ABSTRACT
Four populations of Piptoporus betulinus from Maoer, Changbai, Daxing'an and Xiaoxing'an Mountains were sampled. DNA was isolated and purified from fruiting bodies. Inter simple sequence repeat markers were first used to evaluate the genetic diversity and differentiation of P. betulinus in this study. The results showed that the strains from Xiaoxing'an Mountain had the highest diversity among the four populations, followed by the samples from Maoer and Daxing'an Mountains, whereas the Changbai Mountain samples harboured the lowest value. Cluster analysis and principal coordinates analysis indicated that all of the populations had the same type of geographical distribution (geographical affiliations). These findings will provide useful information for further research and utilization of wood-rotting fungi and will lay the foundation for development of genetic diversity in P. betulinus from various geographic origins.
Introduction
In nature, wood-rotting fungi play an important role in the cyclic utilization of timber. These fungi have a wood biodegradation function due to an efficient enzyme system and include two main basidiomycetes: white-rot fungi, which can break down lignin using a lignin-degrading enzyme, and brown-rot fungi, which hydrolyse cellulose and hemicellulose using cellulase. Piptoporus betulinus is one of the most common of these fungi and can cause brown-rot disease in trees. In addition, this species has been widely used for many centuries in China as a traditional medicine to treat various diseases, including oral ulcer, gastroenteric disorder, hepatocirrhosis, inflammation and cancers [Citation1] and as a source of antioxidant [Citation2], anti-inflammatory [Citation3], antidiabetic and antitumour agents [Citation4]. Therefore, due to its health benefits, P. betulinus has obtained general acceptance worldwide as an effective medicine.
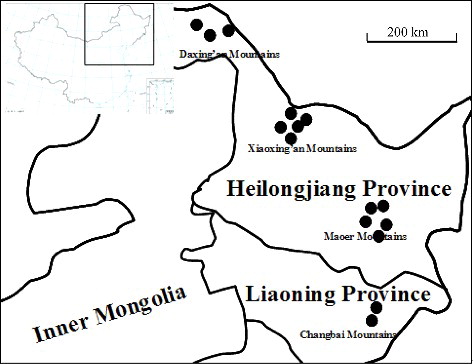
P. betulinus has a global distribution and is found in Europe, Asia, northern and southern Africa and eastern North America [Citation5–7]. In general, the optimal growth temperature of brown-rot fungi is 27–38 °C [Citation6]; thus, P. betulinus is suitably grown in northern China with good distribution, such as in the Heilongjiang, Inner Mongolia and Liaoning Provinces. This brown-rot fungus is especially parasitic on Betula platyphylla [Citation8]. In particular, this fungus can be found in dead, standing trees. This species is easy to identify based on its external fruiting body and has a good distribution in China, making it amenable to population genetics research.
To date, many studies have assessed genetic variation in fungi using molecular marker techniques [Citation9–11]. Among these techniques, inter simple sequence repeat (ISSR) markers are co-dominant traits following Mendelian patterns. This method has the advantages of random amplified polymorphic DNA and amplified fragment-length polymorphism. Accordingly, it shows simplicity, reliability, a moderate through-put ratio and easy sequencing of selected bands [Citation12]. Therefore, ISSR markers have been applied for map construction [Citation13], comparative genetics [Citation14] and genetic diversity analyses [Citation15].
Currently, there are large numbers of reports on genetic diversity using ISSR markers in different fungi, such as Chinese Lentinula [Citation16], Solanum nigrum L. [Citation17] and Tilletia controversa Kühn [Citation18]. To increase the efficiency of characterization and utilization of P. betulinus germplasm resources, an ISSR marker was first used in this investigation to analyse the genetic variability and differentiation of this fungus, collected mainly from north-eastern China, for further effective protection, management and use of P. betulinus. Therefore, this work attempts to establish suitable media and to lay the foundation for further study and reasonable application of wood-degrading fungi.
Materials and methods
Strains
The four populations of P. betulinus were sampled from Maoer Mountain (Heilongjiang, China), Changbai Mountain (Jilin, China), Daxing'an Mountain (Heilongjiang, China) and Xiaoxing'an Mountain (Heilongjiang, China) (). Detailed locations of the studied populations are provided in . Fruiting bodies were cut into small pieces (0.5 mm), surface sterilized in ethyl alcohol (75.0%, w/v) for 1 min, rinsed 4–5 times in sterile deionized water and cultured on potato dextrose agar at 28 °C in order to obtain mycelia. The mycelia were stored at 4 °C for genomic DNA extraction [Citation19].
Table 1. Sampling sites of P. betulinus.
DNA extraction
For each sample, genomic DNA was extracted from 0.1 g of mycelium using the cetyl trimethylammonium bromide method of Doyle [Citation20]. The integrity and purity of isolated DNA was checked by gel electrophoresis in a 1.0% agarose gel. The DNA concentration was determined at 260 nm using a spectrophotometer (UV-1800, Shimadzu, Kyoto, Japan). Then, the DNA samples were diluted to 50 ng/μL and stored at −20 °C for polymerase chain reaction (PCR) amplification.
ISSR amplification
The ISSR reaction was performed as previously described by Meng et al. [Citation21] with minor modifications. A total of 100 primers used in this study were obtained from the University of British Columbia, Vancouver, Canada (www.ubc.ca/). ISSR--PCR amplification was performed in a 200 μL centrifuge tube containing 50 ng of template DNA, 10 pmol primers, 0.25 mmol/L deoxyribonucleoside triphosphates, 0.5 U of Taq polymerase (TAKARA, Kyoto, Japan), 1× PCR buffer and 2.5 mmol/L Mg2+. DNA amplification was carried out in a Perkin–Elmer Cetus DNA 480 thermal cycler (Perkin-Elmer Cetus, Norwalk, CT, USA). The cycling programme was as follows: 1 cycle of 3 min at 94 °C; then 40 cycles of 1 min at 94 °C, 1 min at 55 °C and 1 min at 72 °C, followed by a final cycle of 10 min at 72 °C. The amplification products were detected in a 1.5% agarose gel in 1× Tris–acetate–ethylenediaminetetraacetic acid buffer for 1 h at 100 V and photographed by Bio Image Systems (GG/D2-GeneGenius2, Frederick, MD, USA), and their molecular weights were estimated based on the DL 2000 Marker (TAKARA, Kyoto, Japan).
Data analysis
Each primer was used to amplify all of the samples, and the band patterns with high resolution, good repeatability and reproducibility were scored manually as present (1) or absent (0) to produce a set of binary code for ISSR analysis. To estimate and embody the genetic diversity level of P. betulinus, several parameters were calculated using PopGen32 software (version 1.32), including the observed number of alleles (Na), the effective number of alleles (Ne), Nei's gene diversity (h), Shannon's information index (I), the number of polymorphic loci (PL) and percentage of polymorphic loci (PPL) of populations, Nei's genetic differentiation index (Gst), the total gene diversity (Ht), the genic diversity within a population (Hs), the gene flow (Nm), Nei's gene diversity (H) and Nei's genetic distance (D) [Citation22]. Analysis of molecular variance was used to determine the genetic differentiation and relationship between populations [Citation23,Citation24]. The unweighted pair group method with arithmetic average (UPGMA) dendrogram construction based on Jaccard's similarity coefficients was performed with the SHAN program [Citation25,Citation26]. Finally, principal coordinates analysis (PCoA) was performed to generate three-dimensional (3D) scatter plots and evaluate the genetic distribution of individual accessions [Citation27].
Results and discussion
In the present study, 7 out of 100 ISSR primers with clear high-intensity and relatively high polymorphism bands were selected and used to amplify 15 P. betulinus strains from four populations. A total of 74 reproducible fragments were obtained with an average of 10.57 fragments per primer, and the band size varied from 150 to 2000 bp. The number of PL ranged from 7 (I857) to 14 (I825) (). All of these amplified bands were polymorphic (100%).
Table 2. PCR products amplified by using each primer in P. betulinus based on ISSR markers.
As shown in , the observed number of alleles (Na), the effective number of alleles (Ne), Shannon's index (I) and Nei's gene diversity (h) in population 1 (Xiaoxing'an Mountain) were the highest among the four populations (Na = 1.7435, Ne = 1.4418, I = 0.3896, h = 0.2591). The above four parameters were the lowest in population 3 from Maoer Mountain (Na = 1.1892, Ne = 1.1338, I = 0.1144, h = 0.0784). PL and PPL are important indicators for estimating the level of variation and are also significant for the diversity among species. Species harbouring a high PPL have strong environmental adaptiveness and competitiveness, whereas those with a low PPL have a weak environmental adaptiveness and may even be eliminated in long-term evolution. By analysing the number of alleles and PPL using ISSR, we found that the PL ratio in the four locations ranged from 18.9% to 74.3%, indicating high genetic variation of P. betulinus in these locations ().
Table 3. Nei's gene diversity analysis in P. betulinus from different collections.
The highest values of PPL, PL and I observed through ISSR analysis in population 1 from Xiaoxing'an Mountain indicate higher genetic diversity of P. betulinus from Xiaoxing'an Mountain than in the population from Changbai Mountain, where these indices were the lowest. This difference might be due to the geographical location ( and ). Changbai Mountain, with relatively abundant rainfall, has the highest altitude of the four sampled areas and is affected by low temperature and ultraviolet stress, resulting in the relative isolation of P. betulinus. Indeed, similar results have been reported for Saccharomyces cerevisiae [Citation28] and marine fungi [Citation29]. Persson et al. [Citation30] reported that the genetic diversity among populations of Lilium martagon L. was positively correlated with the total number of samples in a plant. However, 2–5 samples from each area, providing a total of 15 samples, might affect the estimate of diversity of the populations to a certain degree. It is noteworthy that the propagation characteristics of fungi are markedly different from those of plants, mainly depending on wind power; thus, further study of the relation between the population and diversity of fungi is needed.
Here, we found that the genetic identity of P. betulinus ranged from 0.1526 to 0.2289, and the distance from 0.7954 to 0.8585 (). These genetic data indicate that the four populations were related but not exactly the same. It could be expected that populations with lower genetic distance must have a higher genetic similarity.
Table 4. Nei's genetic similarity (above diagonal) and genetic distance (below diagonal) coefficient of groups of P. betulinus.
Genetic similarity and distance reflect differentiation in different ways (). A high level of differentiation could be caused by genetic drift and gene flow [Citation31]. There are multiple explanations for this, including that the diffusion and spread of conidiophores are important factors influencing gene flow, which further affects the genetic structure and diversity of populations [Citation32,Citation33]. In addition, wind power plays a vital role in the spreading process. The distance of spore dissemination is restricted by wind strength and environmental conditions (e.g. geographical isolation), resulting in gene exchange among different groups. On the other hand, P. betulinus is a major parasite that is found on dead standing trees or fallen trees of birch and related hosts, which are relatively few and scattered in these four sites, causing slow self-renewal and limited population expansion. Therefore, low gene flow, limited spore spreading and limited population sizes may be considered to have caused the genetic differentiation of the studied P. betulinus populations.
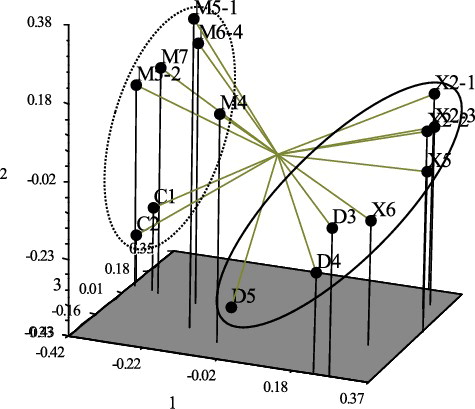
With a common node at a 60% Jaccard's similarity coefficient, the dendrogram clustered the different strains into two independent groups (). Group I included all of the samples from population 1 and population 2. Except for D5, the samples from population 3 and population 4 were placed in the same group (Group II). This result suggests that the clustering depended on the sample collection, indicating a similar genetic background in a collection area. Interestingly, the PCoA clearly separated the 15 accessions into two groups, in accordance with the UPGMA cluster analysis (). These results show the relationship between the genetic characteristics and the geographical distribution of P. betulinus.
Figure 2. UPGMA dendrogram of 15 P. betulinus strains based on Jaccard's coefficient of ISSR marker.
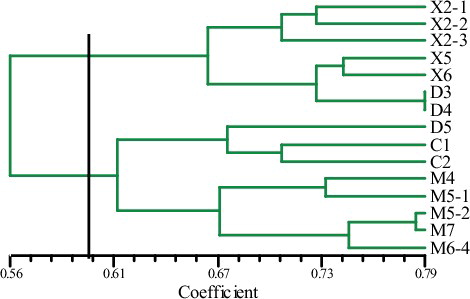
Many previous reports have shown that the genetic diversity of species is markedly related to their geographical distribution [Citation21,Citation27,Citation34]. In agreement with these findings, in this work, 15 individuals were separated into two independent clusters according to UPGMA and PCoA clustering analyses ( and ), reflecting the type of geographic distribution of these fungi. The 15 individual strains collected from four places in north-eastern China could represent the whole forest ecosystem of the Northeast. Group I represents the Songnen Plain, with fertile soil and an extremely low mean annual temperature. Group II samples were mainly found in the Liaodong Peninsula, with a relatively abundant rainfall and high altitude. These results clearly indicate a distinct genetic differentiation between P. betulinus from various geographic origins. Grouping by the two clustering methods revealed geographical affiliations [Citation35]. Therefore, the differentiation of P. betulinus gene pools from different areas might lead to reproductive isolation and divergent natural selection arising from wide geographic separation. This study builds the basis for further effective protection, management and use of P. betulinus germplasm resources in genetics and ecosystems research.
Conclusions
The results from this study showed that, overall, ISSR markers are an effective tool for evaluating the genetic diversity of P. betulinus grown in north-eastern China. Cluster analysis and PCoA indicated that all of the populations had the same type of geographical distribution (geographical affiliations). This study could provide insight into the high level of genetic differentiation and a basis for further, more detailed investigations to understand the contribution to fungi conservation.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Chen W, Zhao Z, Chen SF, et al. Optimization for the production of exopolysaccharide from Fomes fomentarius in submerged culture and its antitumor effect in vitro. Bioresour Technol. 2008;99:3187–3194.
- Lee JS. Effects of Fomes fomentarius supplementation on antioxidant enzyme activities, blood glucose, and lipid profile in streptozotocin-induced diabetic rats. Nutrition Res. 2005;25:187–195.
- Park YM, Kim IT, Park HJ, et al. Anti-inflammatory and anti-nociceptive effects of the methanol extract of Fomes fomentarius. Biol Pharm Bull. 2004;27:1588–1593.
- Lemieszek M, Langner E, Kaczor J, et al. Anticancer effect of fraction isolated from medicinal birch polypore mushroom, Piptoporus betulinus (Bull.: Fr.) P. Karst. (Aphyllophoromycetideae): in vitro studies. Int J Med Mushrooms. 2009;11:351–364.
- Pegler DN. Mushrooms and toadstools of Britain and Europe. London: Pan Macmillan; 1990.
- Schmidt O, Czeschlik D. Wood and tree fungi: biology, damage, protection, and use. Berlin: Springer; 2006.
- Schwarze FW, Engels J, Mattheck C. Fungal strategies of wood decay in trees. Berlin: Springer; 2000.
- Meng F, Liu X, Wang Q. Identification of wood decay related genes from Piptoporus betulinus (Bull. Fr.) Karsten using differential display reverse transcription PCR (DDRT-PCR). Biotechnol Biotechnol Equip. 2012;26:2961–2965.
- Wang Y. Optimization of TRAP-PCR system and a primary study on genetic variation of genes related to lignocellulose enzyme of three wood-rotting fungi. Forestry Sci Technol. 2012;37:4–8.
- Velásquez VB, Cárcamo MP, Meriño CR, et al. Intraspecific differentiation of Chilean isolates of the entomopathogenic fungi Metarhizium anisopliae var. anisopliae as revealed by RAPD, SSR and ITS markers. Genet Mol Biol. 2007;30:89–99.
- Yan D, Hui C, Ming W, et al. ITS and SSR analyses of cultured Hypsizygus marmoreus strains [J]. Acta Agric Shanghai. 2009;3:59–64.
- Li G, Quiros CF. Sequence-related amplified polymorphism (SRAP), a new marker system based on a simple PCR reaction: its application to mapping and gene tagging in Brassica. Theor Appl Genet. 2001;103:455–461.
- Zong C, Song Z, Chen H, et al. Construction of the first genetic linkage map of Salvia miltiorrhiza Bge. using SSR, SRAP and ISSR markers. Acta Pharm Sin. 2015;50:360–366.
- Abdollahi MB, Sadigh P, Azizi H, et al. Comparative assessment of IRAP, REMAP, ISSR, and SSR markers for evaluation of genetic diversity of Alfalfa (Medicago sativa L.). J Agric Sci Technol. 2015;17:999–1010.
- Chiu TH, Kuo CW, Lin HC, et al. Genetic diversity of ivory shell (Babylonia areolata) in Taiwan and identification of species using DNA-based assays. Food Control. 2015;48:108–116.
- Liu J, Wang ZR, Li C, et al. Evaluating genetic diversity and constructing core collections of Chinese Lentinula edodes cultivars using ISSR and SRAP markers. J Basic Microb. 2015;55:749–760.
- Khateeb WA, Al-Qwasemeh H. Cadmium, copper and zinc toxicity effects on growth, proline content and genetic stability of Solanum nigrum L., a crop wild relative for tomato; comparative study. Physiol Mol Biol Plants. 2014;20:31–39.
- Li G, Yu H, Han W, et al. Development of a SCAR marker for molecular detection and diagnosis of Tilletia controversa Kühn, the causal fungus of wheat dwarf bunt. World J Microb Biotechnol. 2014;30:3185–3195.
- Soliman K, Badeaa R. Effect of oil extracted from some medicinal plants on different mycotoxigenic fungi. Food Chem Toxicol. 2002;40:1669–1675.
- Doyle JJ. Isolation of plant DNA from fresh tissue. Focus. 1990;12:13–15.
- Meng F, Wang R, Peng M, et al. Evaluation of genetic diversity among Kongpo Monkshood (Aconitum kongboense L.) germplasm accessions revealed by inter simple sequence repeat markers. Hortscience. 2015;50:940–943.
- Yeh FC, Yang RC, Boyle T, et al. POPGENE, version 1.32: the user friendly software for population genetic analysis. Edmonton: Molecular Biology and Biotechnology Centre, University of Alberta; 1999.
- Excoffier L, Smouse PE, Quattro JM. Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics. 1992;131:479–491.
- Waterman MS, Smith TF. On the similarity of dendrograms. J Theor Biol. 1978;73:789–800.
- NTSYS-pc RF. Numerical taxonomy and multivariate analysis system version 2.0. Setauket (NY): Exeter Publishing; 2000.
- Sneath PH, Sokal RR. Numerical taxonomy: the principles and practice of numerical classification. San Francisco (CA): WF Freeman and CO.; 1973.
- Wang C, Li G, Zhang Z, et al. Genetic diversity of castor bean (Ricinus communis L.) in Northeast China revealed by ISSR markers. Biochem Syst Ecol. 2013;51:301–307.
- Versavaud A, Courcoux P, Roulland C, et al. Genetic diversity and geographical distribution of wild Saccharomyces cerevisiae strains from the wine-producing area of Charentes, France. Appl Environ Microb. 1995;61:3521–3529.
- Richards TA, Leonard G, Mahé F, et al. Molecular diversity and distribution of marine fungi across 130 European environmental samples. Proc R Soc B [Internet]. 2015 [cited 2016 Jul 28];282:20152243. Available from: http://rspb.royalsocietypublishing.org/content/282/1819/20152243.
- Persson HA, Lundquist K, Nybom H. RAPD analysis of genetic variation within and among populations of Turk's‐Cap Lily (Lilium Martagon L.). Hereditas. 1998;128: 213–220.
- Wright S. The genetical structure of populations. Ann Eugenics. 1951;15:323–354.
- Irwin DE. Phylogeographic breaks without geographic barriers to gene flow. Evolution. 2002;56:2383–2394.
- Slatkin M. Gene flow and the geographic structure of natural populations. Science. 1987;236:787–792.
- Meng F, Peng M, Wang R, et al. Analysis of genetic diversity in Aconitum kongboense L. revealed by AFLP markers. Biochem Syst Ecol. 2014;57:388–394.
- Niño-Vega GA, Calcagno AM, San-Blas G, et al. RFLP analysis reveals marked geographical isolation between strains of Paracoccidioides brasiliensis. Med Mycol. 2000;38:437–441.