ABSTRACT
In this work, methyl jasmonate (MeJA), as an exogenous elicitor, was used to investigate the feasibility of enhancing triterpenoid biosynthesis in Inonotus baumii. The results showed that the appropriate concentration of MeJA to be added was 150 μmol/L, and the triterpenoid yield was 12.61 mg/g dry weight (DW), which was 4.05-fold higher than that in the control with water. Moreover, quantitative real-time polymerase chain reaction was performed to measure the transcript levels of several genes in the triterpenoid pathway, including those encoding hydroxy-3-methylglutaryl-Coenzyme A synthase (hmgs), hydroxy-3-methylglutaryl-Coenzyme A reductase (hmgr), farnesyl pyrophosphate synthase (fpps), squalene synthase (sqs), squalene epoxidase (se) and lanosterol synthase (ls). The results demonstrated that these genes were mostly up-regulated by MeJA, although the levels of induction differed. For hmgs, hmgr, fpps and se, the highest transcript levels were at 150 μmol/L MeJA, and were approximately 3.0, 2.5, 2.0 and 2.0-fold higher than those in the control, respectively. However, the sqs and ls genes appeared to be repressed by MeJA. To the best of our knowledge, this is the first time to assess the use of MeJA to elicit triterpenoid biosynthesis in I. baumii, and the results indicated that MeJA was indeed a potent inducer of triterpenoid biosynthesis.
| Abbreviations | ||
| MeJA | = | Methyl jasmonate |
| DW | = | Dry weight |
| MVA | = | Mevalonate |
| HMGS | = | Hydroxy-3-methylglutaryl-Coenzyme A synthase |
| HMGR | = | Hydroxy-3-methylglutaryl-Coenzyme A reductase |
| FPPS | = | Farnesyl pyrophosphate synthase |
| SQS | = | Squalene synthase |
| SE | = | Squalene epoxidase |
| LS | = | Lanosterol synthase |
| FPP | = | Farnesyl diphosphate |
| IPP | = | Isopentenyl diphosphate |
| DMAPP | = | Dimethylallyl diphosphate |
Introduction
Inonotus baumii (synonym Phellinus baumii) is a mushroom that belongs to the Hymenochaetaceae family, and it has been traditionally used as a food source and medicine in East Asia for many centuries [Citation1]. Extracts from this mushroom have been demonstrated to be effective in treating a diversity of diseases, due to their anti-cancer, antioxidant, anti-diabetes, anti-inflammatory characteristics, etc. [Citation2–6]. Moreover, it was reported recently that the extracts have been found to possess anti-influenza properties [Citation7].
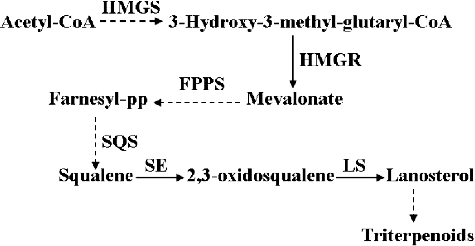
The triterpenoids, one of the main bioactive components found in I. baumii and other fungi belonging to genera Inonotus and Phellinus, play an important role in immune regulation with demonstrated anti-tumour and anti-inflammatory effects [Citation8–10]. Our previous work showed that triterpenoids from I. baumii possess inhibitory activity on breast cancer cells (MCF-7) in vitro [Citation11]. In addition, our results indicated that triterpenoids in I. baumii could also inhibit the activity of the lung cancer cell line A549, the colon cancer cell line Caco-2 and the cervical cancer cell line Hela (data not shown). Despite these important properties of I. baumii triterpenoids, the molecular mechanism of their biosynthesis in I. baumii is still unknown. As a type of terpenoids, triterpenoids are synthesized through the mevalonate (MVA) pathway, where acetyl-CoA is converted through a series of chemical reactions to lanosterol (). Then, lanosterol forms various triterpenoids after a series of oxidation and reduction reactions.
In our previous work, 12 candidate genes of the triterpenoid biosynthetic pathway were obtained from the transcriptome of I. baumii [Citation12]. Moreover, some genes encoding the catalytic enzymes responsible for triterpenoid synthesis within the MVA pathway were isolated and cloned such as se [Citation13], hmgs, sqs and ls (data not published). This allows us to further explore the relationship between triterpenoid biosynthesis and methyl jasmonate (MeJA) and reveal pharmacological mechanisms of triterpenoid biosynthesis in I. baumii at the molecular level.
MeJA has been described as an important, plant-specific, endogenous signalling molecule that can induce plant defence responses to various biotic or abiotic stresses [Citation14–16]. It is well documented that MeJA is also involved in the accumulation of secondary metabolites, such as paclitaxel [Citation17], ginsenosides [Citation18], astragaloside [Citation19], total phenols, flavonoids and coumarins [Citation20] in plants. In fungi, a study reported that MeJA could promote the growth of Aspergillus parasiticus and increase the production of the secondary metabolite aflatoxin B1 (AFB1) [Citation21]. In addition, MeJA acts as a hormone that can induce ganoderic acid (GA) biosynthesis in the basidiomycetous fungus Ganoderma lucidum [Citation22]. Until now, much less is known about the function of MeJA on fungi than on plants. To the best of our knowledge, it is still unknown whether MeJA can induce triterpenoid biosynthesis in I. baumii.
In this work, the effects of MeJA on triterpenoid biosynthesis in liquid culture were investigated. Additionally, the transcript levels of the triterpenoid biosynthetic pathway genes hmgs, hmgr, fpps, sqs, se and ls were analysed in response to MeJA stimulation.
Materials and methods
Mushroom strain
Inonotus baumii, strain DL101, was collected from Syringa reticulata at Liangshui Nature Reserve, Lesser Xing'an Mountains in Yichun City, Heilongjiang Province, China, in July 2009, and was identified according to its ITS (internal transcribed spacer) sequence alignment (GenBank accession number KP974834).
Fermentation conditions
The obtained mycelia of I. baumii were maintained on potato dextrose agar slants, preserved at 4 °C and cultured once every three months. First, the seed culture was incubated using a rotary shaker incubator (180 r/min) at 25 °C for 8–10 days in liquid potato dextrose (PD) medium. Afterwards, the second set of experiments was performed in 500 mL flasks containing 200 mL of PD medium after inoculation with 10% (v/v) of the seed culture. The flasks were then shaken at 180 r/min at 25 °C for 6 days.
MeJA stimulation of cultures
MeJA (Sigma-Aldrich, St. Louis, MO, USA) is an oil miscible liquid that could not be directly dissolved in the aqueous phase of the culture media. Therefore, MeJA was dissolved in absolute ethanol and sterilized by filtration (0.22 μm Millipore) before its addition to the medium on Day 6 of fermentation. The final concentrations of MeJA were 0, 50, 100, 150, 200 and 250 μmol/L, and control cultures received equal volumes of absolute ethanol or sterile water. The duration of treatment with different MeJA concentrations was 48 h, and the experiments were performed in triplicates.
Triterpenoids measurement
Triterpenoids were extracted and quantified according to previous reports [Citation11,Citation23]. To extract the triterpenoids of I. baumii, 100 mg dried mycelia were solubilized with 2 mL of 60% ethanol (v/v), and then placed in an ultrasonic chamber for 30 min at 100 W of ultrasound (40 kHz). Afterwards, the mycelia were removed by centrifugation at 13,000 ×g for 5 min, and the supernatant was dried at 70 °C in a thermostatic water bath. To quantify the amount of triterpenoids, the residues were resuspended in 5% vanillin-acetic acid and perchloric acid, incubated at 70 °C for 20 min and cooled rapidly. Then, 4 mL of ethyl acetate was added, and the absorbance at 551 nm was measured with a spectrophotometer (Guangpu, Shanghai, China). The triterpenoid content was calculated as betulin equivalent from a standard curve using betulin.
RNA isolation and cDNA preparation
The I. baumii mycelia were collected from the culture media and frozen at −80 °C. Total RNA was extracted using an RNAprep pure Plant Kit (Tiangen, China) in accordance with the manufacturer's instructions. The quality and quantity of RNA were assessed by agarose gel electrophoresis and determined with a BioPhotometer D30 (Eppendorf, Hamburg, Germany), respectively. Subsequently, total RNA (1 μg) was reverse-transcribed to cDNA using a PrimeScript™ RT reagent Kit (TaKaRa, Dalian, China), according to the manufacturer's instructions. Finally, the synthesized cDNA (20 μL) was diluted to 200 μL with deionized water and used as the template for subsequent experiments.
Quantitative real-time polymerase chain reaction
Quantitative real-time polymerase chain reaction (qRT-PCR) was performed with the Mx3000P Sequence Detection System (Agilent Technologies, Santa Clara, CA, USA). Primer pairs were designed using Primer 5.0 software (PREMIER Biosoft Co., Palo Alto, CA, USA) and the sequences are listed in . The transcript levels of hmgs, hmgr, fpps, sqs, se and ls were evaluated and α-tubulin and β-tubulin were used as reference genes. PCR reactions were carried out using the SYBR Premix Ex Taq™ II (TaKaRa, Dalian, China) following the manufacturers’ directions. The amplification procedure for all genes was set as follows: initial denaturation at 95 °C for 3 min, followed by 40 cycles of denaturation at 95 °C for 30 s, annealing at 60 °C for 1 min, and extension at 72 °C for 30 s. The transcript levels were evaluated by RT-qPCR according to the 2−△△CT method described by Livak and Schmittgen [Citation24].
Table 1. Primer sets used for quantitative real-time PCR.
Statistical analysis
The results were subjected to variance analysis and multiple comparisons (the least significant difference (LSD) method) using the SPSS Version 17.0 statistical package for Windows (SPSS Inc., Chicago, IL, USA).
Results and discussion
Triterpenoid profiles in response to MeJA elicitation
To investigate the effect of MeJA on triterpenoid biosynthesis in I. baumii, MeJA was added to I. baumii culture at different concentrations and five levels were determined. As controls, we included equal volumes of absolute ethanol or sterile water. A remarkable induction of triterpenoid biosynthesis by MeJA applied at a concentration of 150 μmol/L was observed (). There was a significant difference in triterpenoid production between the control and experimental groups (treated with 100–250 μmol/L of MeJA), which suggested that MeJA could potently stimulate triterpenoid biosynthesis in I. baumii. When MeJA was added at the optimal concentration of 150 μmol/L, the triterpenoid levels were 4.05 and 3.44-fold higher than those in the water control group and the ethanol control group, respectively. Interestingly, the significant difference between the water control and the ethanol control indicated that ethanol may have a positive effect on triterpenoid biosynthesis in I. baumii. Therefore, absolute ethanol was used as the only control in subsequent experiments.
Table 2. Triterpenoids content in I. baumii treated with different concentrations of MeJA for 48 h.
Transcriptional responses to MeJA stimulation at different concentrations
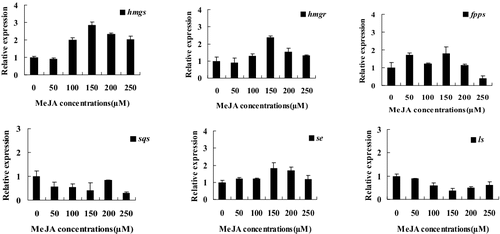
Given the stimulatory effect of MeJA on triterpenoid accumulation (), we speculated that MeJA might activate the expression of genes involved in the triterpenoid biosynthetic pathway. Therefore, the transcript levels of the genes in the triterpenoid biosynthetic pathway in I. baumii were investigated under various concentrations of MeJA stimulation. Overall, these genes were up-regulated by MeJA, although the levels of induction differed. The only exception was sqs and ls. For hmgs, hmgr, fpps and se, the highest transcript levels were at 150 μmol/L MeJA and were approximately 3.0, 2.5, 2.0 and 2.0-fold higher than the control, respectively (). The results indicated that hmgs, hmgr, fpps and se might be important genes in the triterpenoid biosynthetic pathway in response to MeJA. Our results are consistent with previous reports. For instance, Cunillera et al. [Citation25] reported that FPPS catalyses the synthesis of farnesyl diphosphate (FPP) from isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP). This reaction is considered to be a rate-limiting step in isoprenoid biosynthesis. Han et al. [Citation26] reported that squalene epoxidase (SE), which converts squalene to 2,3-oxidosqualene, is considered a rate-limiting step in sterol and triterpenoid biosynthesis. In addition, up-regulation of the genes in the GA biosynthetic pathway, hmgs, hmgr and fpps, was also observed in G. lucidum in response to MeJA [Citation22]. However, the sqs and ls genes appeared to be repressed by MeJA in I. baumii ().
Transcriptional responses to MeJA stimulation at different time points
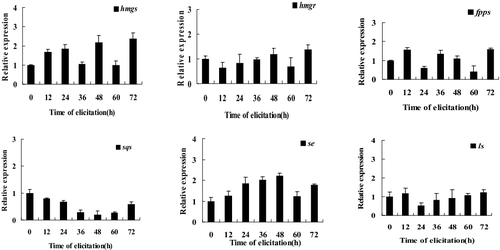
When the concentration of MeJA was at 150 μmol/L and the duration of MeJA treatment was 48 h, the triterpenoid yield of I. baumii was highest (); concomitantly, most of the genes in the triterpenoid biosynthestic pathway, such as hmgs, hmgr, fpps and se, reached their highest transcript levels (). Therefore, we examined the temporal dynamics (12, 24, 36, 48, 60 and 72 h) of gene expression in response to induction with 150 μmol/L MeJA in order to determine the optimal induction time. The genes in the MVA pathway of I. baumii responded with different dynamics. For instance, the expression of hmgs, hmgr and fpps increased 2.4, 1.4 and 1.6-fold compared to the control at 72 h, respectively. For se, the transcript levels increased at 12–72 h compared to the control, and reached the highest level (2.2-fold) at 48 h after induction (), which is consistent with se expression following 150 μmol/L MeJA induction observed previously (). In comparison, the sqs expression decreased at all induction times. Overall, this study confirmed that MeJA could induce triterpenoid biosynthesis in I. baumii. Our continuing studies will be focused on mechanisms of triterpenoid accumulation induced by MeJA at different growth stages of I. baumii. Moreover, we will further explore the interactions of genes in the triterpenoid biosynthetic pathway.
Conclusions
To the best of our knowledge, this study demonstrated for the first time that MeJA could be used as an elicitor to enhance triterpenoid production of liquid cultures of I. baumii. Furthermore, this work suggested that exogenously supplied MeJA induces triterpenoid biosynthesis via induction of the expression of the triterpenoid biosynthetic pathway genes in I. baumii.
Acknowledgments
The authors are grateful to Professor Xuemei Chen (Department of Botany and Plant Sciences, University of California, Riverside, CA, USA) for her helpful suggestions and critical reading of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Wu SH, Dai YC, Hattori T, et al. Species clarification for the medicinally valuable ‘sanghuang’ mushroom. Bot Stud. 2012;53:135–149.
- Xue Q, Sun J, Zhao MW, et al. Immunostimulatory and anti-tumor activity of a water-soluble polysaccharide from Phellinus baumii mycelia. World J Microbiol Biotechnol. 2011;27:1017–1023.
- Ge Q, Mao JW, Zhang AQ, et al. Purification, chemical characterization, and antioxidant activity of a polysaccharide from the fruiting bodies of sanghuang mushroom (Phellinus baumii Pilát). Food Sci Biotechnol. 2013;22:301–307.
- Hwang HJ, Kim SW, Lim JM, et al. Hypoglycemic effect of crude exopolysaccharides produced by a medicinal mushroom Phellinus baumii in streptozotocin-induced diabetic rats. Life Sci. 2005;76:3069–3080.
- Jang BS, Kim JC, Bae JS, et al. Extracts of Phellinus gilvus and Phellinus baumii inhibit pulmonary inflammation induced by lipopolysaccharide in rats. Biotechnol Lett. 2004;26:31–33.
- Yayeh T, Lee WM, Ko D, et al. Phellinus baumii ethyl acetate extract alleviated collagen type II induced arthritis in DBA/1 mice. J Nat Med. 2013;67:807–813.
- Hwang BS, Lee IK, Choi HJ, et al. Anti-influenza activities of polyphenols from the medicinal mushroom Phellinus baumii. Bioorg Med Chem Lett. 2015;25:3256–3260.
- Taji S, Yamada T, Wada SI, et al. Lanostane-type triterpenoids from the sclerotia of Inonotus obliquus possessing anti-tumor promoting activity. Euro J Med Chem. 2008;43:2373–2379.
- Wang GJ, Tsai TH, Chang TT, et al. Lanostanes from Phellinus igniarius and their NOS inhibitory activities. Planta Med. 2009;75:1602–1604.
- Xie JN, Song SF, Li X, et al. 桑黄总三萜的提取及其体外抗脑胶质瘤U251活性 [Extraction of total triterpenoids from Phellinus igniarius and evaluation its inhibitory activity on human glioblastoma U251 Cells in vitro]. Chin J Exp Traditional MedFormulae. 2012;5:24–26. Chinese.
- Zhang LF, Sun TT, Zou L. 鲍姆纤孔菌总三萜的提取及其体外抗乳腺癌细胞 (MCF-7) 活性 [Extraction of total triterpenoids from Inonotus baumii and its inhibitory activity on breast cancer cells (MCF-7) in vitro]. Drug Eval Res. 2015;38:497–502. Chinese.
- Zou L, Sun TT, Li DL, et al. De novo transcriptome analysis of Inonotus baumii by RNA-seq. J Biosci Bioeng. 2016;121:380–384.
- Sun TT, Zou L, Zhang LF, et al. 桑黄鲨烯环氧酶基因克隆与序列分析 [Cloning and sequence analysis of squalene epoxidase gene from Inonotus baumii]. Chin Traditional Herbal Drugs. 2015;46:2768–2773. Chinese.
- Cheong JJ, Choi YD. Methyl jasmonate as a vital substance in plants. Trends Genet. 2003;19:409–413.
- Dar TA, Uddin M, Khan MMA, et al. Jasmonates counterplant stress: a review. Environ Exp Bot. 2015;115:49–57.
- Qian ZG, Zhao ZJ, Xu YF, et al. Novel chemically synthesized hydroxyl-containing jasmonates as powerful inducing signals for plant secondary metabolism. Biotechnol Bioeng. 2004;86:809–816.
- Ketchum REB, Gibson DM, Croteau RB, et al. The kinetics of taxoid accumulation in cell suspension cultures of Taxus following elicitation with methyl jasmonate. Biotechnol Bioeng. 1999;62:97–105.
- Hu FX, Zhong JJ. Jasmonic acid mediates gene transcription of ginsenoside biosynthesis in cell cultures of Panax notoginseng treated with chemically synthesized 2-hydroxyethyl jasmonate. Process Biochem. 2008;43:113–118.
- Jiao J, Gai QY, Wang W, et al. Enhanced astragaloside production and transcriptional responses of biosynthetic genes in Astragalus membranaceus hairy root cultures by elicitation with methyl jasmonate. Biochem Eng J. 2016;105:339–346.
- Duč aiová Z, Sajko M, Mihalič ová S, et al. Dynamics of accumulation of coumarin-related compounds in leaves of Matricaria chamomilla after methyl jasmonate elicitation. Plant Growth Regul. 2016;79:81–94.
- Meimaroglou DM, Galanopoulou D, Markaki P. Study of the effect of methyl jasmonate concentration on aflatoxin B 1 biosynthesis by Aspergillus parasiticus in yeast extract sucrose medium. Int J Microbiol [Internet]. 2009 [cited 2016 Apr 20];2009:842626. Available from: https://www.hindawi.com/journals/ijmicro/2009/842626/
- Ren A, Qin L, Shi L, et al. Methyl jasmonate induces ganoderic acid biosynthesis in the basidiomycetous fungus Ganoderma lucidum. Bioresource Technol. 2010;101:6785–6790.
- Liang J, Sun MY, Zhang T, et al. 响应曲面法优化桑黄菌丝体中三萜的微波提取工艺 [Optimization of extracting triterpene from Phellinus igniarius by response surface methodology]. Chin Agric Sci Bull. 2011;27:235–238. Chinese.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-△△CT method. Methods. 2001;25:402–408.
- Cunillera N, Arró M, Delourme D, et al. Arabidopsis thaliana contains two differentially expressed farnesyldiphosphate synthase genes. J Biol Chem. 1996;271:7774–7780.
- Han JY, In JG, Kwon YS, et al. Regulation of ginsenoside and phytosterol biosynthesis by RNA interferences of squalene epoxidase gene in Panax ginseng. Phytochemistry. 2010;71:36–46.