ABSTRACT
We aimed to construct a highly efficient, lentivirus-mediated vector for RNA interference (RNAi) silencing of the Id1 gene and to examine whether silencing of this gene promotes the neural differentiation of mesenchymal stem cells (MSCs). Three short hairpin RNA sequences targeting the coding region of the Id1 gene and one negative control (NC) sequence were designed. After monophosphorylation, these sequences were introduced into lentiviral vectors containing enhanced green fluorescent protein (EGFP) using the restriction enzyme BsmBI to construct the recombinant vectors pWSLV-EGF-shId1 (1-3) and pWSLV-EGF-NC. These recombinant lentivirus vectors were transfected into H9C2 cells to assess the silencing effect of the shId1 (1-3) sequences on the Id1 gene. Subsequently, pWSLV-EGF-NC and pWSLV-EGF-shId1 which exhibited the strongest silencing effect were transfected into the packaging cell line 293 FT. After determining the viral titers, the cultured MSCs were infected with the lentivirus (multiplicity of infection, MOI = 10). Real-time polymerase chain reaction (PCR) and Western blotting were used to examine the amounts of Id1 mRNA and protein, respectively, in the transfected MSCs, and the results revealed that Id1 expression was markedly inhibited. Finally, MSCs were treated with the growth factors EGF and bFGF in conjunction with ligustrazine to induce their differentiation into neuron-like cells. The expression of NSE/MAP-2/Nestin was detected using immunocytochemistry and real-time PCR, which revealed that the rate of neural differentiation in the pWSLV-EGF-shId1 group was higher than that of the other groups, as was NSE/MAP-2 expression. Thus, Id1 gene silencing promotes the neural differentiation of MSCs.
Introduction
Mesenchymal stem cells (MSCs) are a type of adult stem cells characterized by self-replication and multipotency. They have the potential to differentiate into several types of cells, including endothelial cells [Citation1], male germ-like cells [Citation2], cardiomyocytes [Citation3] and diverse neuronal lineages [Citation4–7]. At present, stem cell-mediated therapies represent potential clinical treatments for degenerative diseases of the nervous system, including Parkinson's disease and spinal cord injury [Citation8,Citation9]. MSCs have the advantages of easy isolation, self-renewal and low immunogenicity, which facilitate allogenic transplantation without the need for immunosuppressive drugs [Citation10,Citation11]. Therefore, they are attractive candidates for clinical applications in the repair or regeneration of damaged tissues. Cell transplantation has been studied as a treatment for neurodegenerative disorders for several decades, and many reports on the differentiation capacity of MSCs in vivo demonstrated great achievements had been made [Citation12,Citation13]. However, the survival and differentiation rate of allogeneic MSCs in vivo is relatively low. It is, therefore, essential to establish a more reliable protocol for their differentiation.
Basic helix-loop-helix (bHLH) proteins are important transcriptional regulators that can activate the expression of relevant genes to promote cell differentiation, including neural differentiation [Citation14] by binding to the E box [Citation15] – DNA response element, which has been found to regulate gene expression in neurons, muscles and other tissues. ID (Inhibitor of DNA differentiation) proteins lack a DNA-binding motif and act as negative regulators of bHLH and other transcription factors by preventing DNA binding or by sequestering their heterodimerization partners [Citation16]. To date, four members of this family (ID1-4) have been identified in mammals [Citation17,Citation18], and the studies have shown that ID proteins are involved in the development of the nervous system, muscle genesis, tumorigenesis, cell cycle regulation and cell apoptosis [Citation19–23]; in particular, ID1-3 expression has been found in dividing neuroblasts of the central nervous system during development [Citation24,Citation25].
To improve the neuronal differentiation rate of MSCs in vitro, we constructed lentiviral vectors to direct RNA interference (RNAi) against the Id1 gene to decrease endogenous Id1 expression in MSCs and facilitate bHLH-mediated neuronal differentiation. In this study, we investigated the changes in the neural differentiation of MSCs upon Id1 knockdown with the goal of providing a theoretical basis for the treatment of neurodegenerative diseases.
Materials and methods
MSC culture and identification
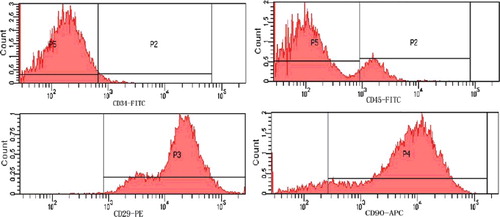
Animal experiments followed the recommendations of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Ethics Committee of Hebei North University. Four-week-old male SD rats were anesthetized by intraperitoneal injection of 0.5 mg/g chloral hydrate. MSCs were isolated and harvested from femora by flushing the shafts with low-glucose Dulbecco's Modified Eagle Medium (L-DMEM; Gibco, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS; Gibco, Carlsbad, CA, USA) using a 5 mL syringe needle. After being stirred softly, the cell suspensions were plated in 25 cm2 culture flasks (Becton Dickinson, Franklin Lakes, NJ, USA). The cultures were maintained at 37 °C in a humidified atmosphere plus 5% CO2. The medium was replaced every three days, and non-adherent cells were gradually removed by changing the medium several times. When the cell monolayer reached 80%–90% confluence, the cells were trypsinized using 0.05% (w/v) trypsin (Gibco, Carlsbad, CA, USA), and the cell suspension was divided into two flasks for further culture. This procedure was repeated until the third generation. To analyse the surface markers of MSCs, flow cytometry (FCM) was performed. MSCs were collected at the third passage, and their density was adjusted to approximately 106 cells/mL. Next, 8 μL CD29-PE, CD34-FITC, CD45-FITC and CD90-APC rat-specific antibodies (BD Pharmingen, San Diego, CA, USA) were added to the tubes. After being well mixed, the cells were incubated at room temperature for 30 min in the dark. Positive cells were detected by FCM (Becton-Dickinson, Franklin Lakes, NJ, USA). Rat IgG1-FITC and IgG1-PE (BD Biosciences Pharmingen, San Diego, CA, USA) were used as isotype controls. Finally, the cell suspensions were washed with D-Hanks buffer to remove any unbound antibody and were resuspended to analyse their surface antigens. After characterization of cell surface markers, the MSCs were infected with RNAi Lv-vector pseudovirions.
Construction and validation of recombinant Lv-shRNAs
According to the cDNA sequence of the Rattus Id1 gene (GenBank: NC_005102.4), RNAi target sequences (shRNA1-3) and one negative control (NC) sequence were designed, and four pairs of oligonucleotide sequences were synthesized (). After monophosphorylation, four pairs of sequences were ligated into pWSLV-EGFP vectors (cut by BsmBI endonuclease) by T4 ligase.
Table 1. Oligonucleotide sequences of Id1-specific shRNAs and NC sequence.
The ligated products were used to infect DH5α cells, and positive clones were sequenced. After culturing, the positive clones were verified by DNA sequencing, recombinant plasmids were extracted and were used to infect H9C2 cells using Lipofectamine 2000. Total RNA was extracted from transfected H9C2 cells and was reverse transcribed into cDNA, which was used as a template to examine the effect of Id1 silencing using real-time polymerase chain reaction (PCR). Real-time PCR was performed in a 25 mL reaction system containing 600 ng cDNA, 20 mmol/L primers specific for the Id1 gene (), 12.5 mL of the Power SYBR Green PCR Master Mix (TaKaRa, Dalian, China) and ddH2O to 25 mL. PCR parameters included pre-denaturation at 94 °C for 5 min; followed by 35 cycles of 94 °C degeneration for 40 s, 60 °C annealing for 30 s and 72 °C extension for 2 min, followed by a 72 °C extension step for 10 min (Applied Biosystems, Foster City, CA, USA).
Table 2. Real-time PCR primer sequences.
Virus packaging and MSC transfection
After the examination of Id1 silencing, pWSLV-EGF-NC and pWSLV-EGF-shId1 which exhibited the most robust silencing effect were, respectively, co-transfected with auxiliary plasmids into 293 FT packaging cells (Invitrogen, Carlsbad, CA, USA) using Lipofectamine 2000. Following 72 h of culture, the virus supernatant was collected, and a concentrated viral solution was made via ultracentrifugation. After the viral titer was determined, the MSCs were infected with packaged virus (multiplicity of infection, MOI = 10). Stable cell lines were created with the vectors pWSLV-EGFP-shId1 and pWSLV-EGFP-NC and were used in subsequent experiments. After infection with lentivirus for 72 h, green fluorescent protein (GFP)-positive cells were selected by FCM for further culture.
Analysis of Id1 gene expression in MSCs
After selection using FCM and amplification in culture, the transfected MSCs were collected and total RNA and protein were extracted. Real-time PCR was carried out to detect the expression level of Id1 in MSCs as described above. After determining the protein concentration by BCA (bicinchoninic acid) assay, equivalent amounts of proteins from each sample mixed with SDS (sodium dodecyl sulphate) loading buffer were separated using 11% SDS-PAGE (polyacrylamide gel electrophoreis). Next, the separated proteins were transferred to PVDF (polyvinylidene difluoride) membranes and blocked with 10% non-fat milk at room temperature for 2 h to inhibit nonspecific protein binding. The membranes were then incubated with rabbit-anti-rat ID1 (1:500) antibodies overnight at 4 °C. After washing with TBS-T (Tris-buffered saline, 0.1% Tween 20), goat anti-rabbit secondary antibody conjugated to horseradish peroxidase was added at a 1:1000 ratio. Anti-β-actin antibody (1:1000) was used as an internal control.
Cell grouping and neural differentiation
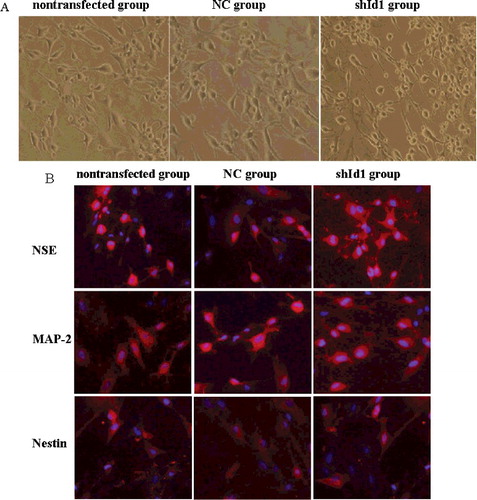
Neural differentiation was examined in three treatment groups: nontransfected group (normal control), NC group (infected with pWSLV-EGFP-NC) and shId1 group (infected with pWSLV-EGFP-shId1). To induce preneural differentiation, MSCs were cultured with pre-induction media: L-DMEM supplemented with 5% FBS, 10 ng/mL bFGF (basic fibroblast growth factor) and 10 ng/mL EGF (epidermal growth factor). After one day, the pre-induction medium was replaced with neural induction media – DMEM/F12 supplemented with 1.5 g/L ligustrazine [Citation26] to induce neural differentiation. During induction, the cell phenotype was closely observed under a light microscope and photos were taken.
Immunocytochemistry analysis
To characterize the differentiation maturity of MSCs, the expression of neuronal markers was assessed by immunocytochemistry after 48 h of induction. The differentiated cells were rinsed three times in PBS (phosphate-buffered saline) buffer and were fixed with 4% paraformaldehyde for 10 min at room temperature, followed by permeabilization with 0.3% Triton X-100 in PBS for 20 min. The cells were then blocked with 2% BSA (bovine serum albumin) (Sigma, Cambridge, UK) for 20 min and incubated with primary antibodies (1:300 dilutions in PBS, Abcam, San Francisco, CA, USA) at 4 °C overnight. The primary antibodies included rabbit monoclonal anti-NSE, anti-MAP-2 and anti-Nestin. After being washed three times in PBS, the cells were incubated with goat anti-rabbit secondary antibody-PE (Abcam, 1:200 diluted in PBS) for 30 min and were washed three times in PBS. Finally, Hoechst 33258 was used to stain nuclei, and the superfluous dye was washed away using PBS. The neuronal markers were detected under a Nikon 90-i multi-microscope and were processed using electronics and a computer module (Real-Time control system). At least 10 fields of view from three independent experiments were used to count the number of NSE-, MAP-2- and Nestin-positive cells.
Expression analysis of neuronal marker genes by real-time PCR
To further investigate whether Id1 silencing promotes the neural differentiation of MSCs, real-time PCR was used to examine the expression patterns of the NSE, MAP-2 and Nestin genes after the addition of neural induction media for 0, 24, 36, 48 and 72 h. TRIzol reagent (Invitrogen, Carlsbad, CA, USA) was used to extract total RNA, which was used as a template to synthesize cDNA. The real-time PCR reactions were performed as described above, and the primers are listed in . The procedures were set as follows: pre-denaturation at 94 °C for 10 min; followed by 35 cycles of 94 °C for 30 s, annealing (temperatures provided in ) for 30 s, and 72 °C for 60 s; and finally, a 72 °C extension for 10 min. β-actin was used as an internal control (Applied Biosystems, Foster City, CA, USA). Every reaction was performed in triplicate, and the average was used for statistical analysis.
Data analysis
All experiments were performed at least in triplicate. The statistical analysis was done using SPSS 17.0 software. The data were analysed by one-way analysis of variance and were presented as means with standard deviation (means±SD). Values among the groups were considered significantly different at P < 0.05.
Results and discussion
MSC culture and cell-surface antigen determination by FCM
Following 24 h of primary culture, most cells had adhered to the surface of the culture flasks and had elongated. As the culture period progressed, adherent cells gradually exhibited spindle-shape morphology, and at 90% confluence, the cells acquired fibroblast-like morphology (). Surface antigen detection of cultured cells stained with rat-specific monoclonal antibodies by FCM showed that the cultured cells were strongly positive for typical MSC markers: CD29 and CD90, which were expressed by 99.8% and 89.1% of cells, respectively; the cells were largely negative for CD34 and CD45, which marked 1.8% and 14.8% of cells, respectively. Thus, the cultured cells exhibited the basic properties of MSCs ().
Validation of silencing by recombinant LV-shRNAs
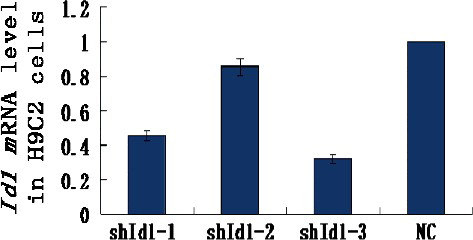
The recombinant vectors pWSLV-EGFP-Id1 (1-3) and pWSLV-EGFP-NC were used to infect H9C2 cells, and total RNA was extracted to analyse the silencing effect of the Id1 gene. Real-time PCR revealed that the level of Id1 mRNA in the pWSLV-EGFP-Id1-3 group was only 32% of the pWSLV-EGFP-NC group (), indicating that Id1 was silenced by RNAi. In our later experiments, pWSLV-EGFP-Id1-3 was used to silence the Id1 gene in MSCs. So far, many methods have been employed for genome editing, including RNAi, CRISPR/Cas9, TALEN and ZFN. However, CRISPR/Cas9, TALEN and ZFN have their limitations. For example, CRISPR/Cas9 has severe off-target effects and its difficulty coefficient is higher [Citation27,Citation28]; TALEN is vulnerable to the effects of DNA methylation, and its design is very complicated [Citation29]; and due to the interaction between adjacent ZF sequences, ZFN exhibits frequent drift in sequence recognition [Citation30]. Compared to these newer methods, RNAi technology has unique advantages in its low cost, ease of use and high silencing efficiency [Citation31].
Virus packaging and MSC transfection
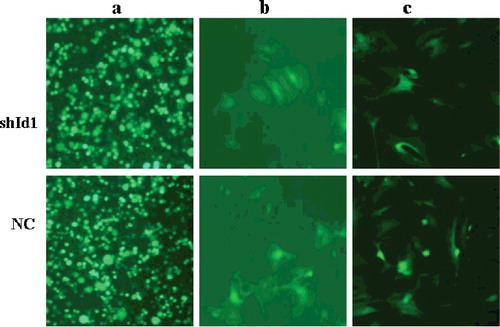
After 72 h co-transfection of recombinant lentivirus and its packaging plasmids, 293 FT cells of the shId1 and NC groups showed strong green fluorescence (), indicating that recombinant lentivirus vectors had been delivered into the cells. MSCs were seeded in 6-well plates and grown to 50%–60% confluence; lentivirus (MOI = 10) was then added to the medium to infect the MSCs. After 72 h of infection, green fluorescence was observed (); GFP-positive cells were sorted by FCM, and the purity of the transfected cells markedly increased.
Figure 4. Virus packing in 293 FT cells and MSCs transfection (×10). GFP fluorescence imaging showed that 293 FT cells were transfected by viral vector after 72 h (a); GFP fluorescence imaging showed that MSCs were infected by the packaged virus (b); strong GFP fluorescence imaging showed that transfected MSCs were sorted by FCM (c).
Expression of the Id1 gene in MSCs
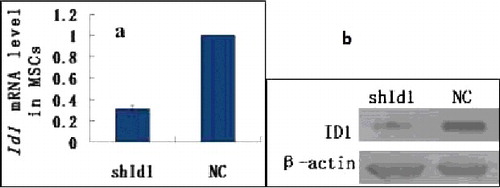
Real-time PCR showed that the amount of Id1 mRNA in the shId1 group was 0.314 ± 0.028, indicating that the silencing efficiency is more than 68%. The amount of Id1 mRNA in the NC group was regarded as 1.00. The Id1 mRNA level in the NC group was statistically higher than that of the silencing group (P < 0.01) (). Based on the Western blotting, the ID1 protein in the shId1 group was significantly suppressed compared to the NC group. Thus, the recombinant shId1 vector efficiently silences the Id1 gene in MSCs.
Neural differentiation and immunocytochemistry
After 24 h of induction treatment, 30.43% ± 3.18% of transfected MSCs exhibited multipolar morphology, but a few bipolar cells were observed in other groups. As induction proceeded, more and more MSCs in all groups differentiated into neurons. However, the neural differentiation rate in the shId1 group was higher, and 85.27% ± 5.43% of transfected MSCs appeared multipolar with projections connected in a network after 48 h of neural induction, whereas only 57.38% ± 2.84% and 56.39% ± 5.36% of MSCs in the NC and nontransfected groups, respectively, exhibited multipolar or bipolar morphologies ((A)). Thus, it appears that silencing the Id1 gene can improve the neural differentiation rate of MSCs, consistent with a report that neural differentiation can be increased by suppressing Id1 expression in zebrafish [Citation32]. It has been reported that high expression of Id1 promotes cell proliferation and inhibits cell differentiation [Citation33,Citation34], which is in agreement with the reports that overexpression of Id1 maintains the undifferentiated state by sustaining pluripotency markers [Citation35] and preventing commitment to a neural fate [Citation36].
Figure 6. Neuronal differentiation of MSCs in vitro (×10): morphological phenotype of neuron-like cells under light microscope (A); neuronal marker expression in three groups (B): high expression in NSE and MAP-2, but low in Nestin.
The bHLH family consists of two classes of transcription regulators: inhibitors (such as Hes and Id) and activators (such as Mash1, Math1 and NeuroD). These two types of bHLH factors coordinate with each other and exist in a state of dynamic equilibrium [Citation37–40]. The balance of these factors plays an important role in neural differentiation in vivo, [Citation41], and it is possible that a change in the bHLH expression pattern can regulate neural differentiation, laying a foundation for the treatment of disease by cell transplantation. For instance, Mash1 protein promotes the generation of neural precursor cells and the neural differentiation of multipotent stem cells [Citation42]. The expression of neurogenin2 (ngn2) in neural progenitor cells of the central and peripheral nervous systems can accelerate the differentiation of these progenitor cells into neurons [Citation43,Citation44]. In vitro Ngn2 overexpression directs 95% of transfected neural stem cells to differentiate into neuron-like cells [Citation45]. One study demonstrated that, in the absence of Ngn2 and Mash1 function, most mutant progenitors that fail to commit to a neuronal fate produce astrocytes rather than remaining undifferentiated [Citation46]. In addition, the ectopic expression of NeuroD in Xenopus embryos results in the premature differentiation of neuronal precursors. Furthermore, NeuroD can convert presumptive epidermal cells into neurons and can act as a neuronal determination gene [Citation47].
Immunocytochemical experiments revealed that after 48 h of neural induction, the neuron-like cells differentiated from MSCs exhibited high levels of NSE and MAP-2 expression, but low levels of Nestin. The incidence of NSE-, MAP-2- and Nestin-positive cells is presented in . As shown, more than 80% of MSCs in the shId1 group were immunopositive for NSE and MAP-2, but only 18% of MSCs were positive for Nestin ((B)).
Table 3. Rate of neural marker-positive cells (%).
Analysis of neuronal marker expression
To further investigate the effects of Id1 gene silencing on the neural differentiation of MSCs, real-time PCR was performed to detect the expression of NSE, MAP-2 and Nestin after 0, 24, 36, 48 and 72 h of induction (). As treatment progressed, the expression of NSE and MAP-2 in shId1 cells rapidly increased and reached their respective peaks at 48 and 72 h; there were significant differences in comparison with the other groups (P < 0.01). The expression pattern of Nestin throughout induction differed from those of NSE and MAP-2; its mRNA gradually increased during the first 0–36 h of induction and then dropped sharply. This reduction was especially rapid in the shId1 group (P < 0.05).
Figure 7. Real time-PCR analysis of the expression of NSE, MAP2 and Nestin in differentiated cells during induction course.
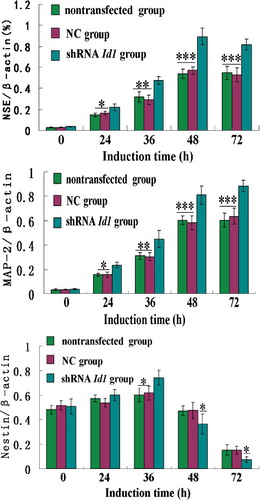
Nestin is expressed in neural precursor cells and neural stem cells. NSE is an acid proteinase specific to neurons and neuroendocrine cells, and it is regarded as a marker of mature neurons. MAP-2 is a thermostable phosphoprotein that is mainly expressed in the cell body and dendrites of neurons [Citation48]. Thus, our research demonstrates that Id1 gene silencing can improve the maturation of differentiated neurons across a range of induction times. However, prior to neuronal differentiation, the differentiated cells progress through a neural precursor stage.
Conclusions
In summary, using RNA interference combined with lentiviral packing allowed us to silence the Id1 gene and generate a stable MSC pWSLV-EGFP-shId1 cell line. After knockdown of Id1 gene expression, the transfected MSCs were easily differentiated into neuron-like cells. By 72 h after induction, the differentiated neuron-like cells exhibited high levels of NSE and MAP-2 expression, but low levels of Nestin expression.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Lu YS, Zhou ZH, Tao J, et al. Overexpression of stearoyl-CoA desaturase 1 in bone marrow mesenchymal stem cells enhance the expression of induced endothelial cells. Lipids Health Dis. 2014;13:1–8.
- Nejad NA, Amidi F, Hoseini MA, et al. Male germ-like cell differentiation potential of human umbilical cord Wharton's jelly-derived mesenchymal stem cells in co-culture with human placenta cells in presence of BMP4 and retinoic acid. Iran J Basic Med Sci. 2015;18:325–333.
- Zhang J, Ho JCY, Chan YC, et al. Overexpression of myocardin induces partial transdifferentiation of human-induced pluripotent stem cell-derived mesenchymal stem cells into cardiomyocytes. Physiol Rep [ Internet]. 2014 [ cited 2016 Aug 5];2:e00237. Available from: http://physreports.physiology.org/content/2/2/e00237.long
- Zhao HB, Ma H, Ha XQ, et al. Salidroside induces rat mesenchymal stem cells to differentiate into dopaminergic neurons. Cell Biol Int. 2014;38:462–471.
- Soleimani MM, Chian MF. Cysteine: a novel neural inducer for rat bone marrow mesenchymal stem cells. Cell J. 2014;16:195–202.
- Takeda YS, Xu Q. Neuronal differentiation of human mesenchymal stem cells using exosomes derived from differentiating neuronal cells. PLoS ONE [ Internet]. 2015 [ cited 2016 Aug 5];10:e0135111. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4527703/
- Kim EY, Lee KB, Yu J, et al. Neuronal cell differentiation of mesenchymal stem cells originating from canine amniotic fluid. Human Cell. 2014;27:51–58.
- Yin XF, Xu HM, Jiang YX, et al. The effect of lentivirus-mediated PSPN genetic engineering bone marrow mesenchymal stem cells on Parkinson's disease rat model. PLoS ONE [ Internet]. 2014 [ cited 2016 Aug 5];9:e105118. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4132064/
- Zhang Z, Dai GH, Liu XB, et al. Umbilical cord mesenchymal stem cell transplantation for spinal cord jury. J Chin Pract Diagn Ther. 2015;29:478–480.
- Lennon DP, Caplan AI. Isolation of rat marrow-derived mesenchymal stem cells. Exp Hematol. 2006;34:1606–1607.
- Han SM, Han SH, Coh YR, et al. Enhanced proliferation and differentiation of Oct4- and Sox2-overexpressing human adipose tissue mesenchymal stem cells. Exp Mol Med. 2014;46:e101–e109.
- Baksh D, Song L, Tuan RS. Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med. 2004;8:301–316.
- Wu J, Sun Z, Sun HS, et al. Intravenously administered bone marrow cells migrate to damaged brain tissue and improve neural function in ischemic rats. Cell Transplant. 2008;16:993–1005.
- Zhu GX, Wu YX, Qiao L, et al. Expression of neurogenin2 and math6 in the embryonal and postnatal mouse inner ear and their molecular regulation. Chinese J Otology. 2012;10:96–100.
- Kageyama R, Ohtsuka T, Hatakeyama J, et al. Roles of bHLH genes in neural stem cell differentiation. Exp Cell Res. 2005;306:343–348.
- Aloia L, Gutierrez A, Caballero JM, et al. Direct interaction between Id1 and Zrf1 controls neural differentiation of embryonic stem cells. EMBO Rep. 2015;16:63–70.
- Yun K, Marianne B. To proliferate or to die: role of Id3 in cell cycle progression and survival of neural crest progenitors. Genes Dev. 2005;19:744–755.
- Perk J, Iavarone A, Benezra R. Id family of helix-loop-helix proteins in cancer. Nat Rev Cancer. 2005;5:603–614.
- Kremer D, Aktas O, Hartung HP, et al. The complex world of oligodendroglial differentiation inhibitors. Ann Neurol. 2011;69:602–618.
- Norton JD. ID helix-loop-helix proteins in cell growth, differentiation and tumorigenesis. J Cell Sci. 2000;113:3897–3905.
- Lasorella A, Benezra R, Iavarone A. The ID proteins: master regulators of cancer stem cells and tumour aggressiveness. Nat Rev Cancer. 2014;14:77–91.
- Anido J, Sáez-Borderías A, Gonzàlez-Juncà A, et al. TGF-beta receptor inhibitors target the CD44(high)/Id1(high) glioma-initiating cell population in human glioblastoma. Cancer Cell. 2010;18:655–668.
- Ohtani N, Zebedee Z, Huot TJ, et al. Opposing effects of Ets and Id proteins on p16INK4a expression during cellular senescence. Nature. 2001;409:1067–1070.
- Jen Y, Manova K, Benezra R. Each member of the Id gene family exhibits a unique expression pattern in mouse gastrulation and neurogenesis. Dev Dyn. 1997;208:92–106.
- Neuman T, Keen A, Zuber MX, et al. Neuronal expression of regulatory helix–loop–helix factor Id2 gene in mouse. Dev Biol. 1993;160:186–195.
- Du XM, Wei HP, Zhang AL, et al. Research of ligustrazine induced effects of long-term to rat bone marrow of mesenchymal stem cells. Chin Med Mod Distance Educ China. 2012;10:157–158.
- Kuscu C, Arslan S, Singh R, et al. Genome-wide analysis reveals characteristics of off-target sites bound by the Cas9 endonuclease. Nat Biotechnol. 2014;32:677–683.
- Pattanayak V, Lin S, Guilinger JP, et al. High-throughput profiling of off-target DNA cleavage reveals RNA programmed Cas9 nuclease specificity. Nat Biotechnol. 2013;31:839–843.
- Bultmann S, Morbitzer R, Schmidt CS, et al. Targeted transcriptional activation of silent oct4 pluripotency gene by combining designer TALEs and inhibition of epigenetic modifiers. Nucleic Acids Res. 2012;40:5368–5377.
- Pruett-Miller S, Connelly J, Maeder M, et al. Comparison of zinc finger nucleases for use in gene targeting in mammalian cells. Mol Therapy. 2008;16:707–717.
- Zhao YH, Liang H, Liu MQ, et al. The application of gene knockout technologies in big domestic animals. Acta Veterinaria et Zootechnica Sinica. 2014;45:1–8.
- Gao H, Bu Y, Wu Q, et al. Mecp2 regulates neural cell differentiation by suppressing the Id1 to Her2 axis in zebrafish. J Cell Sci. 2015;128:2340–2350.
- Otoole PJ, Inoue T, Emerson L, et al. ID proteins negatively regulate basic helix-loop-helix transcription factor function by disrupting subnuclear compartmentalization. J Biol Chem. 2003;278:45770–45776༎
- Zhang HL, Yin W, Tao Y, et al. Effect of retinociacdi combined extracts from testudinis carapacis et plastron on proliferating in MSCs and its mechanism. J Chin Med Mater. 2014;37:87–90.
- Ying QL, Nichols J, Chambers I, et al. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115:281–292.
- Malaguti M, Nistor PA, Blin G, et al. Bone morphogenic protein signalling suppresses differentiation of pluripotent cells by maintaining expression of E-Cadherin. eLife [ Internet]. 2013 [ cited 2016 Aug 5];2:e01197. Available from: http://dx.doi.org/10.7554/eLife.01197
- Vetter M. A turn of the helix: preventing the glial fate. Neuron. 2001;29:559–562.
- Lee SK, Pfaff SL. Transcriptional networks regulating neuronal identity in the developing spinal cord. Nat Neurosci. 2001;4:1183–1191.
- Cai L, Morrow EM, Cepko CL. Misexpression of basic helix-loop-helix genes in the murine cerebral cortex affect cell fate choices and neuronal survival. 2000;127:3021–3030.
- Bae S, Bessho Y, Hojo M, et al. The bHLH gene Hes6, an inhibitor of Hes1, promotes neuronal differentiation. Development. 2000;127:2933–2943.
- Wang JB, Liu WP, Zhang X, et al. Expression of bHLH genes in differentiation of neural stem cells. Chin J Neurosurg Dis Res. 2007;6:199–202.
- Hamada M, Yoshikawa H, Ueda Y, et al. Introduction of the MASH1 gene into mouse embryonic stem cells leads to differentiation of motoneuron precursors lacking Nogo receptor expression that can be applicable for transplantation to spinal cord injury. Neurobiol Dis. 2006;22:509–522.
- Fode C, Gradwohl G, Morin X, et al. The bHLH protein neurogenin2 is a determination factor for epibranchial placode-derived sensory neurons. Neuron. 1998;20:483–494.
- Ma Q, Fode C, Guillemot F, et al. Neurogenin1 and neurogenin2 control two distinct waves of neurogenesis in developing dorsal root ganglia. Genes Dev. 1999;13:1717–1728.
- Falk A, Holmström N, Carlén M, et al. Gene delivery to adult neural stem cells. Exp Cell Res. 2002;279:34–39.
- Nieto M, Sehuurmans C, Britz O, et al. Neural bHLH genes control the neuronal versus glial fate decision in cortical progenitors. Neuron. 2001;29:401–413.
- Lee JE, Hollenberg SM, Snider L, et al. Conversion of Xenopus ectoderm into neurons by NeuroD, a basic helix-loop- helix protein. Science. 1995;268:836–844.
- Luo SS, Yu J, Wang XY, et al. Differentiation of rat bone marrow mesenchymal stem cells into neuron-like cells induced by Xinmailong injection. J Shandong Univ (Health Sci). 2012;50:32–36.