 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Zucchini yellow mosaic virus (ZYMV) has a wide distribution in Mecca regions and is considered to be a serious threat on watermelon production. The aim of this study was to evaluate the antiviral activity of five medicinal plant extracts, against ZYMV-infecting watermelon plants. The experiment consisted of four application of soaking seeds, in vitro and in vivo experiments. The results of greenhouse experiments with individually treatments by decoction and infusion of Nigella sativa (NS) seeds in soaking and in-vitro experiment, bougainvillea and neem in pre-inoculation and thuja extract following nigella decoction in post inoculation were efficient to reduce and\or inhibit symptoms expression infecting watermelon seedling, whereas, decoction exhibited higher antiviral activity against ZYMV than infusion. The decoction and infusion of NS seed increased the total photosynthesis pigments, total soluble intracellular protein and total phenol contents. In conclusion, the results of the present investigation propose that the bougainvillea and neem extracts may perhaps play a central function in the mechanism of acquired systemic resistance and may produce pathogenesis-related proteins that play a significant role in watermelon seedling resistance against ZYMV.
Introduction
Watermelon (Citrullus lanatus L.; family Cucurbitaceae) is grown widely in the Mecca region (KSA) and is considered as an important crop for agriculture in the country [Citation1]. This crop is infected by a dozen of viruses [Citation2] of which Zucchini yellow mosaic virus (ZYMV) that belongs to the genus Potyvirus, family Potyviridae [Citation3] is regarded as one of the most destructive viruses. Watermelon infected plants exhibit symptoms that vary from mild to server mosaic, mottling and bubbling followed by leaf deformation and blister [Citation4]. ZYMV has a positive single-stranded RNA and flexuous filamentous particles [Citation3].
The antiviral activity of the products including plant extracts and synthetic chemicals is connected to their components which may acts directly by interaction with virus particles in the early stage of infection and block the liberation of its nucleic acid that could finally lead to stopping the virus multiplication [Citation5,Citation6].
Also, these compounds may act indirectly as a trigger of inducing systemic resistance agents in the plants against the virus [Citation5,Citation6]. The Ranunculaceae family includes Nigella sativa (NS), L. plant called black seed (Bailey, 1953). The seeds contain fixed oil, volatile oil and proteins [Citation7–11].
Thuja orientalis (family- Cupressaceae) was used in various forms of traditional medicines. T. orientalis preparations have active component thujone that can be efficiently used against microbial/worm infection, antioxidant, anticancer, antiviral and anti-inflammatory agent [Citation6,Citation12–15].
The inducers such as neem (Azadirachta indica) and Bougainvilla spectabilis extracts were effective in reducing the concentration of viruses in each indicator and host plants, these results were confirmed by ELISA [Citation16]. Therefore, the objective of this study was to evaluate antiviral activity of some medicinal plant extracts against ZYMV, in vitro and in vivo on the watermelon. ZYMV identification was confirmed by double antibody sandwich - enzyme linked immunosorbent assay (DAS-ELISA), ultrathin section by transmission electron microscopy and reverse transcription - polymerase chain reaction (RT-PCR). Also, the effect of the medicinal plant extracts on the virus concentration, infection percentage, severity of disease and physiological characteristics were studied.
Material and methods
Virus sources and isolation
No special permission to access watermelon locations was needed. Noteworthy mentioning that fields in Jeddah, region, subject of this study, did not include endangered or protected species.
During the winter of 2014, samples of watermelon plants exhibiting venial yellowing leaves, mottling, blisters, severe deformation and severe stunting were collected from Jeddah, Kingdom of Saudi Arabia. The presence of ZYMV and other viruses normally present in watermelon (watermelon mosaic virus (WMV); cucumber mosaic virus (CMV) and squash mosaic virus (SqMV) were checked in the collected samples by serological means using DAS-ELISA technique according to the manufacturer's instructions (Sanofi-Santi animal, France), as described by [Citation17] and using optical density at λ = 405 nm in an ELISA micro-well reader (using Dynatech Immunoassay MR 7000). Samples were considered positive when the reaction absorbency was at least twice that of the healthy control.
ZYMV purification and propagation
ZYMV was mechanically transmitted into herbaceous hosts (Chenopodium amaranticolor Coste and Reyn plants) as described by [Citation18], then it was propagated and maintained in squash plants (Cucurbita pepo L.), following the protocol of [Citation19].
Twenty days post inoculation (DPI), the symptoms were recorded and the infected leaves were frozen and used as an inoculums source in further experiments [Citation20].
ZYMV identification using reverse transcription polymerase chain reaction (RT-PCR) technique
RNA extraction
Total RNA was extracted from leaves showing typical symptoms of ZYMV and positive in ELISA using AccuZol™ reagent (Bioneer, Alameda, CA) according to the manufacturer's instructions. After precipitation with ethanol, total RNA was re-solubilized in 25 μL of RNase-free water.
RT-PCR amplification
In this procedure, oligonucleotide primers (reverse primer: 5′-ATGTCGAGTATCACATTTCC-3′: 8200–8220 and forward primer 5′ GGTTCATGTCCCACCAAGC-3′: 8800–8819) were designed to amplify a fragment of the Nuclear inclusion bodies (NIb) and coat protein (CP) coding regions of ZYMV (about 600 bp), overlapping the variable N-terminal part of the CP [Citation21]. These primers were synthesized by MWG-Biotech. Co. (Germany).
RT-PCR was performed in a two-step format using the extracted total RNA. Reverse transcription reaction was done in 25 µL volumes containing 4 µL of template RNA, 1 µL of the reverse primer RT (100 pmol/µL) and 1 µL of Revert Aid™ M-MuLV reverse transcriptase (Promege, USA). This reaction was carried out using a top-heating thermal cycler at 42 °C for 60 min and stopped by incubation at 70 °C for 10 min, as suggested by the manufacturer. For PCR amplification in 25 reaction volumes, 1 µL of the primers forward and reverse (100 pmol/µL), 2.5 µL of 10X Taq reaction buffer (200 mmol/L Tris-HCl, 500 mmol/L KCl, pH 8.4), 0.75 µL MgCl2 (50 m mol/L), 0.5 dNTPs (10 m mol/L) and GoTag polymerase (2.5 U μL) (Promege, USA) were added to each 5 µL of first-strand cDNA reaction mixture. The PCR program consisted of a 3 min heating step at 94 °C, followed by 35 cycles of amplification step of 30 sec at 94 °C, 30 sec at 55 °C and 30 sec at 72 °C. Then, 7 min at 72 °C was performed [Citation22].
PCR products and DNA ladder (GeneRuler™ 250 bp DNA Ladder Plus,(Promege, USA) were analysed by electrophoresis through 1% agarose gels in the presence of 1 μg mL−1 ethidium bromide using 1 X Tris-Borate EDTA (TBE) buffer (89 mmol/µL Tris, 89 mmol/µL boric acid, 2 mmol/µL Na2EDTA, pH 8.3). Gels were visualized and photographed with UV-illuminator.
After electrophoresis, the amplified DNA fragments were purified with DNA purification kit (Amersham Pharmacia Biotech Inc. USA), according to manufacturer's procedure.
Electron microscope
For ultra-thin sectioning of infected and healthy mesophyll tissues, pieces were fixed and stained according to a standard procedure described by [Citation23]. Ultra-thin sections were viewed with a JOEL-JEA100 CX electron microscope Unit.
Medicinal plants
Leaves and fruit samples of T. orientalis in flowering and fruiting stage were collected from the Faculty of Agriculture gardens (Suez Canal University, Egypt) and lyophilized as described by [Citation13]. Accordingly, 100 g of plant powder was added to 300 mL of 80% ethyl alcohol in flask with 1000 mL capacity. The mixture was shaken for 24 hrs by a magnetic stirrer and filtrated by Whatman filter paper (0.2µm). In a water bath at 42 °C, the filtrate was concentrated and stored at −20 °C. Solutions at 6 g/L from the concentrated extracts were prepared in distilled water amended with 0.1% Tween-20 [Citation13].
Ten grams of tested medicinal plants were taken from aqueous infusion prepared in 100 mL distilled water then left for 24 hours at room temperature with occasional shaking, after that clear infusion was obtained by filtration. For the aqueous decoction preparation, 10 g of tested medicinal plants were boiled in 100 mL distilled water in a flask for 20 min and then cooled at room temperature. To obtain clear decoction, the content of the flask was filtered as described by [Citation24].
Extracts of plants from neem (10% w/v) and bougainvillea were prepared following a standard procedure [Citation16].
Plant materials and treatments
Seeds of watermelon susceptible cultivar (Charleston Gray No.B3 USA) were soaked in water for 36 hrs and composted in warm place for up to 72 hrs until embryonic roots were visible and afterward grown in plastic pots (30 cm diameter × 30 cm height) containing sterilized soil.
Plants with the same age (14 days) and size were chosen for planting and separated into five treatments, together with healthy and infected control treatments. Each treatment included 4 replicates and each one was composed of 12 healthy seedlings. The treatments were denoted as following: Treatment 1: (T1) control healthy; Treatment 2: (T2) control infected with ZYMV; Treatment 3: (T3) ZYMV + thuja extract; Treatment 4: (T4) ZYMV + nigella infusion; Treatment 5: (T5) ZYMV + nigella decoction; Treatment 6: (T6) ZYMV + bougainvillea leaf extract; Treatment 7: (T7) ZYMV + neem leaf extract. Twenty one DPI data were recorded. All the experiments were repeated twice with similar results at two different times to confirm their validity.
Equal volumes of medicinal plant extraction and ZYMV inoculums were mixed together in test tubes for 15 min. and 100 μL of the mixture was used as a source of inoculum for direct inoculation on cotyledonary and first leaves of watermelon seedlings. The numbers of plants showing symptoms was counted after 21 DPI and the mean of 48 plants / treatment was calculated. Healthy control plants were inoculated with buffer only. Viral control plants were inoculated by ZYMV only.
Inoculated seedlings were treated by medicinal plant extracts 24 hrs after viral infection with 100 µL/ leaf. The developing symptoms were recorded after 21 DPI. The positive controls were watermelon plants inoculated with ZYMV only, whereas the healthy controls were treated with phosphate buffer.
Seedlings were treated with 100µl/leaf with medicinal plant extracts 48 hrs before viral inoculation. The symptoms were recorded after 21 DPI.
The watermelon seeds were soaked in medicinal plant extracts for 36 hrs and composted in warmth for 72 hrs until appearing of embryonic root and were then grown in plastic pots. Fourteen days after plantation, the cotyledonary and first leaves were inoculated with ZYMV inoculum (100 μL/ leaf). The viral controls of watermelon seeds were soaked in distilled water and inoculated with the virus in the seedling stage.
Virus concentration measurements, disease severity and growth parameters
ZYMV concentration in leaves was determined by ELISA. Twelve different samples per replicates were collected after 21 DPI and grounded in 50 mmol/L carbonate buffer, pH 9.6 [Citation25].
Disease index was recorded based on a scale of 0–4 as follows: 0 = no appearance of external symptoms; 1 = light mottling and vein clearing; 2 = soft mosaic and vein banding; 3 = severe mosaic and blisters; and 4 = deformation followed by stunting. We can calculate disease severity (DS) values by the following formula according to [Citation26] and Yang et al. [Citation27].
Total soluble protein contents were identified through Bradford (1976) using standard bovine serum albumin. Total photosynthesis pigments were determined using spectrophotometer method after extraction by 80% acetone as solvent as described in [Citation28].
For determining the total phenols in different leaf samples, a modified Folin–Ciocalte method was used [Citation29].
The optical densities were measured using a Beak man DK-2 spectrophotometer at a wavelength of 650 nm. Concentration of total phenols in the extracts was calculated as mg/gm (D.Wt.) using a pyrogallol standard curve.
Data were analysed using one-way analysis of variance (ANOVA) and the least significant difference (LSD) test using COSTAT software program (C0Hort Computer Software, Berkeley, CA, USA). All data presented are the mean values using LSD test at 5% probability.
Results and discussion
Zucchini yellow mosaic virus sources, isolation and propagation
Naturally, watermelon infected plants exhibiting typical symptoms of ZYMV, i.e. yellowing, mottling, blisters, severe deformation and stunting of leaves, were collected from Jeddah fields, Saudi Arabia (). One of the most important viral pathogens in the world is ZYMV which leads to serious and injurious effects in most types of cucurbit [Citation21,Citation30–35] and one of the first potyviruses that is identified [Citation3].
Figure 1. Symptoms caused by natural ZYMV infection on watermelon leaves observed from Mecca region (a) (b) (c) & (d) showing yellowing, mild to severe mosaic, mottling and bubbling followed by leaf deformation with blistering.

Leaves resulted DAS-ELISA positive to ZYMV solely were collected and used as inoculum sources. Samples found infected with other viruses such as WMV, CMV and SqMV were discarded. After successive single local lesion transferred on C. amaranticolor Coste and Reyn, the resulting virus isolate was propagated as a pure ZYMV source in propagation host such as Cucurbita pepo (squash CVs. vegetable marrow white bush). The developed symptoms were observed on squash leaves.
RT-PCR
RT-PCR was used for the detection of ZYMV coat protein (cp) gene in infected watermelon. PCR fragment of the expected size 600 bp was amplified. Electrophoresis of PCR amplicons is shown in (A). Safaeizadeh [Citation22] and Yakoubi et al. [Citation35] used the same technique to isolate ZYMV and reported that there was no specific product obtained from healthy material and no bands were found when the PCR assay was performed without the initial RT step.
Figure 2. (A): Agarose gel electrophoresis analysis of amplified ZYMV- cp gene fragment Lanes (1– 5). RT-PCR products of five ZYMV samples showing amplified ZYMV-cp, Gene fragment of the correct size 600 bp (arrow) in lanes (1,2,3 and 4). Lane (5). Healthy watermelon. M: DNA ladder marker. (B): Ultrathin section of a Zucchini yellow mosaic virus-infected watermelon mesophyl cell. A,B,C,E and F: Showing laminated aggregate of crystalline particles (CP), pinwheel inclusions (pw), cristal inclusion bodies (CIB), scrolls and viral particles (VP), VP. = viral particles.
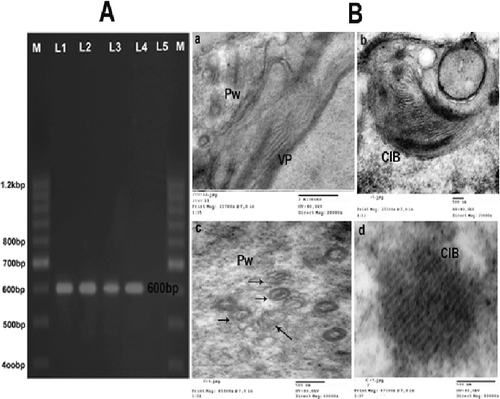
Electron microscope
(B) illustrates the part of a typical ultrathin section of ZYMV-infected cell watermelon. Inclusion bodies, consisting of pinwheels, scrolls and the laminated aggregate of crystalline particles coated in the ultrathin section of infected watermelon cells by ZYMV. Also, elongated flexuous particles were easily detected in infected watermelon cells; the modal length of the particles was close to 750 nm. The same result was obtained in other studies [Citation26,Citation36–40].
In vitro and in vivo screening of antiviral activities of five medicinal plants on ZYMV
The effect of five medicine plants on ZYMV infection was designed and evaluated in this current work. Typical symptoms of ZYMV were observed on inoculated watermelon plants compared with non-inoculated. The effect of medicine plant extracts on inhibition of ZYMV symptoms is presented in . A higher significant effect of the virus was mentioned in control infected compared with other treatments.
Table 1. The efficiency of five medical plants extracts of virus concentration, percentage of infection and diseases severity of infected watermelon cultivar (Charleston Gray No.B3 USA) under four periods from ZYMV inoculation and greenhouse condition.
The results of soaking of watermelon seeds and in vitro experiment revealed that decoction and infusion of NS seeds reduce the ZYMV symptoms on watermelon seedling. In addition, the application of decoction and infusion of black seeds not only reduce the virus symptoms, but also, decrease the virus concentration, infection percentage and disease severity of inoculated seedling, in comparison with healthy and infected control. This result agreed with that obtained by Abdel-Shafi [Citation5]. The results in show that the soaking seeds and in-vitro experiment with decoction showed the highest activity against ZYMV compared with infusion black seeds treatment. The decoction showed lowering in virus concentration, infection percentage and disease severity (0.135. 10.42% and 00.00%), respectively, after soaking for 36 hrs and (0.133, 14.58 and 00.00) of in-vitro experiment with decoction, while infusion gave (0.142, 31.25 and 7.81) and (0.153, 16.67 and 4.17), respectively, compared with infected control (1.394, 97.92 and 97.92), respectively. On the other hand, seedlings treatments by medical plant extracts from bougainvillea and neem before 48 hrs from viral inoculation had a virus concentration, infection percentage and disease severity considerably lower than other treatments in the pre-inoculation experiment.
Also, data in showed that the thuja extract following NS decoction had decreased virus concentration, infection percentage and disease severity in post-inoculation experiment compared with other extracts in this treatment.
The physiological responses of watermelon plants infected with ZYMV and treated by five medical plant extracts
Data shown in (A) and showed that the soaking seeds application of decoction and infusion from NS seeds led to an increase in total photosynthesis pigments (chlorophyll a, chlorophyll b, chlorophyll a + b and carotenoids), total soluble intracellular protein and total phenols contents of watermelon leafs compared to the viral control seedling. The application of NS decoction increased the total photosynthesis pigments in all experiments compared to the viral control where it was 0.647, 0.387, 1.034 and 0.158 compared to 0.310, 0.092, 0.402 and 0.076, respectively. The total soluble intracellular protein significantly increased upon treatment with decoction and infusion of NS seeds comparing to the viral control where it was 45.976, 44.361compared to 43.885, respectively. The application of NS decoction and infusion were increased the total phenol compounds in all experiments compared to viral control where it was 33.998, 32.970 compared to 30.661, respectively.
Figure 3. The effectiveness of medical plant extracts on estimation of physiological changes (photosynthesis pigments) of watermelon cultivar (Charleston Gray No.B3 USA) in the presence Zucchini yellow mosaic virus under greenhouse condition.
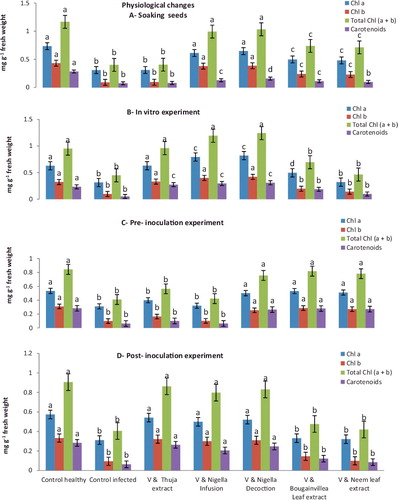
Figure 4. The effectiveness of medical plant extracts on estimation of physiological changes (total soluble protein and total phenols) of watermelon cultivar (Charleston Gray No.B3 USA) in the presence Zucchini yellow mosaic virus under greenhouse condition.
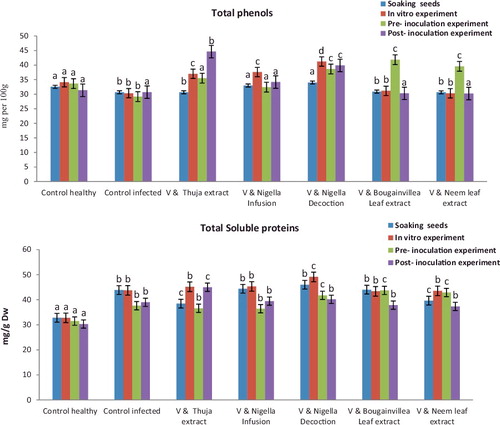
Data shown in (B) and demonstrate that in vitro application of decoction and infusion of NS seeds led to an increase of total photosynthesis pigments, total soluble intracellular protein and a total phenol content of watermelon leafs compared to viral control seedlings. The application of NS decoction increased the total photosynthesis pigments in all experiments compared to viral control where it was 0.821, 0.421, 1.242 and 0.310 compared to 0.315, 0.099, 0.449 and 0.053, respectively. The total soluble intracellular protein significantly increased upon treatment with decoction and infusion of NS seeds compared to viral control where it was 49.110, 45.321 compared to 43.742, respectively. While, in vitro application of decoction and infusion of NS seeds led to an increase in total phenols contents of watermelon plants compared to viral or healthy control plants were 41.205 and 37.592 compared to 30.331, respectively.
Moreover, results of the physiological parameters of watermelon seedlings in pre-inoculated experiments in (C) and revealed that the total photosynthesis pigments, total soluble intracellular protein and contents of total phenols were significantly increased in bougainvillea Leaf extract, neem leaf extract and decoction of NS seeds which give (0.532, 0.287, 0.819, 0.280, 43.732 and 41.851); (0.511, 0.272, 0.783, 0.272, 42.884 and 39.561) and (0.502, 0.255, 0.757, 0.265, 41.754 and 38.631) more than the viral control (0.311,. 0.099, 0.410, 0.063 37.637 and 29.163), respectively.
Data in (D) and showed that the post-inoculation treatment of thuja extract, decoction and infusion of NS seeds led to an increase in chlorophyll a (0.542, 0.521 and 0.499), chlorophyll b (0.321, 0.310 and 0.299), chlorophyll a+b (0.863, 0.831 and 0.798), carotenoids (0.263, 0.246 and 0.205), total soluble intracellular protein (44.994, 40.151 and 39.440) and total phenols contents (44.622, 39.873 and 34.182) of watermelon plants compared to viral control plants (0.311, 0.094, 0.405, 0.063, 38.975 and 30.685),respectively.
In conclusion, the results of the present investigation propose that the bougainvillea and neem extracts may perhaps play a central function in the mechanism of acquired systemic resistance and may product pathogenesis-related proteins play a significant role in watermelon seedling resistance against ZYMV. Similar results were noticed in [Citation16,Citation41–43].
In a while, the thuja extract following NS decoction in post application were to inhibit the development of symptoms and decreased concentration of virus, infection percentage and disease severity from infected seedling, compared with control infected and other extracts in this application.
In addition, thuja extract and NS decoction increased the total photosynthesis pigments; total soluble intracellular protein and total phenols contents in post application compared with any of the other extracts in this treatment and control infected. Similar results were obtained in previous studies concerning the use of plant extracts to supervise virus disease in both animals and plants [Citation2,Citation13,Citation44,Citation45].
The antiviral action of the products used in this study is linked to their components that may act directly by interaction with the virus particles in the early stage of the infection and block the liberation of its nucleic acid that eventually lead to stopping viral replication. The curative action of these products may support this intention [Citation13,Citation46–49].
The resistance, stimulated or non-induced, is characterized by the low concentration of the virus accompanied with retardation of virus symptoms development.
Rao et al. [Citation50] argued that the protein content was increased in the plants infected with the virus due to increase activity of RNA synthesizer or RNA polymerase. The treated plants also show high protein content compared to viral control [Citation51,Citation52].
The accumulation of the phenolic compounds and their derivatives may be considered as a defence mechanism or as a hypersensitive reaction. Acquire systemic resistance response associated with changes in cell metabolism reactions [Citation10].
Conclusion
In conclusion, five medical plant extracts were examined for their abilities against ZYMV infection of watermelon seedlings under four application of soaking, in-vitro, pre and post application. Individual treatment by decoction and infusion of NS seeds in soaking and in-vitro application, bougainvillea and neem before 48 hrs from viral inoculation and thuja extract following nigella decoction in post application showed inhibitory effect against ZYMV disease symptoms. Percentage of infection, disease severity and virus concentration were correlated with disease symptoms. Evaluation of total photosynthesis pigments; total soluble intracellular protein and total phenol contents, were recorded and showed a height increase compared with infected control.
Disclosure statement
No potential conflict of interest was reported by the author.
Additional information
Funding
References
- Fenny D, Jiarong L. Diversity and origin of cultivated and citron type watermelon (Citrullus lanatus). Genet Resour Crop Evol. 2006;54(6):1255–1265.
- Papayiannis LC, Ioannou N, Boubourakas IN, et al. Incidence of viruses infecting cucurbits in Cyprus. J Phytopathol. 2005;153:530–535.
- Desbiez C, Lecoq H. Zucchini yellow mosaic virus. Plant Path. 1997;46:809–829.
- Lisa V, Lecoq H. Zucchini yellow mosaic virus. CMI/AAB Descriptions of Plant Viruses no. 282. Slough: Commonwealth Agricultural Bureaux; 1984.
- Abdel-Shafi S. Preliminary studies on antibacterial and antiviral activities of five medicinal plants. J Plant Pathol Microb. 2013;4:190(1–8). doi:10.4172/2157–7471.1000190
- Al-Ani RA, Mustafa AA, Kareem AH. Antiviral activity of Vit-org, 2-nitromethyl phenol and Thuja extract against eggplant blister mottled virus (EBMV). Afr J Microbiol Res. 2011a;5(21):3555–3558.
- E1-Alfy TS, E1-Fatatry HM, Toama MA. Isolation and structure assignment of an anti microbial principle from the volatile oil of Nigella sativa, L. seeds. Pharmazie. 1975;30(2):109–111.
- Hashim FM, El-Kiey MA. Nigella sativa seeds of Egypt. J Pharm Sci United Arab Reb. 1962;3:121–133.
- Toghyani M, Toghyani M, Gheisari A. Growth performance, serum biochemistry and blood hematology of broiler chicks fed different levels of black seed (Nigella sativa) and peppermint (Mentha piperita). Livestock Sci. 2010;129:173–178.
- Mohamed, EF. Inhibition of broad bean mosaic virus (BBMV) using extracts of Nigella (Nigella sativa L.) and Zizyphus (Zizyphus spina-christi Mill.) plants. J Am Sci. 2011;7(12):727–734.
- AL-Beitawi NA, EL-Ghousein SS, Nofal AH. Replacing bacitracin methylene disalicylate by crushed Nigella sativa seeds in broiler rations and its effects on growth, blood constituents and immunity. Livestock Sci. 2009;125(2–3):304–307.
- Al-Ani RA, Hassan SA. [Effect of henna, thuja, and tamarisk extracts on the multiplication of Tomato yellow leaf curl virus (TYLCV)]. Jerash J Res Stud. 2002;6(2):135–148. ( in Arabic).
- Al-Ani RA, Diwan SNH, Adhab MA. Efficiency of Thuja orientalis and Artimisia campestris extracts to control of potato leaf roll virus (PLRV) in potato plants. Agric Biol J North Am. 2014;1(4):579–583.
- Al-ani RA, Mustafa AA, Hamad SAH, et al. Tomato yellow leaf curl virus (TYLCV), identification, virus vector relationship, strains characterization and a suggestion for its control with plant extracts in Iraq. Afr J Agr Res. 2011b;6(22):5149–5155.
- Srivastava P, Kumar P, Singh DK. Biological properties of Thuja Orientalis Linn. Adv Life Sci. 2012;2(2):17–20.
- Madhusudhan KN, Nalini MS, Prakash HS, et al. Effect of inducers against tobamovirus infection in tomato and bell pepper. Int J Bio. 2005;1:59–61.
- Adams MF, Adams AN. Characteristics of the Microplate method of enzyme-linked immunosorbent assay for the detection of plant viruses. J Gen Virol. 1977;34:475–483.
- Kuhn CW. Separation of cowpea virus mixture. Phytopathology. 1964;54:739–740.
- Faccioli G, Capponi. Antiviral factor present in plants of Chenopodium amaranticolor locally infected by tobacco necrosis virus: 1- extraction, partial purification, biological and chemical properties. Phytopathology. 1983;106:289–301.
- Yarwood CE. Mechanical transmission of Apple mosaic virus. Hilgardia. 1955;23:613–28.
- Lecoq, H, Desbiez C, Kheyr-Pour A, et al. International training course on modern techniques for plant virus diagnosis. Tehran: PPDRI. 2004.
- Safaeizade M. Comparative biological and molecular variability of Zucchini yellow mosaic virus in Iran. Asian J Plant Pathol. 2008;2:30–39.
- Martelli GP, Russo M. Use of thin sectioning for visualization and identification of plant viruses. Methods Virol. 1984;8:143–224.
- Saeed S, Tariq P. In vitro antibacterial activity of clove against gram negative bacteria. Pakistan J Bot. 2008;40:2157–2160.
- Garcia-Ruiz H, Murphy JF. Age-related resistance in bell pepper to Cucumber mosaic virus. Ann Appl Biol. 2001;139:307–317.
- Radwan MR, Khalaf AF, Sabry YM, et al. Physiological and metabolic changes of Cucurbita pepo leaves in response to zucchini yellow mosaic virus (ZYMV) infection and salicylic acid treatments. Plant Physiol Biochem. 2007;45:480–489.
- Yang X, Liangyi K, Tien P. Resistance of tomato infected with Cucumber mosaic virus satellite RNA to potato spindle tuber viroid. Ann Appl Biol. 1996;129:543–551.
- Chappelle EW, Kim MS, McMurtrey JE. Ratio analysis of reflectance spectra (RARS): an algorithm for the remote estimation of the concentrations of chlorophyll a, chlorophyll b, and carotenoids in soybean leaves. Remote Sens Environ. 1992;39:239–247.
- William H, Chichilo PAC, Reynolds H. Official methods of analysis of the association of agricultural chemists. 10th ed. Washington ( DC): Association of Official Agricultural Chemists; 1965; p. 158.
- Aisan G, Nemat SB, Nahid M. Sequencing part of Watermelon mosaic virus genome and phylogenetical comparison of 5 isolates with other isolates from world. J Agric Food Tech. 2012;2:93–101.
- Blua MJ, Perring TM. Effect of zucchini yellow mosaic virus on development and yield of cantaloupe (Cucumis melo). Plant Dis. 1989;73:317–320.
- Gal-On A. Zucchini yellow mosaic virus: insect transmission and pathogenicity the tails of two proteins. Mol Plant Pathol. 2007;8(2):139–150.
- Charoensilp C, Pongsopee A, Mila J, et al. Sequencing and haracterization of Thai Papaya Ringspot Virus Isolate Type P (PRSV th P). Sci Asia. 2003;29:89–94.
- Nameth ST, Dodds JA, Paulus AO, et al. Zucchini yellow mosaic virus associated with severe diseases of melon and watermelon in southern California desert valleys. Plant Dis. 1985;69:785–788.
- Yakoubi S, Desbiez C, Fakhfakh H, et al. Molecular, biological and serological variability of Zucchini yellow mosaic virus in Tunisia. Plant Pathol. 2008;57:1146–1154.
- Fraser RSS. Biochemistry of virus infected plants. Letchworth: Research Studies Press / New York (NY): John Wiley and Sons; 1987.
- Lisa V, Boccardo G, D'Agostino G, et al. Characterization of a potyvirus that causes zucchini yellow mosaic. Phytopathology.1981;71:667–672.
- Tucci M, Nygard K, Farber HW et al. Modulation of IGF and IGF binding protein expression by hypoxia in cultured vascular endothelial cells (EC). Pediatr Res (Abstract 2333). 1996;39:392A.
- Zechmann B, Zellnig G. Rapid TEM diagnosis of plant virus diseases. J Virol Methods. 2009;162:163–169.
- Zellnig G, Pöckl MH, Möstl S, et al. Two and three dimensional characterization of Zucchini Yellow Mosaic Virus induced structural alterations in Cucurbita pepo L. plants. J Struct Biol. 2014;186:245–252.
- Deepthi N, Madhusudhan KN, Uday Shankar AC, et al. Effect of plant extracts and acetone precipitated proteins from six medicinal plants against tobamovirus infection. Int J Virol. 2007;3:80–87.
- Ramesh CK, Prabhan MN, Deepak SA, Madhusudhan KN. Screening of antiviral property against tobamoviruses in latex of Euphorbia tirucalli L. Biotechnol Indian J. 2009;3:1–7.
- Zida EP, Sereme P, Leth V, et al. Effect of aqueous extracts of Acacia gourmaensis A. Chev and Eclipta alba (L.) hassk. on seed healthy, seedling vigour and grain yield of sorghum and pearl millet. Asian J Plant Pathol. 2008;2:40–47.
- Wannang NN, Ndukwe HC, Nnabuife C. Evaluation of the analysis properties of the Datura metel seeds aqueous extracts. J Med Plants Res. 2009;3(4):192–195.
- Yarmolinsky L, Zaccai M, Ben Shabat S, et al. Antiviral activity of ethanol extracts of Ficus binjamina and Lilium candidum in vitro. New Biotechnol. 2009;26(6):307–313.
- Hammerschmidt R. Induced disease resistance: how do induced plants stop pathogens. Physiol Mole Plant Pathol. 1999;55:77–84.
- Shi Z, Wang F, Zhou W, et al. Application of osthol induces a resistance response against Powdery mildew in pumpkin leaves. Int J Mole Sci. 2007;8:1001–1012.
- Walters DR, Walsh D, Newton AC, et al. Induced resistance for plant disease control: maximizing the efficacy of resistance elicitors. Phytopathology. 2005;95:1368–1373.
- Al-Ani RA, Al-Essawi UN, Al-Mashaikhy SA. [Isolation of proteins from Datura stramonium have ability to inhibition the multiplication of Potato virus Y (PVYn)]. Jerash J Res Stud. 2002;7(1):9–21. ( in Arabic).
- Rao AL, Cooper B, Deom CM. Defective movement of viruses in the family Bromoviridae is differentially complemented in Nicotiana benthamiana expressing tobamovirus or dianthovirus movement protein. Phytopathology. 1998;88:666–672.
- Abdel-Shafi S. Biological studies on antiviral activities of some bacterial isolates [PhD thesis]. Egypt: Department of Botany and Microbiology, Faculty of Science, Zagazig University; 2005
- Deya Eldeen MR, Lu G, Khalaf AF, et al. Protective action of salicylic acid against bean yellow mosaic virus infection in Vicia faba leaves. J Plant Physiol. 2010;165:845–857.
