ABSTRACT
Glutamate dehydrogenase (GDH) protein of Streptococcus suis can be used for detection of S. suis infection and protection of pigs against S. suis infection. Acetate is a primary inhibitory metabolite for cell growth and formation of GDH protein. In this study, the ptsG gene, which encodes the integral membrane permease IICBGlc in the phosphotransferase system, was deleted and the effect of this deletion on the expression of GDH protein was investigated. The plasmids containing glfZ. mobilis (encoding glucose facilitator)–glkE. coli (encoding glucokinase) or galPE. coli (encoding galactose permease)–glkE. coli were transformed into ptsG mutant cells to recover the cell growth and glucose utilization of ptsG mutant. The mutants with deletion of ptsG decreased the accumulation of acetate; and higher cell density and GDH protein concentration were obtained with the ptsG mutants containing glf–glk or galP–glk. When the ptsG mutant containing glf–glk (SSGGFK) was used for expression of GDH protein, the cell density (optical density OD600 of 2.68) and the concentration of GDH protein (42.34 mg/L) were highest, with an increase by 12.61% and 14.84%, respectively, compared with the parental strain (SSG). The acetate accumulation was reduced to 2.35 g/L, i.e. a 37.33% decrease compared with the SSG strain. High concentration of GDH protein was obtained with reduction of acetate accumulation through gene modification of the phosphotransferase system. This can decrease the production cost of the subunit vaccine of GDH protein and provide theoretical foundation for high-level expression of other recombinant proteins.
Introduction
The glutamate dehydrogenase (GDH) of Streptococcus suis is conserved and antigenic, as it reacts with serum from animals with S. suis type 2 infection, leading to it being utilized as a serological assay for diagnosis of S. suis infection [Citation1,Citation2]. It has been reported that the subunit vaccination based on GDH protein of S. suis is an effective strategy for protecting pigs against S. suis infection [Citation3] but its market is limited because of the high cost. Improvement of the GDH protein by Escherichia coli can decrease the production cost and expand the application market of GDH subunit vaccine, leading to protection of the pig production industry and public health. The accumulation of acetate in the culture process is an important problem in the expression of recombinant proteins, since acetate inhibits cell growth and protein synthesis [Citation4], and becomes more inhibitory to recombinant protein-producing cells than to wild-type cells [Citation5]. There are examples where the expression of recombinant protein was significantly improved by decreasing the accumulation of acetate [Citation4]. The accumulation of acetate is due to the combined high rates of glucose uptake and glucose catabolism by the Embden–Meyerhof–Parnas pathway, resulting in a rate of acetyl-coenzyme A synthesis surpassing the capacity of the tricarboxylic acid (TCA) cycle to completely consume this metabolite [Citation6,Citation7]. In addition, modification of the glucose transport capacity is a successful approach for decreasing the excretion of acetate under aerobic conditions [Citation7].
In E. coli, the phosphoenolpyruvate–sugar phosphotransferase system (PTS) participates in the transport and phosphorylation of several sugars [Citation7]. The glucose-specific enzyme IIGlc complex of the glucose-specific PTS containing the soluble IIAGlc enzyme (encoded by crr) and the integral membrane permease IICBGlc (encoded by ptsG) [Citation8], and inactivation of ptsG are evaluated as effective strategies to reduce the glucose uptake capacity [Citation7]. However, the PTS− strain is not suitable for industrial production because of its low growth and glucose utilization rate [Citation8]. The expression of galactose permease (encoded by galP) of E. coli or glucose facilitator protein (encoded by glf) of Zymomonas mobilis, together with glucokinase (encoded by glk), is the most common approach for recovery of the cell growth and glucose utilization of a PTS− strain [Citation9]. When the PTS−Glc+ strain, where glucose is transported and phosphorylated by an alternative transport system (GalP and Glk), is used for L-phenylalanine production, the yield from glucose in the synthesis of phenylalanine can increase by 57% as compared to the isogenic PTS+ strain [Citation10]. In addition, the PTS− strains recruiting Glf of Z. mobilis have higher glucose utilization rates than PTS− strains using E. coli GalP, and the glucose utilization rate of PTS− strains decreases with replacing E. coli Glk with the isoenzyme of Z. mobilis [Citation9].
In the present study, the ptsG mutant was constructed and the plasmids containing glf–glk or galP–glk were transformed to the ptsG mutant. In addition, the effect of genetic modification of the PTS system on the expression of GDH protein by recombinant E. coli was studied to select the strain for higher expression of GDH protein.
Materials and methods
Bacterial strains, plasmids and primers
All bacterial strains, plasmids and primers used here are listed in .
Table 1. Strains, plasmids and primers used in this study.
Media
The media used for generating and propagating recombinant strains and plasmids were prepared according to published procedures [Citation12]. The seed medium Luria–Broth contained the following components: 10 g/L tryptone, 5 g/L yeast extract and 5 g/L NaCl. The fermentation medium for producing GDH protein had the following composition: 5 g/L glucose, 10 g/L yeast extract, 2 g/L (NH4)2SO4, 2.5 g/L MgSO4·7H2O and 1.5 g/L KH2PO4. The pH of both seed and fermentation media was adjusted to 7.0 with 4 mol/L NaOH.
Culture methods
The culture conditions used for the construction of recombinant strains and plasmids were controlled according to published procedures [Citation12]. A 500-mL baffled flask containing 100 mL seed medium was inoculated with a single colony of E. coli SS2-GDH and cultivated at 37 °C with shaking at 200 r/min for 12 h. The culture grown in the baffled flask was inoculated aseptically (2% v/v) into 5 L of production medium in a 10-L fermenter (GRJ-10D Fermentor System, Zhenjiang, China). The temperature was maintained at 37 °C, and the pH was adjusted to 7.0 with 4 mol/L NaOH during the course of the cultivation period. The dissolved oxygen (DO) level was maintained at 20%. When the initial glucose was depleted, glucose solution (50% w/v) was added to the fermentor according to the DO feedback feeding strategy [Citation13]. Once the cell density (optical density at 600 nm, OD600) increased to 0.5–0.6, isopropyl thiogalactose (IPTG) was added into the fermentor to control the concentration of IPTG at 0.5 mmol/L to induce synthesis of the GDH protein.
Construction of plasmids pSTV-FK and pSTV-PK
Genes glfZ.mobilis, galPE. coli and glkE. coli were amplified by polymerase chain reaction (PCR) with the primers glf-P1-glf-P2, galP-P1-galP-P2 and glk-p1-glk-p2, respectively. The PCR products were purified using a purification kit. The purified glkE. coli fragment digested with SacI and PstI was inserted into pSTV28 that was digested with SacI and PstI to construct pSTV-K. The purified glfZ.mobilis and galPE. coli fragments digested with PstI and SphI were inserted into pSTV-K that was digested with PstI and SphI to construct the plasmids pSTV-FK and pSTV-PK, respectively [Citation12].
Assays of plasmids pSTV-FK and pSTV-PK
The expression of genes glf and galP in pSTV-FK and pSTV-PK was determined by comparing the glucose consumption rate of PAR, PARG, PARGFK and PARGPK. The expression of glkE. coli was monitored by assay of the glucokinase activity of PAR, PARG, PARGFK and PARGPK. Glucokinase activity was determined by monitoring the formation of nicotinamide adenine dinucleotide phosphate (NADPH) through coupling with glucose-6-phosphate dehydrogenase as described previously [Citation8]. The assay solution included 100 mmol/L Tris-HCl buffer (pH 7.5), 60 mmol/L MgCl2, 1 mmol/L DL-dithiothreitol, 0.5 mmol/L NADP+, 2 mmol/L adenosine triphosphate, 2 U glucose-6-phosphate dehydrogenase and 15 mmol/L glucose.
Plasmid manipulations
Single- and multi-gene deletion mutants were constructed as described previously [Citation12]. Disruption of ptsG was performed using the Red helper plasmid, pKD46. The appropriate DNA fragment was obtained by PCR using primers ptsG-P1 and ptsG-P2 with helper plasmid pKD3. To eliminate the CmR gene from the integrated locus, the cells were transformed with plasmid pCP20 carrying the Flippase recombination enzyme (FLP) recombinase gene. All test PCRs were used with primers ptsG-P3 and ptstG-P4.
Analysis of substrates and by-products
The DO, pH and temperature were measured automatically with electrodes attached to the fermenters. The cell density was determined as described previously [Citation14]. The glucose concentration was monitored using an SBA-40E Biosensor Analyzer (Biology Institute of Shandong Academy of Sciences, China). The concentration of acetate was determined using an Agilent 1206 (Agilent Technologies, Santa Clara, CA, USA) high-pressure liquid chromatography system. The concentration of GDH protein was determined as described previously [Citation15].
Statistical analysis
All experiments were conducted in triplicate, and the data were averaged and presented as means with standard deviation (±SD). Statistical analysis was done using one-way analysis of variance followed as Dunnett's multiple comparison test, and statistical significance was defined as P < 0.05.
Results and discussion
Construction of ptsG mutants
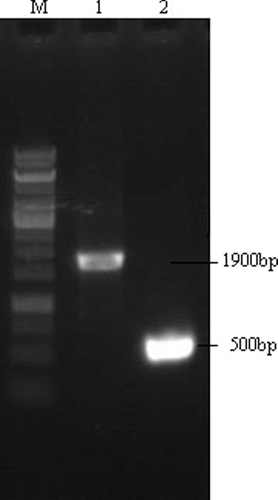
The deletion of ptsG in E. coli was confirmed by colony PCR using primers ptsG-P3-ptsG-P4, and the PCR results are presented in . The lengths of the fragments agreed with their theoretical lengths, indicating that mutants with deletion of ptsG were constructed [Citation16].
Construction of plasmids pSTV-FK and pSTV-PK
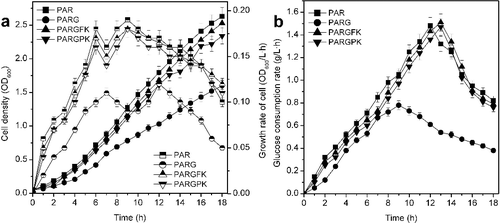
The glucokinase activity of PARGFK and PARGPK was 2.17- and 2.15-fold higher than that of PAR and PARG, which suggested that glkE. coli was actively expressed in plasmids pSTV-FK and pSTV-PK [Citation8], and there was no difference in the glucokinase activity of PAR and PARG. The cell density (OD600), cell growth rate and glucose consumption rate with the strains PRA, PARG, PARGFK and PARGPK are presented in . The mutant with lesion in ptsG showed the lowest growth rate, cell density and glucose consumption rate. The deletion of ptsG impaired the capacity for cell growth and glucose consumption, which resulted in PARG not being used as a host strain for GDH protein expression [Citation9]. The capacity for cell growth and glucose consumption of the ptsG mutant containing the plasmids pSTV-FK or pSTV-PK were higher than those of the ptsG mutant, and the plasmids pSTV-FK or pSTV-PK could recover the cell growth rate and glucose uptake of the ptsG mutant. This indicated that the plasmids pSTV-FK and pSTV-PK expressed the active glkZ.mobilis and galPE. coli, respectively. The glucose consumption rate of PARGFK was higher than that of PARGPK, which was due to the higher glucose transport velocity and lower energy cost of Glf [Citation17].
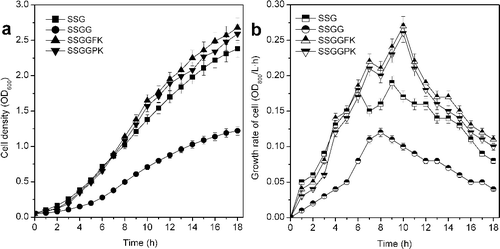
GDH expression with the parental strain and mutants with modification of PTS
Cell density and growth rate
shows the cell density and cell growth rate of SSG and the mutants. The cell density and growth rate of SSGG were lowest because of the deletion of ptsG, and the cell density of SSGG was 1.22 (OD600), which was a decrease by 48.74% compared with that of SSG (OD600 of 2.38) [Citation7]. The cell density of SSGGFK and SSGGPK were 1.13- and 1.09-times higher than that of SSG. The cell growth rate of SSGGFK and SSGGPK were also higher than that of SSG during the later fermentation phase. The highest cell density and growth rate were obtained with SSGGFK. Overexpression of glf is more feasible for increasing the glucose uptake rate of E. coli than galP, which resulted in obtaining higher cell density and growth rate [Citation17].
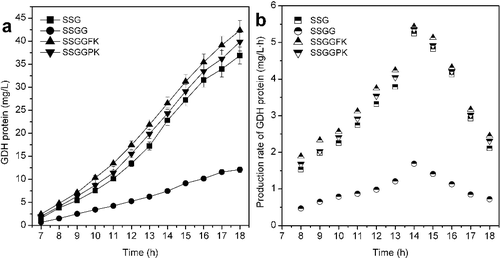
Concentration and production rate of GDH protein
The concentration and production rates of GDH protein with different strains are presented in . Due to the low cell density and growth rate, the lowest concentration and production rate of GDH protein was obtained with SSGG, and the GDH protein concentration of SSGG was 12.12 mg/L. The GDH protein concentrations of SSGGFK and SSGGPK were 42.34 and 39.87 mg/L, which were increased by 14.84% and 8.14% compared with SSG (36.87 mg/L), respectively. A study indicated that cultures of a ptsG-strain growing in complex medium supplemented with 20 g/L of glucose resulted in significant reduction of acetate excretion and more than 50% increase in recombinant protein synthesis compared with the wild-type strain cultures [Citation18]. The production rate of GDH protein of SSGGFK and SSGGPK were higher than that of SSG during the whole induction period.
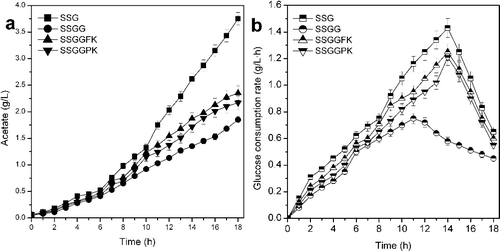
Concentration of acetate and glucose consumption rate
The acetate concentration and glucose consumption rate of different strains are displayed in . The deletion of ptsG decreased the accumulation of acetate, and the mutants with lesion in ptsG accumulated lower concentration of acetate [Citation4,Citation7]. The acetate accumulation of SSGG (1.85 g/L) was lowest, and the acetate accumulation of SSGGFK and SSGGPK were 2.35 and 2.17 g/L, which marked a decrease by 37.33% and 42.13%, respectively. Due to the lower accumulation of acetate, higher cell density and GDH protein concentration were obtained with SSGGFK and SSGGPK [Citation4,Citation18]. The glucose consumption rate of SSGG was lowest, which resulted in the lowest cell density and GDH protein concentration when SSGG was used [Citation8]. The expression of pSTV-FK or pSTV-PK increased the glucose consumption rate of the ptsG mutant, and the glucose consumption rates of SSGGFK and SSGGPK were higher than that of SSG. The glucose transport rate of Glf is near five times higher than that of GalP, and Glf does not require metabolic energy in the form of a proton potential, while GalP operates by a sugar-proton symport mechanism [Citation7,Citation9]. Thus, application of Glf is more energetically favourable than GalP, and the glucose utilization rate of SSGGFK was higher than that of SSGGPK. The concentration of GDH protein was increased with reduction of acetate accumulation through genetic modification of the PTS. Coordinated expression of glf and glkE. coli was required for improving glucose utilization and the expression of the GDH protein, which can provide theoretical foundation for genetic modification of the PTS in E. coli and high-level expression of other recombinant proteins.
Conclusions
In the present study, the accumulation of acetate was decreased by the deletion of ptsG. Higher cell density and concentration of GDH protein were obtained by the ptsG mutants containing the plasmids pSTV-FK or pSTV-PK, and the cell density and GDH protein concentration of SSGGFK were highest. The enhancement of the expression of GDH protein can decrease the production cost and expand the application market for the subunit vaccine of the GDH protein, which may aid in the control and prevention of diseases caused by S. suis, and thus promote the development of the pig industry. In addition, the construction strategy of the strain in this study can provide the theoretical basis for expression of other recombinant proteins.
Acknowledgments
This work was supported by the Doctoral Program Foundation of Shandong Binzhou Animal Science and Veterinary Medicine Academy under grant BS201402, Development of Science and Technology Plan Program of Binzhou under grant 2015ZC0107, 2013GG0304, Shandong Province Natural Science Foundation of China under Grant ZR2014CQ009 and Innovation Program of Modern Agricultural Industry System of Shandong under grant SDAIT-08-17, SDAIT-10-07.
The research of this manuscript was conducted in Shandong Binzhou Animal Science and Veterinary Medicine Academy, Shandong Binzhou Research, Development and Promotion Center for Livestock and Poultry Propolis Vaccine, Shandong Lvdu Bio-science and Technology Co. Ltd.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Okwumabua O, Persaud JS, Reddy PG. Cloning and characterization of the gene encoding the glutamate dehydrogenase of Streptococcus suis serotype 2. Clin Diagn Lab Immunol. 2001;8(2):251–257.
- Kutz R, Okwumabua O. Differentiation of highly virulent strains of Streptococcus suis serotype 2 according to glutamate dehydrogenase electrophoretic and sequence type. J Clin Microbiol. 2008;46(10):3201–3207.
- Cheng LK, Fan WX, Lei LC, et al. Application of dissolved oxygen control and substrate feeding strategy to improve production of glutamate dehydrogenase protein of Streptococcus suis in Escherichia coli. J Chem Pharm Res. 2016;8(6):223–230.
- Eiteman MA, Altman E. Overcoming acetate in Escherichia coli recombinant protein fermentations. Trends Biotechnol. 2006;24(11):530–536.
- March JC, Eiteman MA, Altman E. Expression of an anaplerotic enzyme, pyruvate carboxylase, improves recombinant protein production in Escherichia coli. Appl Environ Microbiol. 2002;68:5620–5624.
- Chang DE, Shin S, Rhee JH, et al. Acetate metabolism in a pta mutant of Escherichia coli W3110 importance of maintaining acetyl coenzyme A flux for growth and survival. J Bacteriol. 1999;181(21):6656–6663.
- Gosset G. Improvement of Escherichia coli production strains by modification of the phosphoenolpyruvate: sugar phosphotransferase system. Microb Cell Fact. 2005 [cited 2016 Oct 21];4:14. DOI:10.1186/1475-2859-4-14
- Lu J, Tang JL, Liu Y, et al. Combinatorial modulation of galP and glk gene expression for improved alternative glucose utilization. Appl Microbiol Biotechnol. 2012;93:2455–2462.
- Tang JL, Zhu XN, Lu J, et al. Recruiting alternative glucose utilization pathways for improving succinate production. Appl Microbiol Biotechnol. 2013;97:2513–2520.
- Báez-Viveros JL, Osuna J, Hernandez-Chavez G, et al. Metabolic engineering and protein directed evolution increase the yield of L-phenylalanine synthesized from glucose in Escherichia coli. Biotechnol Bioeng. 2004;87:516–524.
- Cherepanov PP, Wackernagel W. Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene. 1995;158(1):9–14.
- Liu Q, Cheng YS, Xie XX, et al. Modification of tryptophan transport system and its impact on production of L-tryptophan in Escherichia coli. Bioresour Technol. 2012;114:549–554.
- Cheng LK, Wang J, Xu QY, et al. Effect of feeding strategy on L-tryptophan production by recombinant Escherichia coli. Ann Microbiol. 2012;62:1625–1634.
- Cheng LK, Wang J, Fu Q, et al. Optimization of carbon and nitrogen sources and substrate feeding strategy to increase the cell density of Streptococcus suis. Biotechnol Biotechnol Equip. 2015;29(4):779–785.
- Lin CW, Cheng LK, Wang J, et al. Optimization of culture conditions to improve the expression level of beta1-epsilon toxin of Clostridium perfringens type B in Escherichia coli. Biotechnol Biotechnol Equip. 2016;30(2):324–331.
- Han C, Zhang WC, You S, et al. Knockout of the ptsG gene in Escherichia coli and cultural characterization of the mutants. Chin J Biotechnol. 2004;20(1):16–20.
- Siedler S, Bringer S, Blank LM, et al. Engineering yield and rate of reductive biotransformation in Escherichia coli by partial cyclization of the pentose phosphate pathway and PTS-independent glucose transport. Appl Microbiol Biotechnol. 2012;93:1459–1467.
- Chou CH, Bennett GN, San KY. Effect of modified glucose uptake using genetic engineering techniques on high-level recombinant protein production in Escherichia coli dense cultures. Biotechnol Bioeng. 1994;44:952–960.