ABSTRACT
Variety of health technologies applied in medical and dental practice are associated with formation of fine water droplets/aerosols. This presents a potential risk of both the patients and the personnel acquiring Legionnaires' disease. The aim of this study was to assess the presence of Legionella antibodies in the personnel (medical and dental) and a control group. The serum antibody index (Ab index) to L. pneumophila Sg1-6 (IgG + IgM) was determined using enzyme-linked immunosorbent assay. L. pneumophila Sg 1-6 antibodies (Ab index > 5) were found in 27/66 (40.91%) of the personnel vs. 7/90 (7.78%) of the control subjects (χ2 = 24.55, p < 0.0001). There was no statistically significant difference in the seropositivity levels in the groups in terms of sex, concomitant chronic diseases, intake of medications affecting the immune response, smoking and history of pneumonia. Association was observed with the professional categories of the personnel (χ2 = 6.836, df = 2, p < 0.05): more than 50% of the physicians were seropositive for L. pneumophila. The logistic regression analysis proved the role of seropositivity associated factors such as age, use of protective equipment and workplace (building with proven presence of L. pneumophila in the water system). The seroprevalence rate of Legionella antibodies in the personnel can be associated with occupational risk exposure, especially in the absence of systematic and regular use of protective equipment during work and the lack of an established system for regular monitoring and preventive maintenance of the water systems in the healthcare facilities.
Introduction
Legionella spp. are ubiquitous inhabitants of natural (water and soil) and artificial aquatic environments. They demonstrate a strong tendency to contaminate man-made water systems (water heaters, pipes, showers, sinks, pools, etc.). There, under favourable conditions, they reproduce to high concentrations and can cause illness in humans. The disease is spread by inhaling fine water droplets/aerosols (sized < 5 µm) containing legionella or aspiration. A variety of health technologies applied in medical and dental practice are associated with formation of such aerosol: e.g. using the air-water syringe, ultrasonic scalers and turbines in dental surgery, baths and hydrotherapy showers, air humidifiers, output water from dental unit waterlines, etc. All these present a potential risk both for the patients and the personnel, especially the dental personnel [Citation1]. Over time, the prevalence of legionellosis, or Legionnaires' disease (LD), has risen, which might indicate a greater awareness and reporting of the disease [Citation2]. Although only about 8% of the recorded cases of LD in Europe are the result of nosocomial transmission [Citation3], this is a reason for rigorous investigations as to the probable transmission factors in hospital settings.
The clinical significance of Legionella, especially L. pneumophila Sg1, primarily relates to severe life-threatening pneumonia known as LD. The lethality in LD can reach 20%, but in the nosocomial form it is considerably higher.
A predominant proportion of the individuals exposed to Legionella spp. develop subclinical infection which can remain unidentified, especially in cases of regular exposure [Citation4,Citation5], such as the occupational type. This is evidenced by the fact that during outbreaks only 0.1%–5% of those exposed have been diagnosed with LD [Citation3]. The risk of infection in immune competent healthcare workers is assessed as low, provided that the levels of L. pneumophila in the water systems are low [Citation6,Citation7]. On the other hand, the routinely available diagnostic tests do not offer the desired sensitivity [Citation2].
The aim of this study was to assess the presence of Legionella antibodies among hospital personnel (medical and dental) and the associated risk factors and compare the results with a control group of people not subjected to occupational exposure.
Subjects and methods
Survey design
This prospective seroepidemiological survey was conducted in the period May–November 2015 in the Plovdiv Province (the administrative region with the second largest population in Bulgaria). The cohort included a total of 156 persons allocated in two groups: Group I, healthcare (medical/dental) personnel (n = 66), and Group II, control group (n = 90).
The inclusion criteria for Group I participation were: age over 25 (at least one year of service at a health facility), work involving operation of medical/dental devices generating water aerosol and absence of history of pneumonia within the last six months. The average age of the participants in the first group was 46.39 years with a standard deviation (±SD) of ± 12.09 (age range from 26 to 71 years) and the female/male ratio was 4.5:1. In addition, the serum samples for the first group were selected among personnel working at health facilities with water monitoring for the presence of Legionella spp. According to the official guidelines, a water safety plan is required, although such was not active in these healthcare facilities/practices. The monitoring of the water in the medical devices and the overall plumbing system in these settings is part of a research project (No. 1/2013), at the Medical University of Plovdiv.
The inclusion criteria for Group II participation were: age over 20, absence of history of pneumonia and no dental manipulations within the last six months prior to the survey. The average age ± SD of the control group was 50.06 ± 13.84 years (age range from 24 to 80 years) with a female/male ratio of 1.04:1.
All survey participants underwent testing after having signed informed consent forms and having completed a questionnaire whose forms had been presented to and approved by the Scientific Ethics Committee at the Medical University of Plovdiv (protocol No. 5/31.10.2013).
Questionnaire
All participants were surveyed using a standard questionnaire, specifically developed for the purposes of the survey, whereby the following information was collected: demographics (age, gender, etc.); epidemiological data for habits such as smoking; concomitant chronic diseases (diabetes, chronic lung diseases, etc.); use of medications which affect the immune response; and history of pneumonia episodes. Additionally, data were collected for the health employee group regarding the workplace (building), work activity profile (medical or dental), personnel category (physicians, nurses and orderlies) and use of personal protective equipment at work.
Enzyme-linked immunosorbent assay (ELISA)
ELISA was used to determine the serum antibody index (Ab index) to L. pneumophila Sg1-6 IgG + IgM (Vircell, Santa Fe Granada, Spain). Prior to participants (personnel and control group) testing, an Ab index cut-off determination was carried out by establishing the mean Ab index ±SD in serum samples of individuals from the same region, tested at а private diagnostic laboratory for various medical conditions. They met the following criteria: age over 20, no history of pneumonia in the last two years, living in a single-family house without district heating and having a job that does not involve risks of LD. This yielded an Ab index cut-off of ≥5. Based on the cut-off, a scale was introduced for grouping the actual results obtained in the seroepidemiological survey of the personnel and the control group: samples with low Ab index (<5), with intermediate Ab index (5–11) and with high Ab index (>11). The survey was conducted at the National Reference Laboratory High Medical Risk Infections, National Center of Infectious and Parasitic Diseases (NCIPD), Sofia.
Data analysis
For statistical analysis, the quantitative variables were expressed as mean values with standard deviations (±SD) and 95% confidence intervals (95% CI); qualitative variables were presented as proportion with pooled sample standard error (±Sp). Comparisons between groups were performed by the chi-square test or Fisher's exact test for categorical variables, and by the t-test for quantitative variables. A P-value of less than 0.05 was considered to indicate statistically significant differences. Logistic regression analysis was performed to determine the risk factors for Legionella seropositivity in the blood donors. Data were stratified by the place of work of the donors. The variables included age, sex, hot water supply system and previous episodes of illness. Univariate analysis was performed for all variables in order to identify potential risk factors, which were included in the multivariate logistic regression model. Statistical analysis was performed using SPSS v.22.0 (SPSS Inc., Chicago, IL, USA).
Results and discussion
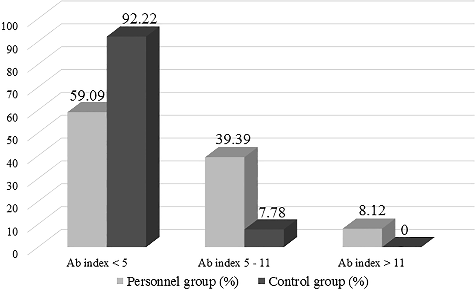
L. pneumophila Sg 1-6 (IgG+IgM) antibodies were found in all tested samples within a wide range: Ab index between 0.607 and 12.127 in the personnel and between 0.84 and 8.03 in the control group. This result is indicative of the wide spread of Legionella spp. and in particular of L. pneumophila Sg 1-6 in our environment as well as of the frequent exposure during everyday activities. The distribution of the subjects from the two groups (personnel and control group) as per Ab index according to the scale adopted by us is presented in .
The proportion of controls with Ab index < 5 was bigger 92.22% vs. 59.09% in the personnel group. On the contrary, the percentage of positive tests with intermediate Ab index (5–11) was higher within the personnel group 39.39% vs. 7.78% among the controls. In both cases, there was statistically significant difference, U = 4.96, P < 0.001 and U = 4.76, P < 0.001, respectively. These results indirectly point to a significantly more frequent contact with Legionella at the workplace, which has resulted in higher levels of antibodies in the personnel.
Only one case (1.51%, 1/66), a dentist was found to have an Ab index of 12.127, which is indicative of an ongoing infection, according to the test manufacturer's chart (Ab index >11). With regard to the serology results, a single high antibody titre for Legionella is only suggestive evidence of a legionellosis diagnosis [Citation8]. In the absence of clinical symptoms and recent history of pneumonia, this finding is indicative of continuous accumulation of antibodies in the course of regular exposure. Legionella infections often remain unidentified and making a diagnosis at the early stages of the disease is based on the use of specialized microbiological tests often in combination with: urinary antigen test, isolation of L. pneumophila or detection of Legionella nucleic acid in samples from the lower respiratory tract [Citation9].
In the current practice, the use of serological tests to detect the presence of Legionella antibodies in order to prove LD is considered not reliable. The main disadvantages are: delayed antibody production, particularly due to chronic underlying conditions; requirement of double testing of serum samples to prove the diagnosis; the test kit characteristics and, although less often, the possibility of false-positive results occurring because of cross-reactions with other bacteria, including Bacteroides fragilis, Pseudomonas sp., Stenotrophomonas sp. and Flavobacterium sp. [Citation9]. That is why a single isolated serological positive result is not to be interpreted as a marker for disease in case of absence of comparable clinical data or recent pneumonia. Despite these disadvantages, the serological tests are suitable for retrospective diagnosis of LD and for the needs of seroepidemiological research to identify groups at risk and to establish potential factors for seropositivity.
Serum samples with an index above the cut-off of 5 were determined as positive, and samples below the cut-off of 5, as negative, so as to more precisely analyse the risk factors in both groups (personnel and controls). Presence of L. pneumophila Sg 1-6 antibodies (Ab index >5) was found in 27/66 (40.91%) of the personnel compared to 7/90 (7.78%) in the control group (χ2 = 24.55, p < 0.0001; Fisher's exact test p < 0.0001). The univariate analysis confirmed the role of occupational exposure as a potential risk factor for seropositivity (OR = 8.21) among the personnel. The few seroprevalence studies in the available English-language literature also confirm the occupational risk among healthcare workers compared to the control groups [Citation4,Citation10–12], especially among dental staff [Citation4,Citation10,Citation12]. This is associated with the fact that the design and interior of small-diameter water lines and devices are ideal location for the formation of a biofilm, proliferation and persistence of Legionella spp., as well as for the formation of a water-air spray with suitable dimensions (0.2–5.0 μm) in close proximity to the respiratory tract, providing the main transmission route for this microorganism [Citation4–6,Citation13–15]. Interesting data have been reported by Borella et al. [Citation11], who within a single study conducted in Italy, found geographical differences in the seroprevalence levels of health professionals and dentists compared to that in the control group. Borella et al. [Citation11] associated the lack of significant difference between the two groups in some areas of Italy with the development and implementation of daily disinfection procedures to prevent the contamination of the water in the dental unit with Legionella spp. A study conducted by Pankhurst et al. [Citation7] among 246 general dental practitioners in London and Northern Ireland did not find a significant difference in the seroprevalence levels compared to the control group, but established that the titers of Legionella antibodies in dentists are significantly lower, i.e. the risk of occupational exposure to Legionella appears to be minimal. It should be noted that, in the United Kingdom and in other developed countries, responsible prevention of LD has been regulated for decades.
However, our results could not define the occupational risk as low. This was not surprising, given the fact that the healthcare facilities employing the surveyed healthcare/dental personnel lack prevention programmes for avoiding contamination of the water systems – including dental unit water systems – with Legionella. This could also explain the significantly higher seroprevalence levels in the personnel compared to the control group (40.91% vs. 7.78%).
Some of the risk factors commonly associated with LD include older age, male gender, smoking, diabetes mellitus, other underlying chronic diseases and medication affecting the immune response [Citation16–18]. Based on the data from our study, we were unable to prove statistically significant differences in the seropositivity levels in each of the groups (personnel and control group) in terms of sex, concomitant chronic diseases, intake of medications that affect the immune response, smoking and history of pneumonia (). Despite the higher seropositivity levels in the personnel group (), our data did not show increased incidence of past pneumonia in this group. We attribute this to the fact that Legionella infection is largely asymptomatic and often remains unidentified [Citation3,Citation4,Citation19].
Table 1. Seroprevalence of L. pneumophila Sg 1-6 in the personnel and the control group.
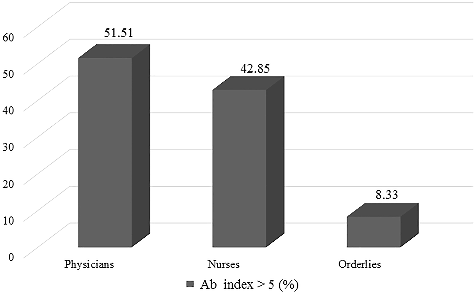
Based on the data collected additionally among the healthcare and dental personnel, we revealed a statistically significant association between the share of positive samples and the professional position (χ2 = 6.836, df = 2, P = 0.033), with more than 50% of the physicians being seropositive for L. pneumophila Sg 1-6 (IgG+IgM) antibodies (). The high levels of seropositivity among them are most likely due to the fact that physicians perform diagnostic and treatment procedures associated with the generation of a fine water aerosol, i.e. they are subject to cumulative and frequent exposure to the causative agent. These results are in agreement with previous data [Citation7,Citation10] and reports that have pointed out the importance of the length of service in the dental office [Citation20].
The logistic regression analysis () proved the role of seropositivity-associated factors such as age, use of protective equipment and workplace (buildings with proven presence of L. pneumophila in the water system).
Table 2. Odds ratio (OR) and 95% confidence interval (CI) for association between L. pneumophila Sg 1-6 seropositivity and relevant factors.
We established an OR of 9.77 (95% CI of 1.44–60.97) in terms of the age factor, which demonstrates a higher probability of Ab index > 5 in the personnel under 49 years. This is in contrast to previous reports [Citation16–18] about the existence of association between morbidity of LD and more advanced age. A possible explanation could most likely be sought in the relatively small number of participants included in our survey and the fact that they are predominantly under 50 years of age (the average personnel age is 46.39 ± 12.09 years). The other two factors resulting from the logistic regression analysis – protective equipment and workplace – are essential for the daily practice, as well as for the prevention and control of LD.
The use of the full range of protective equipment by the healthcare personnel (mask, goggles/face shield and gloves) during work was found to be associated with a significantly lower seropositivity risk (): OR = 63.868 (95% CI of 4.04–1007.81) when using only a single piece of protective equipment and OR = 3.796 (95% CI of 0.48–29.82) when using two pieces of protective equipment.
The presence of Legionella in the plumbing system of the building is an essential risk factor. The risk to patients and personnel is higher in buildings with Legionella contamination, especially with L. pneumophila Sg1, as well as the use of risk devices directly connected to such pipelines. Factors such as water temperature, configuration and age of the water distribution systems, physicochemical constituents of the water and plumbing materials may favour the growth of legionellae [Citation21,Citation22]. Risk is also posed by devices operating with distilled water if the containers are not handled properly during cleaning and refilling.
It has to be noted, however, that the present study has some limitations: the number of respondents in the personnel group was small; there was some imbalance between the units of observation in the two groups (personnel and control) and the female/male ratio was different due to the fact that the majority of the personnel in the enrolled medical/healthcare units is dominated by females. Finally, despite these limitations, our study is an important attempt towards drawing attention to the issue and improving the awareness of medical/dental professionals in local healthcare facilities.
Conclusions
The seroprevalence rate of Legionella antibodies in the personnel enrolled in this study could be associated with occupational risk exposure, especially in the absence of systematic and regular use of protective equipment during work and the absence of an established system for regular monitoring and preventive maintenance of the water systems in the healthcare facilities. The existence of and compliance with the water safety plan in terms of Legionella is essential for the prevention of LD.
Acknowledgements
The authors wish to express their gratitude to the personnel at Ramus Private Medical Diagnostic Laboratory (Plovdiv, Bulgaria) for their support in collecting serum samples from the control group and the group tested during the preparatory stage of the study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Dallolio L, Scuderi A, Rini MS, et al. Effect of different disinfection protocols on microbial and biofilm contamination of dental unit waterlines in community dental practices. Int J Environ Res Public Health. 2014;11:2064–2076.
- Cunha BA, Burillo A, Bouza E. Legionnaires' disease. The Lancet. 2016;387(10016):376–385.
- European Centre for Disease Prevention and Control. Legionnaires’ disease in Europe, 2012. Stockholm: ECDC; 2014. Available from: http://ecdc.europa.eu/en/publications/Publications/legionnaires-disease-surveillance-2012.pdf
- Rudbeck M, Viskum S, Mølbak K, et al. Legionella antibodies in a Danish hospital staff with known occupational exposure. J Environ Public Health. 2009 [ cited 2016 Sep 26];812829;[ 6 pages]. Available from: http://dx.doi.org/10.1155/2009/812829
- Szymańska J. Risk of exposure to Legionella in dental practice. Ann Agric Environ Med. 2004;11:9–12.
- Ditommaso S, Giacomuzzi M, Ricciardi E, et al. Cultural and molecular evidence of Legionella spp. colonization in dental unit waterlines: Which is the best method for risk assessment? Int J Environ Res Public Health. 2016 [ cited 2016 Sep 26];13(2):211–220. Available from: http://dx.doi.org/10.3390/ijerph13020211
- Pankhurst CL, Coulter W, Philpott-Howard JJ, et al. Prevalence of legionella waterline contamination and Legionella pneumophila antibodies in general dental practitioners in London and rural Northern Ireland. Br Dent J. 2003;195:591–594.
- Review of the prevention and control of Legionella pneumophila infection in Queensland. Chief Health Officer's report. [ place unknown]: Chief Health Officer Branch; September 2013. Available from: www.health.qld.gov.au/publications/clinical-practice/guidelines-procedures/diseases-infection/cho-legionella-report.pdf
- Murdoch DR. Diagnosis of Legionella infection. Clin Infect Dis. 2003;36(1):64–69.
- Reinthaler FF, Mascher F, Stünzner D. Serological examinations for antibodies against Legionella species in dental personnel. J Dent Res. 1988;67(6):942–943.
- Borella P, Bargellini A, Marchesi I, et al. Prevalence of anti-legionella antibodies among Italian hospital workers. J Hosp Infect. 2008;69(2):148–155.
- Fotos PG, Westfall HN, Snyder IS, et al. Prevalence of Legionella-specific IgG and IgM antibody in a dental clinic population. J Dent Res. 1985;64:1382–1385.
- Atlas RM, Williams JF, Huntington MK. Legionella contamination of dental-unit waters. Appl Environ Microbiol. 1995;61(4):1208–1213.
- Veronesi L, Capobianco E, Affanni P, et al. Legionella contamination in the water system of hospital dental settings. Acta Biomed. 2007;78(2):117–122.
- Szymańska J, Sitkowska J. Bacterial hazards in a dental office: An update review. African J Microbiol Res. 2012;6(8):1642–1650.
- Den Boer JW, Nijhof J, Friesema I. Risk factors for sporadic community-acquired Legionnaires’ disease. A 3-year national case-control study. Public Health. 2006;120(6):566–571.
- Ginevra C, Duclos A, Vanhems P, et al. Host-related risk factors and clinical features of community–acquired Legionnaires’ disease due to the Paris and Lorraine endemic strains, 1998–2007, France. Clin Infect Dis. 2009;49:184–191.
- Valciņa O, Pūle D, Lucenko I, et al. Legionella pneumophila seropositivity-associated factors in Latvian blood donors. Int J Environ Res Public Health. 2016 [ cited 2016 Sep 26];13(1):58–65. Available from: http://dx.doi.org/10.3390/ijerph13010058
- Diederen BM. Legionella spp. and Legionnaires' disease. J Infect. 2008;56(1):1–12.
- Estrich CG, Vogt KL, Gruninger SE. Dental practitioners’ risk factors for exposure to Legionella pneumophila. Poster 1237 presented at: 43rd Annual Meeting & Exhibition of the AADR and 28th Annual Meeting of the CADR; 2014 Mar 19–22; Charlotte, NC. Available from: www.researchgate.net/profile/Steve_Gruninger/publication/266790574_Dental_Practitioners_Risk_Factors_for_Exposure_to_Legionella_pneumophila/links/55a548f408ae5e82ab1f9318.pdf
- Borella P, Bargellini A, Marchegiano P, et al. Hospital-acquired Legionella infections: an update on the procedures for controlling environmental contamination. Ann Ig. 2016;28:98–108.
- D'Alessandro D, Fabiani M, Cerquetani F, et al. Trend of Legionella colonization in hospital water supply. Ann Ig. 2015;27:460–466.