ABSTRACT
To gain insights into the function of Cav3.3 T-type calcium channel in dorsal root ganglion neurons, we designed a one-cell model in dorsal root ganglion neurons that specifically inhibits the expression of Cav3.3 mRNA. We designed three different shRNA sequences and then connected them to pYr-1.1 plasmid and labelled them as pYr-1.1-Cav3.3 shRNA1, pYr-1.1-Cav3.3 shRNA2 and pYr-1.1-Cav3.3 shRNA3, respectively. The correct sequence vector (pYr-1.1-Cav3.3 shRNA3) was then recombined with the pAd/PL-DEST vector into pAd-Cav3.3 shRNA the adenovirus vector. The dorsal root ganglion neurons were infected with the pAd-Cav3.3 shRNA adenovirus vector. After detecting the expression of Cav3.3 mRNA and protein, the pAd-Cav3.3 shRNA adenovirus vector was verified by digestion and sequencing. These results showed that the constructed pAd-Cav3.3 shRNA adenovirus vector could infect dorsal root ganglion cells and inhibit the expression of Cav3.3 mRNA or protein. These results established a one-cell model for further functional study of Cav3.3.
Introduction
Low voltage-dependent calcium channels, also known as T-type calcium channels, have fast activation and slow inactivation and can be activated at resting potential (−65 mV to −50 mV) [Citation1–4]. They play a crucial role in many physiological activities and pathological processes. For example, the T-type calcium channel regulates the release of neurotransmitter, hormone secretion and neuronal excitability [Citation5–6]. T-type calcium channels are closely related with neuropathic pain and spinal cord injury induced by local anaesthetics [Citation7–11]. There are three T-type calcium channel subtypes: Cav3.1, Cav3.2 and Cav3.3. Cav3.1 T-type calcium channels are an important factor in SH-SY5Y cell lines injury induced by local anaesthetics in vitro. Cav3.2 and Cav3.3 T-type calcium channels are involved with neuropathic pain in rats. Cav3.3 T-type calcium channels are mainly expressed in the cerebrum, spinal cord, dorsal root ganglion (DRG) neurons, adrenal cortex and thyroid gland. It has been shown that Cav3.3 in nucleus reticularis thalami is involved with the sleep spindles, a hallmark of natural sleep [Citation12]. To date, there is no specific blocking agent for T-type calcium channels. Mibefradil has been used as a T-type calcium channel blocking agent; however, it also inhibits the other calcium channels, such as the high voltage-dependent calcium channels, with increasing dosage [Citation13]. Furthermore, mibefradil has no specificity to Cav3.1, Cav3.2 and Cav3.3. To gain further insights into the functions of T-type calcium channel subtypes, we used methods that knocked down or overexpressed the genes of T-type calcium channel subtypes.
RNA interference (RNAi) technology, which is a new technology that has been widely used to explore gene function, infectious diseases and malignant tumour gene therapy, can specifically remove or inhibit target gene expression [Citation14–16]. The first step in its mechanism is that the double-stranded RNA induces highly conserved and homologous mRNA degradation. shRNA with a tight hairpin turn, namely small hairpin RNA or short hairpin RNA, comprises two short reverse repeat sequences that are separated by a loop sequence. shRNA with hairpin structure is then transformed into short interfering RNA to degrade the target gene.
In this study, we constructed a one-cell model that specifically inhibits the expression of Cav3.3 mRNA in DRG neurons by the RNAi method. We designed the Cav3.3 shRNA sequence and connected the sequence with the pYr-1.1 plasmid. The ligation product of the Cav3.3 shRNA and pYr-1.1 plasmid was recombined with the pAd/PL-DEST vector into the pAd-Cav3.3 shRNA adenovirus vector. We then infected the DRG neurons with pAd-Cav3.3 shRNA adenovirus vector to inhibit the expression of Cav3.3 mRNA in vitro. These results established a one-cell model for functional studies of Cav3.3.
Materials and methods
The shRNA sequence
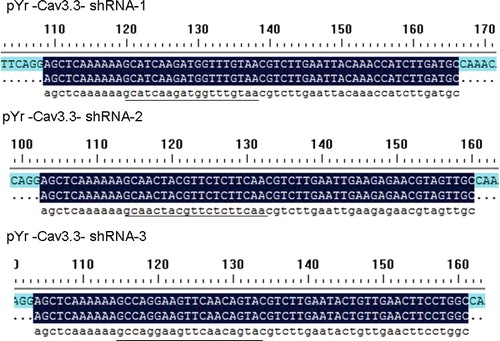
Based on the Cav3.3 gene rats (NM_020084.3), the shRNA sequences were designed using BLOCK-iT™ RNAi Designer (https://rnaidesigner.invitrogen.com/rnaiexpress/). The sequences were as follows: Cav3.3–shRNA1, GCATCAAGATGGTTTGTAA (target of the gene locus 209–227); Cav3.3–shRNA2, GCAACTACGTTCTCTTCAA (target of the gene locus 2420–2438); Cav3.3–shRNA3, GCCAGGAAGTTCAACAGTA (target of the gene locus 6385–5403). The shRNA primer structure was BsaI + sense + loop + antisense + TTTTTT + SacI + BsaI, and the shRNA primers () were synthesized by Jinsirui Ltd. (Nanjing, China).
Table 1. Cav3.3 shRNA primers.
Ligation of shRNA and pYr-1.1 plasmid
The Cav3.3 shRNA primers (1 μg/μL) were mixed as follows: Cav3.3 shRNA-1-forward + Cav3.3 shRNA-1-reverse, Cav3.3 shRNA-2-forward + Cav3.3 shRNA-2-reverse and Cav3.3 shRNA-3-forward + Cav3.3 shRNA-3-reverse. The mixtures were then annealed at 95 °C for 5 min to form the double-stranded shRNA. After digestion with BsaI (Dalian TAKARA Co., Ltd., Dalian, China) and purification, the pYr-1.1 plasmid (Wuhan Biofield Biological Service Co., Ltd., Wuhan, China) was mixed with the annealed double-stranded shRNA and T4 DNA ligase (Dalian TAKARA Co., Ltd., Dalian, China) at 4 °C overnight. The ligation product was then labelled as pYr-1.1-Cav3.3 shRNA-1, pYr-1.1-Cav3.3 shRNA-2 and pYr-1.1-Cav3.3 shRNA-3.
Transformation of the ligation product and the extraction plasmid
The ligation products between the pYr-1.1 plasmid and Cav3.3 shRNA were incubated at 65 °C for 5 min to inactivate the ligase and then cooled on ice for 2 min. Ligation products (5 µL) were added into competent cells DH5а (Dalian TAKARA Co., Ltd., Dalian, China) and the mixtures were put on ice for 10 min. The mixture was then heat shocked at 42 °C water bath for 30 s. It was important that the length of the heat shock was no longer than 30 s; otherwise, it would cause the death of the competent cells. Luria–Bertani (LB) medium (100 μL) was added to the mixture and incubated in 37 °C water bath for 30 min. Then, the competent cells DH5а were coated with Kanamycin (30 µg/mL) LB tablet and incubated at 37 °C in an incubator overnight. We selected a monoclonal colony from the LB tablet and inoculated the colony into 5 mL of LB medium with Kanamycin in 37 °C shaking incubator overnight. We extracted and digested the plasmids with BsaI (Dalian TAKARA Co., Ltd., Dalian, China) following the manufacturer's instructions of the EZNA Plasmid Mini Kit I (Omega Bio-tek, Norcross, GA, USA). The plasmids were then sent to Jinsirui Ltd. (Nanjing, China) for sequencing. The correct plasmid sequence was labelled as pYr-Cav3.3–shRNA.
Restructuring of the adenovirus vector and infection of the 293 cells
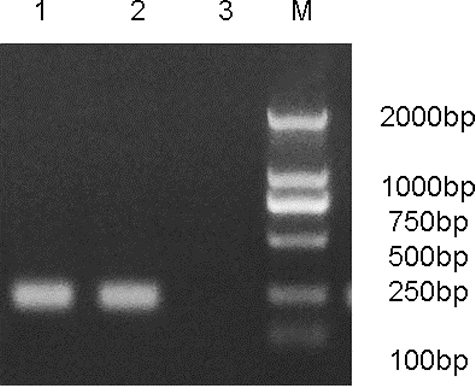
The verified sequence of plasmid pYr-Cav3.3–shRNA was mixed with pAd/PL-DEST vector at 4 °C overnight to prepare for the ligation product. The ligation product was transformed into the competent cells DH5а for amplification. After extraction, the plasmid was then digested with XbaI enzyme and sent for sequencing. The correct monoclonal vector was labelled as pAd-Cav3.3 shRNA restructured plasmid. The pAd-Cav3.3 shRNA restructured plasmid was used to infect 293 cells to generate and amplify the adenovirus. In brief, pAd-Cav3.3 shRNA restructured plasmid was first linearized with PacI enzyme and then transfected into 293 cells using Lipo2000 kits (Thermo Fisher Scientific Inc., Waltham, MA, USA). The medium of the 293 cells infected with the restructured plasmid was collected for identification with polymerase chain reaction (PCR) and sequencing (Jinsirui Ltd., Nanjing, China). The hU6 universal primer sequence was 3'-TACGATACAAGGCTGTTAGAGAG-5', and the reverse primer of the Cav3.3 was 5'-AATACTGTTGAACTTCCTGGCCAAA-3' (Jinsirui Ltd., Nanjing, China). The PCR product size was 244 bp.
Infection of DRG cells with pAd-Cav3.3 shRNA adenovirus
Neonatal one-day-old rats (animal centre of the Zhongnan Hospital of Wuhan University, Wuhan, China) were sacrificed, and their vertebral columns were completely separated. The DRG were carefully separated and incubated at 37 °C in 5% CO2. We carefully separated the DRG with fine dissecting forceps and transferred the DRG cells. The DRG cells with 2–3 × 105/mL density were incubated in neurobasal medium (D-glucose 4.5 g/L, L-glutamine 2 mmol/L, foetal bovine serum 1%, B-27 additive 2%, nerve growth factor 10 μg/L, penicillin 100 U/L and streptomycin 100 μg/L) at 37 °C and 5% CO2. We changed the neurobasal medium every three days. Recombinant pAd-Cav3.3 shRNA adenovirus was then added to the cells with a 3 × 109 TU/mL titre. The infection efficiency was observed using fluorescence microscopy (M165 FC, Leica Inc., Solms, Germany).
Quantitative real-time PCR (qRT-PCR) detection of the expression of Cav3.3 mRNA in DRG cells
The DRG cells infected with pAd-Cav3.3 shRNA adenovirus were collected and the level of Cav3.3 mRNA expression was determined using real-time PCR kits (Invitrogen Ltd., Carlsbad, CA, USA). The sequence of the β-actin primer (Jinsirui Ltd., Nanjing, China) was 5′-CACGATGGAGGGGCCGGACTCATC-3′ (forward primer)and 5′-TAAAGACCTCTATGCCAACACAGT-3′ (reverse primer), and the product size was 240 bp. The sequence of the Cav3.3 primer (Jinsirui Ltd., Nanjing, China) was 5′-GACCAGCAGCCAGTGACGAA-3′ (forward primer) and 5′-CACGACCACGCCCACAAACA-3′ (reverse primer), and the product size was 108 bp. The mRNA levels were recorded as 2−△△Ct, where Ct represented the threshold cycle.
Western blotting detection of Cav3.3 protein expression in DRG cells
The DRG cells infected with pAd-Cav3.3 shRNA adenovirus were collected, and the quantity of Cav3.3 protein was determined using western blotting. In brief, we collected the infected DRG cells and extracted the total protein. We then ran the total proteins (50 µg) through 7.5% sodium dodecyl sulphate polyacrylamide gel electrophoresis and transferred them to polyvinylidene fluoride (PVDF) membrane. The PVDF membrane was blocked with fat-free milk at 4 °C overnight. The next day, the Cav3.3 protein or β-actin primary antibody (1:300, Santa Cruz Biotechnology, Inc., Dallas, TX, USA) was incubated in the PVDF film for 24 h at 4 °C overnight. We then incubated the horseradish peroxidase conjugated rabbit anti-sheep antibody (1:500, Boster Bio-engineering Ltd. Co., Wuhan, China) for 1 h at room temperature and tested for electrochemiluminescence colouration. The quantity of the Cav3.3 protein expression was then normalized against that of the β-actin protein.
Statistical analysis
The results were expressed as the mean values with standard deviation (±SD) from six independent experiments; SPSS16.0 was used for data analysis. The comparison of mRNA and protein expression of the Cav3.3 in the DRG neurons was performed with one-way analysis of variance. P < 0.05 was considered to indicate a statistically significant difference.
Results and discussion
pYr-1.1-Cav3.3 shRNA plasmid digestion and sequencing
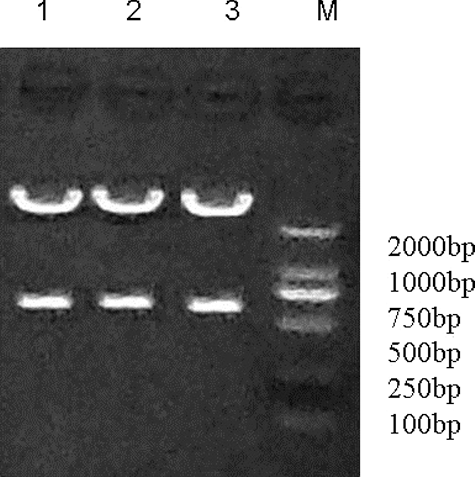
The pYr-1.1-Cav3.3 shRNA plasmid was digested with BasI enzyme and identified by its fragment size. The bacterial colonies of the pYr-1.1-Cav3.3 shRNA1, pYr-1.1-Cav3.3 shRNA2 and pYr-1.1-Cav3.3 shRNA3 plasmids were amplified and extracted. After digestion with BasI, the samples were run on agarose electrophoresis. As shown in , one 698 bp band was observed. In addition to observing the 698-bp band, we sent the pYr-1.1-Cav3.3 shRNA1, pYr-1.1-Cav3.3 shRNA2 and pYr-1.1-Cav3.3 shRNA3 plasmid samples for sequencing. The sequence data showed that the shRNA1, shRNA2 and shRNA3 had been correctly inserted into the plasmids (). We selected the pYr-1.1-Cav3.3 shRNA3 plasmid for further study and labelled it pYr-1.1-Cav3.3 shRNA. We designed two restriction enzyme cutting sites for BasI in the shRNA sequence that matched the pYr-1.1 vector BasI-cutting site. The results showed that the shRNA sequences were inserted into the pYr-1.1 vector. Meanwhile, there was one SacI-cutting site in the pYr-1.1 vector and shRNA sequence. In this study, the band size of the enzyme digestion and the sequencing results showed that the shRNA sequences were correctly inserted into the pYr-1.1 vector.
Identification of recombinant adenovirus by PCR in 293 cells
As shown in , the positive control (Lane 1) showed the presence of the promoter hU6 and pAd-Cav3.3 shRNA recombinant adenovirus (Lane 2) showed the Cav3.3 shRNA band. The negative control (Lane 3) without vector did not display any band. The pAd-Cav3.3 shRNA recombinant was sent for sequencing and we verified that Cav3.3–shRNA had been inserted into pAd-Cav3.3 shRNA recombinant adenovirus.
Efficiency of pAd-Cav3.3 shRNA recombinant adenovirus infection in DRG cells
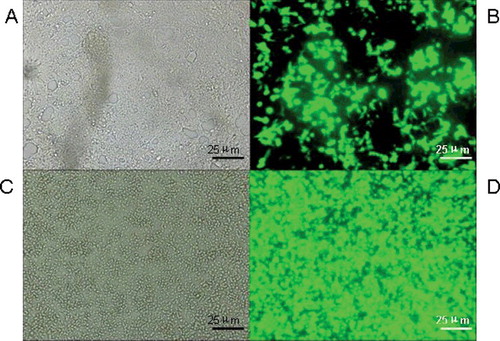
We infected DRG cells with pAd-Cav3.3 shRNA adenovirus for 24 h. The infection efficiency was detected using the fluorescence microscope. The pYr-1.1 plasmid contained the green fluorescence protein EGFP gene. The pAd-Cav3.3 shRNA recombinant adenovirus infection efficiency in DRG cells was estimated based on the green fluorescence. As shown in , the green fluorescence indicated that the DRG cells were infected with pAd-Cav3.3 shRNA adenovirus. When the pAd-Cav3.3 shRNA adenovirus was amplified, the green fluorescence was significantly increased. To observe the infection efficiency, we detected the green fluorescence by EGFP in the pYr-1.1 vector.
Cav3.3 mRNA and protein expression in pAd-Cav3.3 shRNA adenovirus-infected DRG cells
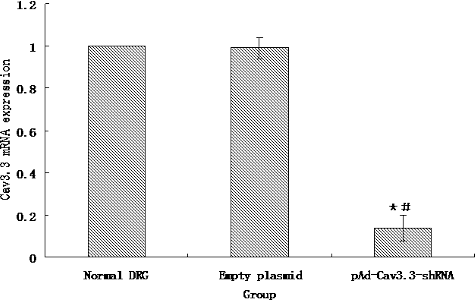
To measure the RNAi efficiency, we detected the expression of Cav3.3 mRNA in DRG cells by real-time PCR. As shown in , we found that the empty plasmid without the Cav3.3 shRNA had no effect on the expression of Cav3.3 mRNA in DRG cells. However, pAd-Cav3.3 shRNA adenovirus significantly inhibited the expression of Cav3.3 mRNA in DRG cells. There was 14% difference in the expression of Cav3.3 mRNA in the normal DRG cells compared to that in the pAd-Cav3.3 shRNA adenovirus-infected DRG cells.
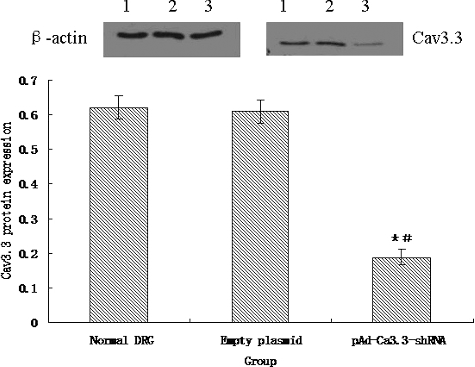
As shown in , there was no difference in the expression of Cav3.3 protein between the normal DRG cells and the empty plasmid DRG cells. However, the infection of pAd-Cav3.3 shRNA adenovirus significantly reduced the expression of Cav3.3 protein in the DRG cells.
Figure 6. Expression of Cav3.3 protein. Lane 1: expression of Cav3.3 protein in normal DRG cells; Lane 2: expression of Cav3.3 protein in empty DRG cells; Lane 3: expression of Cav3.3 protein in pAd-Cav3.3 shRNA adenovirus-infected DRG cells.
We detected the mRNA and protein expression in DRG cells. The results indicated that pAd-Cav3.3 shRNA adenovirus could specifically inhibit the expression of Cav3.3 mRNA and protein in DRG cells. These results are significant because it is important for the functional study of Cav3.3 to establish a DRG cell model that specifically inhibits the expression of Cav3.3 mRNA and protein.
pAd-Cav3.3 shRNA adenovirus-infected DRG cells can contribute to the function research of the DRG cells
The T-type calcium channel plays an important role in the regulation of hormone secretion and neuronal excitability. This channel is involved in many physiological and pathological regulations. Previous studies have shown that T-type calcium channel was closely related to the chronic neuropathic pain and spinal cord neurotoxicity induced by local anaesthetics. Meanwhile, DRG neurons, as the primary afferent neurons, play an important role in the chronic neuropathic pain and local anaesthetics spinal cord neurotoxicity [Citation17–19]. The expression of Cav3.3 calcium channel in DRG neurons was abundant, which indicated that Cav3.3 may play an important role in DRG neurons. Because of the shortage of Cav3.3 blocker, the Cav3.3 T-type calcium channel function in DRG cells remains unclear. Therefore, it is necessary to establish a one-cell model to knock down the expression of Cav3.3 mRNA. In this study, we reconstructed pAd-Cav3.3 shRNA adenovirus vector to infect the DRG cells to specifically inhibit the expression of Cav3.3 mRNA.
Conclusions
Cav3.3 T-type calcium channel in DRG cells may play key roles in signal regulation of the neurons. We established a cell model that specifically inhibits the expression of Cav3.3 mRNA and protein in DRG cells, which would be crucial for further studies of Cav3.3 function in DRG cells.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Perez-Reyes E. Molecular physiology of low-voltage-activated t-type calcium channels. Physiol Rev. 2003;83(1):117–161.
- Feltz A. Low-threshold-activated Ca channel: from molecules to function: over 25 years of progress. Crit Rev Neurobiol. 2006;18(1–2):169–178.
- Cueni L, Canepari M, Adelman JP, et al. Ca2+ signaling by T-type Ca2+ channels in neurons. Eur J Physiol. 2009;457(5):1161–1172.
- Chemin J, Traboulsie A, Lory P. Molecular pathways underlying the modulation of T-type calcium channels by neurotransmitters and hormones. Cell Calcium. 2006;40(2):121–134.
- Iftinca MC, Zamponi GW. Regulation of neuronal T-type calcium channels. Trends Pharmacol Sci. 2009;30(1):32–40.
- Iftinca MC. Neuronal T-type calcium channels: what's new? Iftinca: T-type channel regulation. J Med Life. 2011;4(2):126–138.
- Wen XJ, Xu SY, Chen ZX, et al. The roles of T-type calcium channel in the development of neuropathic pain following chronic compression of rat dorsal root ganglia. Pharmacology. 2010;85(5):295–300.
- Wen XJ, Li ZJ, Chen ZX, et al. Intrathecal administration of Cav3.2 and Cav3.3 antisense oligonucleotide reverses tactile allodynia and thermal hyperalgesia in rats following chronic compression of dorsal root of ganglion. Acta Pharmacol Sin. 2006;27(12):1547–1552.
- Wen XJ, Xu SY, Liu H, et al. Neurotoxicity induced by bupivacaine via T-type calcium channels in SH-SY5Y cells. PLoS One [Internet]. 2013 [cited 2016 Sep 7];8(5):e62942. Available from: http://dx.doi.org/10.1371/journal.pone.0062942.
- Wen XJ, Xu SY, Zhang QG, et al. Inhibitory gene expression of the Cav3.1 T-type calcium channel to improve neuronal injury induced by lidocaine hydrochloride. Eur J Pharmacol. 2016;775:43–49.
- LeBlanc BW, Lii TR, Huang JJ, et al. T-type calcium channel blocker Z944 restores cortical synchrony and thalamocortical connectivity in a rat model of neuropathic pain. Pain. 2016;157(1):255–263.
- Astori S, Wimmer RD, Prosser HM, et al. The Ca(V)3.3 calcium channel is the major sleep spindle pacemaker in thalamus. Proc Natl Acad Sci USA. 2011;108(33):13823–13828.
- Chen YL, Tsaur ML, Wang SW, et al. Chronic intrathecal infusion of mibefradil, ethosuximide and nickel attenuates nerve ligation-induced pain in rats. Br J Anaesth. 2015;115(1):105–111.
- Fakhr E, Zare F, Teimoori-Toolabi L. Precise and efficient siRNA design: a key point in competent gene silencing. Cancer Gene Ther. 2016;23(4):73–82.
- Alagia A, Eritja R. siRNA and RNAi optimization. Wiley Interdiscip Rev RNA. 2016;7(3):316–129.
- Bobbin ML, Rossi JJ. RNA interference (RNAi)-based therapeutics: delivering on the promise? Annu Rev Pharmacol Toxicol. 2016;56:103–122.
- Van Steenwinckel J, Noghero A, Thibault K, et al. The 5-HT2A receptor is mainly expressed in nociceptive sensory neurons in rat lumbar dorsal root ganglia. Neuroscience. 2009;161(3):838–846.
- Yin K, Baillie GJ, Vetter I. Neuronal cell lines as model dorsal root ganglion neurons: a transcriptome comparison. Mol Pain. 2016;12. pii: 1744806916646111. DOI:10.1177/1744806916646111.
- Zhang Y, Yan L, Cao Y, et al. Long noncoding RNA BDNF-AS protects local anesthetic induced neurotoxicity in dorsal root ganglion neurons. Biomed Pharmacother. 2016;80:207–212.