadecanoic acid methyl ester from Nocardiopsis dassonvillei MAD08 by GC–MS analysis. They act as antioxidant, hypocholesterolemic nematicide, pesticide, lubricant, antiandrogenic, flavour, hemolytic and 5-alpha reductase inhibitor. Other constituents of the Streptomyces Chy 4-10 extract detected by GC–MS are n-hexadecanoic acid, squalene and tetracosane [Citation40]. Tetracosane acts as antioxidant [Citation44] and squalene has antibacterial, antioxidant, antitumour, cancer preventive and immunostimulant activity [Citation45].
Nandhini et al. [Citation46] reported antifungal compounds produced by Streptomyces rubralavendulae strain SU1; the antifungal compounds were identified by GC–MS analysis as 2-isopropyl-5-methyl-1-heptanol and eicosane. Eicosane has been reported as an antifungal compound [Citation40]. Shiyamala et al. [Citation47] identified 1-heneicosene from marine Kocuria sp. SRS88 by GC–MS analysis. Using GC–MS analysis, Nandhini et al. [Citation48] identified n-heneicosane from marine Streptomyces as antibacterial agent. Akpuaka et al. [Citation49] analysed octadecanoic acidmethyl ester from Azadirachta indica A. Juss (Neem) leaves by GC–MS analysis. GC–MS of Cytosoria compressa and Spongia officinalis extracts contained fatty acids (hexadecanoic acid and octadecanoic acid) and fatty acid esters (hydroxy ester of hexadecanoic and octadecanoic acids) as the basic components [Citation50]. Fatty acids are known to act as anionic surfactants and to show antibacterial and antifungal activity at low pH. [Citation50] Octadecanoic acid, which has antifungal, antitumour and antibacterial activity [Citation42,Citation50], has been identified to be produced by a novel Streptomyces isolate Chy 2-3 from Chyulu National Park [Citation40].
Another novel Streptomyces isolate from Chyulu National Park, Chy 15-5, was identified by GC–MS analysis to produce docosane, tricosane, octacosane and pentacosane [Citation40]. These compounds have antibacterial activity [Citation51,Citation52] and octacosane has antiviral activity as well [Citation53]. Karanja et al. [Citation40] also identified hexacosane from a novel Streptomyces isolate, RUJ7-1, from Ruma National Park by GC–MS analysis. Hexacosane has been shown to exhibit potent antibacterial activity against 25 phytopathogenic bacterial strains [Citation54] and antioxidant activity [Citation55]. Stearic acid 2-hydroxy-1-(hydroxymethyl) ethyl ester has antifungal activity [Citation56]. Seong [Citation57] identified octadecanoic acid 2-hydroxy-1-(hydroxymethyl) ethyl ester from Paeonia japonica and reported its antifungal activity against C. albicans. There are few reports on the activity of octacosene and tetradecanoic acid tetradecyl ester, although tetradecanoic acid is known to have potential antibacterial and antifungal activity [Citation58,Citation59]. Triacontane has cytotoxic and antimicrobial effect [Citation42].
Overall, the ethyl acetate extract of S. anulatus NEAE-94 with its 22 bioactive compounds identified by GC–MS analysis in this study showed antimicrobial activity comparable to conventional drugs. According to Lancini et al. [Citation60], the activity of an antibiotic is defined and measured in terms of its ability to inhibit microbial growth (bacteria, fungi) and protozoa. The wide range of bioactive constituents in the ethyl acetate extract of S. anulatus NEAE-94 is in good agreement with other recent studies. For example, Nandhini et al. [Citation48] reported the presence of 19 bioactive compounds in the ethyl acetate extract of Streptomyces cavouresis KUV39, using GC–MS analysis. These results make S. anulatus NEAE-94 an attractive strain for further investigation and industrial exploitation.
Conclusions
In the present study, S. anulatus NEAE-94 showed a broad antimicrobial spectrum, as it inhibited Gram-positive, Gram-negative bacteria, as well as Candida albicans. Based on the data analysis, the S. anulatus NEAE-94 extract could be considered to have biological activity as good as other conventional drugs against such micro-organisms. Further studies are suggested in the future to evaluate the pharmacological potentials of the extracted bioactive metabolites. On the basis of the results of this work, we can recommend the wild isolate S. anulatus NEAE-94 as a good source of bioactive metabolites that inhibited Gram-positive, Gram-negative bacteria as well as fungi. It shows promising potential to be employed as antibiotic for treatment of human pathogenic bacterial and fungal infections.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Mayer KH, Hopkins JD, Gilleece ES, et al. Molecular evolution, species distribution, and clinical consequences of an endemic aminoglycoside resistance plasmid. Antimicrob Agents Chemother. 1986;29:628–633.
- Bhavanani SM, Ballow CH. New agents for gram-positive bacteria. Curr Op Microbiol. 2000;3:528–534.
- Chambers HF, DeLeo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nature Rev Microbiol. 2009;7(9):629–641.
- Motta AS, Cladera-Olivera F, Brandelli A. Screening for antimicrobial activity among bacteria isolated from the Amazon Basin. Brazilian J Microbiol. 2004;35:307–310.
- Kieser T, Bibb MJ, Buttner MJ, et al. Practical Streptomyces genetics. 2nd ed. Norwich: John Innes Foundation; 2000.
- Mohamedin AH, El-Naggar NE, Sherief AA, et al. Optimization of bioactive metabolites production by a newly isolated marine Streptomyces sp. using statistical approach. Biotechnol. 2015;14:211–224.
- Sahin N, Ugur A. Investigation of the antimicrobial activity of some Streptomyces isolates. Turk J Biol. 2003;27:79–84.
- Raja A, Prabakarana P. Actinomycetes and drug – an overview. Am J Drug Disc Dev. 2011;1:75–84.
- Hanson JR. Natural products: the secondary metabolites. Cambridge: RSC; 2003.
- Zähner H, Drautz H, Weber W. Bioactive microbial products: search and discovery. New York: Academic Press; 1982.
- Hopkins DW, Bibb MJ, Chater KF, et al. Genetic manipulation of Streptomyces. A laboratory manual. Norwich: The John Innes Institute; 1985.
- El-Naggar NE, Mohamedin AH, Sherief AA, et al. Optimization of fermentation conditions for production of bioactive metabolites effective against Staphylococcus epidermidis by a newly isolated Nocardiopsis chromatogenes strain SH89 using the response surface methodology. J Pure Appl Microbiol. 2016;823–839.
- Miller WL. Recent advances in the photochemistry of natural dissolved organic matter. In: Helz GR, Zepp RG, Crosby DG, editors. Aquatic and surface photochemistry. Ann Arbor (MI): Lewis Publishers; 1994. p. 111–127.
- El-Naggar NE, El-Bindary AA, Nour NS. Statistical optimization of process variables for antimicrobial metabolites production by Streptomyces anulatus NEAE-94 against some multidrug-resistant strains. Int J Pharmacol. 2013;9(6):322–334.
- Berdy J. Bioactive microbial metabolites. J Antibiot. 2005;58:1–26.
- Pathom-aree W, Stach JEM, Ward AC et al. Diversity of actinomycetes isolated from challenger deep sediment (10, 898 m) from the Mariana Trench. Extremophiles. 2006;10:181–189.
- Cho KW, Lee HS, Rho JR, et al. New lactone-containing metabolites from a marine-derived bacterium of the genus Streptomyces. J Nat Prod. 2001;64: 664–667.
- Sanchez-Lopez JM, Martinez IM, Perez BJ, et al. New cytotoxic indolic metabolites from a marine Streptomyces. J Nat Prod. 2003;66:863–864.
- Lee HB, Kim Y, Kim JC, et al. Activity of some aminoglycoside antibiotics against true fungi, Phytophthora and Pythiums pecies. J Appl Microbiol. 2005;99: 836–843.
- Jensen PR, Mince TJ, Williams PG, et al. Marine actinomycete diversity and natural product discovery. J Ant Leeuw. 2005;87:43–48.
- Masuda M, AbeT, Sato S. Diversity of halogenated secondary metabolites in the red alga Laurencia nipponica (Rhodomelaceae, Ceramiales). J Phycol. 1997;33:196–208.
- Lima-Filho JVM, Carvalho AFFU, Freitas SM, et al. Antibacterial activity of extracts of six macroalgae from the north eastern Brasilian coast. Brazi J Microbiol. 2002;33: 311–314.
- Usha NS, Masilamani SM. Bioactive compounds produced by Streptomyces strain. Int J Pharm Pharma Sci. 2013;5(1):176–178.
- Augustine SK, Bhavsar SP, Kapadnis BP. A non-polyene antifungal antibiotic from Streptomycetes albidoflavus PU 23. J Biosci. 2005;30: 201–211.
- Dhanasekaran D, Thajuddin N, Panneerselnam A. An antifungal compound: 4′ phenyl -1- napthyl – phenyl acetamide from Streptomyces sp. Med Biol. 2008;15:7–12.
- Desbois AP, Smith VJ. Antibacterial free fatty acids: activities, mechanisms of action and biotechnological potential. Appl Microbiol Biotechnol. 2010;85:1629–1642.
- Desbois AP. Potential applications of antimicrobial fatty acids in medicine, agriculture and other industries. Recent Pat Antiinfect Drug Discov. 2012;7:111–122.
- Smith KR, Thiboutot DM. Thematic review series: skin lipids. Sebaceous gland lipids: friend or foe? J Lipid Res. 2008;49:271–281.
- Takigawa H, Nakagawa H, Kuzukawa M, et al. Deficient production of hexadecanoic acid in the skin is associated in part with the vulnerability of atopic dermatitis patients to colonization by Staphylococcus aureus. Dermatol. 2005;211:240–248.
- Kenny JG, Deborah W, Elisabet J, et al. The Staphylococcus aureus response to unsaturated long chain free fatty acids: survival mechanisms and virulence implications. PLoS One [ Internet]. 2009 [ cited 2016 Jul 12];4(2):e4344. Available from: http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0004344
- George P, Crozat K, Lauth X, et al. A toll-like receptor 2-responsive lipid effector pathway protects mammals against skin infections with gram-positive bacteria. InfectImmun. 2005;73(8):4512–4521.
- Parsons JB, Yao J, Frank MW, et al. Membrane disruption by antimicrobial fatty acids releases low-molecular-weight proteins from Staphylococcus aureus. J Bacteriol. 2012;194(19):5294–5304.
- Greenway DL, Dyke KG. Mechanism of the inhibitory action of linoleic acid on the growth of Staphylococcus aureus. J Gen Microbiol. 1979;115:233–245.
- Galbraith H, Miller TB. Effect of long-chain fatty acids on bacterial respiration and amino acid uptake. J Appl Bacteriol. 1973;36: 659–675.
- Knapp HR, Melly MA. Bactericidal effects of polyunsaturated fatty acids. J Infect Dis.1986;154:84–94.
- Butcher GW, King G, Dyke KG. Sensitivity of Staphylococcus aureus to unsaturated fatty acids. J Gen Microbiol.1976;94:290–296.
- Chamberlain NR, Mehrtens BG, Xiong Z, et al. Correlation of carotenoid production, decreased membrane fluidity, and resistance to oleic acid killing in Staphylococcus aureus 18Z. InfectImmun. 1991;59:4332–4337.
- Sado-Kamdem SL, Vannini L, Guerzoni ME. Effect of α-linolenic, capric and lauric acid on the fatty acid biosynthesis in Staphylococcus aureus. Int J Food Microbiol. 2009;129:288–294.
- Jennings JA, Courtney HS, Haggard WO. Cis-2-decenoic acid inhibits S. aureus growth and biofilm in vitro: a pilot study. Clin Orthop Relat R. 2012;470:2663–2670.
- Karanja EN, Muigai AW, Wamunyokoli F, et al. Growth characteristics and production of secondary metabolites from selected novel Streptomyces species isolated from selected Kenyan national parks. Nairobi: Jomo Kenyatta University of Agriculture and Technology; 2006.
- Sengol JS, Balasubramanian V, Rajaram R. Chemical characterization and bioactivity evaluation of bacteriocin from marine biofilm-forming bacteria. Afr J Microbiol Res. 2014;8(41): 3617–3624.
- Hsouna AB, Trigie M, Mansour RB, et al. Chemical composition, cytotoxicity effect and antimicrobial activity of Ceratonia silisqua essential oil with preservative effects against Listeria inoculated in minced beef meat. Int J Food Microbiol. 2011;148(1): 66–72.
- Selvin J, Shanmughapriya S, Gandhimathi R. Optimization and production of novel antimicrobial agents from sponge associated marine actinomycetes Nocardiopsis dassonvillei MAD08. Appl Microbiol Biot. 2009;83: 435–445.
- Kalegari M, Philippsen AF, Dias JFG, et al. Antibacterial, allelopathic and antioxidant activity of extracts and compounds from Roureainduta Planch. (Connaraceae). J Appl Pharm Sci. 2012;2(9):61–66.
- Sermakkani M, Thangapandian V. GC-MS analysis of Сassiaitalica leaf methanol extract. Asian J Pharm Clin Res. 2012;5:90–94.
- Nandhini SU, Bharathy PJ, Rekha S. Antifungal compounds from marine Streptomyces. Int J Pharm Pharm Sci. 2014;7(1):207–209.
- Shiyamala DS, Priya P, Sahadevan R. Pyrrolo [1, 2-A] Pyrazine-1,4-dione, hexahydro-3-(2-methylpropyl)- and phenol, 2,4-Bis (1,1-dimethy ethyl) novel antibacterial metabolites from a marine Kocuria sp. SRS88: optimization and its application in medical cotton gauze cloth against bacterial wound pathogens. Int J pharm Res Dev. 2014;6(2):44–55.
- Nandhini SU, Sangareshwari S, Kumari L. Gas chromatography-mass spectrometry analysis of bioactive constituents from the marine Streptomyces. Asian J Pharm Clin Res. 2015;8(2):244–246.
- Akpuaka A, Ekwenchi MM, Dashak DA, et al. Biological activities of characterized isolates of n-hexane extract of Azadirachta Indica A.Juss (Neem) leaves. NY Sci J. 2013;6(6):119–124.
- Abou-Elela GM, Abd-Elnaby H, Ibrahim AHH, et al. Marine natural products and their potential applications as anti-infective agents. World Sci J. 2009;7:872–880.
- Uma B, Parvathavarthini R. Antibacterial effect of hexane extract of sea urchin Emnopleurus alexandri. Int J Pharm Tech Res. 2010;2:1677–1680.
- Mihailovi V, Vukovi N, Niforovi N, et al. Studies on the antimicrobial activity and chemical composition of the essential oils and alcoholic extracts of Gentiana asclepiadea L. J Med Plants Res. 2011;5:1164–1174.
- Helmy WA, Abd-Alla HI, Amer H, et al. Chemical composition and in-vitro antiviral activity of Azadirachta indica A. Juss (Neem) leaves and fruits against Newcastle disease virus and infectious Bursal disease virus. Aust J Basic Appl Sci. 2007;1:801–812.
- Kotan R, Cakir A, Dadasoglu F, et al. Antibacterial activities of essential oils and extracts of Turkish Achillea, Satureja and Thymus species against plant pathogenic bacteria. J Sci Food Agr. 2010;90:145–60.
- Fethi BJ, Samia AM, Karima BS, et al. Antioxidant activities of essential oil from Cotula coronopifolia L. growing in Tunisia. Afr J Microbiol Res. 2012;6:4388–4395.
- Qing FD, Shu FZ, Zhi W, et al. Antifungal activity of crude extracts and fat-soluble constituents of Holotrichia diomphalia larvae. Bioresour Technol. 2008;99:8521–8523.
- Seong I. Antifungal activity of the extract from Paeonia japonica against Candida albicans. Korean JMedMycol. 2006;11:19–26.
- McGaw LJ, Jager AK, Van SJ. Isolation of antibacterial fatty acids from Schotia brachypetala. Fitoterapia. 2002;73:431–433.
- Seidel V, Taylor PW. In vitro activity of extracts and constituents of Pelagonium against rapidly growing mycobacteria. Int J Antimicrob Agents. 2004;23:613–619.
- Lancini G, Parenti F, Gallo GG. Antibiotics-a multidisciplinary approach. 1st ed. New York: Springer; 1995. p. 278.