ABSTRACT
Bacillus subtilis BSD-2 produces small molecule antibiotics that effectively control grey mould. To isolate the genes responsible for these antimicrobial activities, a B. subtilis BSD-2 mutant library was constructed utilizing the pHV1249 plasmid with a mini-Tn10 transposon. The antimicrobial potential of the generated strains was evaluated in the presence of cucumber grey mould and the mutant sequences were cloned by chromosome walking. Among the generated mutants, four mutants showed enhanced inhibitory effects in the presence of grey mould, and one mutant lost its inhibitory effect. The negative 1–19 mutant strains showed 98% sequence homology of the yodF gene, which encodes a Na+/metabolite permease. Next, we constructed a yodF knockout vector (pB-y-k) and used it to transform B. subtilis. A mutant yodF strain lost its inhibitory effect on grey mould, whereas reversion of the mutation restored the fungistatic activity.
Introduction
Bacillus subtilis is widespread in nature. It does not cause disease in humans or animals and does not pollute the environment, but it can produce a variety of antibiotics, such as bacilysin [Citation1], iturin, and fengycin [Citation2], with broad-spectrum antimicrobial/antifungal activity [Citation3,Citation4]. B. subtilis strain BSD-2 can produce small antifungal peptides that inhibit Botrytis cinerea and Verticillium wilt in plants [Citation5].
To characterize the B. subtilis BSD-2 small molecule polypeptide-coding genes and their inhibitory mechanisms at the molecular level, we constructed a mutant library using mini-Tn10 transposons and screened for antimicrobial/antifungal genes. The mini-Tn10 transposon system is commonly utilized in Bacillus research, with gene mutations occurring through transformation, thus greatly reducing the transposition insertion sequence specificity and making it more conducive to generating random mutation in the whole genome [Citation6]. A previous study examining bacilysin production of B. subtilis strain PY79 constructed a mini-Tn10 transposon mutant library and found that yvgW gene mutants displayed a bacilysin-negative phenotype [Citation7]. While conventional physical or chemical mutational methods are relatively simple but do not include a mutation point selection marker, it is difficult to locate and clone the mutated gene [Citation8]. Transposon sequences were identified via a chromosome-walking method based on flanking sequences. In the present study, mini-Tn10 transposon insertion mutagenesis was utilized to construct a mutant library for antibiotic functional enhancement or loss screening. This approach will enable the isolation of potential genes functional in antimicrobial production.
Materials and methods
Bacterial strains and plasmids
The strains and vectors used in this study are listed in . The mini-Tn10 delivery vector pHV1249 (GenBank Accession No.: AF307748.1) was supplied by Guo Qinggang's laboratory at the Plant Protection Institute, Hebei Academy of Agricultural and Forestry Science, China.
Table 1. Strains and plasmids used in this study.
Primers
Primers were designed using Primer Premier 5 (Premier Biosoft, Palo Alto, CA, USA) software and are listed in .
Table 2. Primers for PCR.
Culture medium
Luria–Bertani (LB) medium was utilized for B. subtilis BSD-2 protoplast transformation, and SMMP medium (selective medium for the isolation of Megasphaera and Pectinatus) [Citation9,Citation10] was used for protoplast culturing. Antibiotic concentrations employed in this study for direct selection were as follows: 0.5 μg/mL erythromycin, 5 μg/mL chloramphenicol, and 100 μg/mL ampicillin.
DNA techniques and manipulations
Plasmid DNA from Escherichia coli and chromosomal DNA from B. subtilis BSD-2 were isolated and purified using a SanPrep Column Plasmid Mini-Preps Kit and genomic DNA extraction kit (Sangon Biotech Co., Ltd., Shanghai, China), respectively. Nucleotide sequences were compared with the National Center for Biotechnology Information database using the BLAST search (http://www.ncbi.nlm.nih.gov/BLAST).
B. subtilis BSD-2 protoplast preparation
B. subtilis BSD-2 was cultured in 5 mL of Penassay broth medium at 37 °C overnight, and then 0.5 mL of culture was transferred into 200-mL flasks containing 50 mL of medium. These samples were then incubated at 37 °C with shaking until reaching log phase (optical density at 600 nm (OD600) of approximately 0.4). Cells were collected by centrifugation at 600 × g for 10 min, rinsed in 0.1 mol/L Tris-HCl buffer (pH 8.0), centrifuged as described above, and re-suspended in SMMP medium. The samples were then centrifuged to pellet the cells (1000 g, 15 min), washed once with SMMP, re-suspended in SMMP and incubated in a 37 °C water bath with shaking (100 r/min) for 40 min in the presence of 2 mg/mL lysozyme. Protoplasts were microscopically observed to ensure a 90% protoplast content (dye containing 20% sucrose safranin). B. subtilis protoplast transformation was carried out as previously described [Citation11]. Transformation of E. coli was carried out via chemical transformation.
B. subtilis BSD-2 protoplast transformation
As previously described [Citation9,Citation12,Citation13], 50 μL of plasmid DNA was mixed with an equal volume of 2 × SMM buffer (1 mol/L sucrose, 0.04 mol/L maleic acid, 0.04 mol/L MgCl2, pH6.5); 0.5 mL of a protoplast suspension was immediately added in addition to 1.5 mL of 40% polyethylene glycol solution in SMM. The sample was gently mixed and incubated on a shaker for 2 min at room temperature. Following incubation, 5 mL of SMM solution was added and the sample was centrifuged at 1000 g for 10 min. The obtained protoplasts resuspended in 1 mL of SMMP solution and incubated in a 37 °C water bath with gentle shaking for 90 min. Transformed protoplast suspensions were transferred into regeneration medium containing 0.5 μg/mL erythromycin and 5 μg/mL chloramphenicol and cultured at 30 °C for 24 h.
Plasmid and transposon detection
Transposon mini-Tn10 primers were designed to detect transformant plasmids and mutant genomic DNA. Samples were amplified by polymerase chain reaction (PCR) and sequenced (Sangon Biotech Co.) to determine the presence of transposon mini-Tn10.
Transposon mini-Tn10 transposon induced and inhibitory function mutants screening
Transposon mini-Tn10 transposon induced and inhibitory function mutant screening was performed. As previously described [Citation10,Citation14], single colonies were plated onto LB medium containing 5 μg/mL chloramphenicol and then incubated at 50 ± 1 °C overnight to screen for mutants. Inhibitory abilities were evaluated in the presence of B. cinerea (ACCC 36 448).
Analysis of chromosomal walking in mini-Tn10 transposons
According to the mini-Tn10 transposon sequences, five pairs of primers () were designed to detect specific fragments and clone sequences flanking the insertion site by chromosome walking. Mutant genomic DNA was extracted according to the genome-walking kit (Takara, Shiga, Japan) instructions. DNA was extracted and purified using the TaKaRa Agarose Gel DNA Purification Kit Ver. 2.0, according to the manufacturer's instructions; fragments were sequenced utilizing the AP2 or SP3 primers (Sangon Biotech Co.), and the sequences were evaluated with BLAST.
YodF and kanamycin selectable marker gene amplification
GenBank, which contains 168 B. subtilis gene sequences, shows that the full-length yodF gene sequence is 1490 base pairs (bp). To amplify this sequence, a pair of oligonucleotide primers, P1 and P2 (), were designed, and KpnI and SacI restriction sites (underlined below) were included in the primer. The B. subtilis strain BSD-2 genome was utilized as a template to amplify yodF according to the following PCR protocol: 94 °C, 5 min; 94 °C for 45 s; 60 °C for 45 s; 72 °C for 90 s; 30 cycles; 72 °C for 10 min (using My Cycler thermal cycler of BIO-RAD).
Oligonucleotide primers P4 and P5 () were designed according to the kanamycin resistance gene sequence, with HindIII and BamHI restriction sites included at the ends (shown underlined). The sequence was amplified according to the following PCR protocol: 94 °C for 5 min; 94 °C for 30 s; 59 °C for 45 s; 72 °C for 90 s; 30 cycles; 72 °C for 10 min.
Gene yodF (GenBank accession numbers: KP202250).
Gene ybfB (GenBank accession numbers: KP202251).
Knockout plasmid
Primers P1 and P2 were utilized to amplify the BSD-2 yodF gene (1490 bp). The pBluSKM (Ampr) plasmid and the yodF fragment were digested with the ApaI and SacI restriction enzymes and ligated with T4 ligase to form a new vector pB-y (Ampr). The pEASY-T1 (Ampr, kanar) plasmid was extracted from E. coli DH5α and digested with BamHI and HindIII to recover a small fragment (794 bp), while BamHI/HindIII digestion of the pB-y vector recovered a large fragment (3285 bp). Positive clones were selected and the carrier was denoted as pb-y-k.
Construction of B. subtilis reverse mutation carriers BSD-2 yodF knockdown
Two oligonucleotide primers, P1 and P2, were designed according to the yodF sequence and KpnI and SacI restriction sites (underlined below) were added to the primers (). The B. subtilis strain BSD-2 genome was used as a template to amplify yodF via PCR. The pB-y (Ampr) vector was used to generate yodF knockout B. subtilis. Positive recombinants were obtained and the inserted sequence was confirmed via PCR and enzyme digestion.
Results and discussion
B. subtilis BSD-2 transformation
Protoplast transformation was completed using the BSD-2 strain with the pHV1249 plasmid and validated by gel electrophoresis to establish a consistent plasmid and PCR product size (Supplementary Figure S1a). Following PCR amplification of the transposon, the PCR pHV1249 (CK2) positive control was found to correspond to the generated transposon fragment, while the negative control (CK1) did not correspond to the generated fragment (Supplementary Figure S1b).
B. subtilis BSD-2 mutant library construction
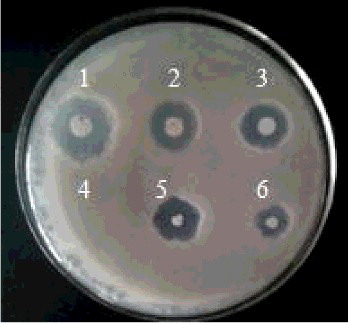
The induction process was performed at a high temperature, thus enabling the mini-Tnl0 transposon to randomly insert into the B. subtilis genome to produce mutants. The recombinant bacteria containing pHV1249 were cultured on selective medium and 3000 mutant strains were selected for antimicrobial activity evaluation in the presence of grey mould. The results showed that four mutants had an enhanced inhibitory effect on grey mould, with the negative mutant 1–19 identified as having completely lost the inhibitory function and the control BSD-2 sample 6 showing an inhibitory effect on grey mould ().
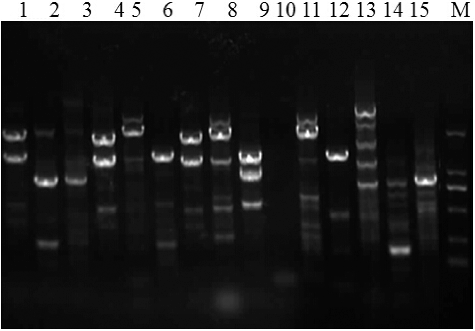
Plasmid and genomic DNA was extracted from mutant templates using the Tn10-5' and Tn10-3' () primers to amplify specific sequences for validation (Supplementary Figure S2). The transformant genome (lane 12), mutant genome (lanes 1–11), and pHV1249 plasmid (lane 13) generated fragments of ∼340 bp, while the B. subtilis BSD-2 template (lane 14) did not show an amplified fragment. These results indicate that the mutant transformants contained the mini-Tn10 transposon, with mutants showing functional changes in their antimicrobial/antifungal activity relative to the B. subtilis BSD-2 control.
Mutant mini-Tn10 transposon insertion site sequence analysis
Using a chromosome-walking technique, five mutant sequences flanking the insertion sites were cloned and sequenced (). Sequences were compared with B. subtilis mode strain 168 (GenBank Accession No.: AL009126.3), and strains 1–18 and 1–9 showed the highest sequence homology with gene 2,372,879–2,373,839 in strain 168, which corresponded to ybfB and encodes an acid transporter protein. The strain 1–24 insertion site fell between ybcL and ybcM, not producing a protein product. Strains 1–19 and 2–59 showed the highest sequence homology to gene 2,130,377–2,131,867, which is located within yodF and encodes a Na+/metabolite permease ().
Table 3. Gene function of identified mutants.
Cloning and sequence analysis of yodF
Because the Bacillus genome is large, the first experimental design generated a PCR product length of 4.5 kb using mega-primers. This product was then used as a template, in conjunction with the P1 and P2 primers, to obtain the target yodF and generate a fragment of approximately 1.5 kb (Supplementary Figure S3a). This gene was also examined in the pMD19-T vector in E. coli, with the plasmid digested to verify (Supplementary Figure S3b) that the digested T vector generated a larger fragment (3 kb), while the smaller fragment (1.5 kb) was yodF.
Construction of plasmid knockout pB-y-k
Based on the pBluescript plasmid, a plasmid which contains a kanamycin resistance gene was constructed to knock out yodF. In this study, the restriction enzymes KpnI and SacI were selected for digestion, but failed to generate the desired fragments because a KpnI recognition site was present in the target yodF; thus, the restriction endonucleases were changed to ApaI and SacI. To verify the reliability of the constructed plasmid, the plasmids were extracted and digested with three different endonuclease combinations (Supplementary Figure S4). The sample in lane 1 was digested with ApaI and SacI to produce a large vector fragment (∼3 kb) and small yodF fragment (1.5 kb), while that in lane 2 was digested with BamHI and HindIII to generate a small kanamycin resistance gene fragment, and the sample in lane 3 was digested with SacI and HindIII, producing consistent results. Furthermore, gene sequencing showed that yodF had inserted into the kanamycin resistance gene.
Currently, gene knockout technology is an important component in functional genomics research. Knockout vectors are comprised of at least two basic components, the target homologous sequence and genome backbone plasmid, therefore, it is very important to select the appropriate plasmid backbone. The recombinant pB-y-k plasmid used in this study provided the backbone required to construct the knockout plasmid vector. First, the pB plasmid does not contain a replicon capable of self-replication in B. subtilis BSD-2. Second, the kanamycin resistance gene followed by recombination carries its own promoter, generating a linearized vector knockout. Once integrated into the BSD-2 chromosome, the transformant will show kanamycin resistance. Finally, the common restriction sites located on both sides of the kanamycin resistance gene are convenient for connecting homologous sequences. In summary, the recombinant pB-y-k plasmid provided a fully functional vector backbone enabling the generation of a knockout construct.
The knockout was generated via homologous recombination, with the probability of generating a mutant strain following completion of this double exchange being very low. To improve the efficacy of deletion mutations, samples were cultured to the mid-logarithmic growth phase prior to inducing mutations. During this growth period, the chromosomal DNA in bacterial cells is often in a relatively loose chromatin state, increasing the probability of homologous pairing regions and pair switching.
YodF deletion strain construction and identification
To extract the knockout pB-yk plasmid for use as a template, primers P1 and P2 were utilized to generate a ∼1.5-kb fragment that was purified and used to transform B. subtilis BSD-2 by protoplast transformation. The transformants were screened for kanamycin resistance to isolate resistant strains. Eight resistant strains were identified and genomic DNA was extracted for PCR testing. Gel electrophoresis showed bands of approximately 800 bp (Supplementary Figure S5), indicating that homologous recombination had occurred.
YodF deletion strain inhibitory effect on grey mould
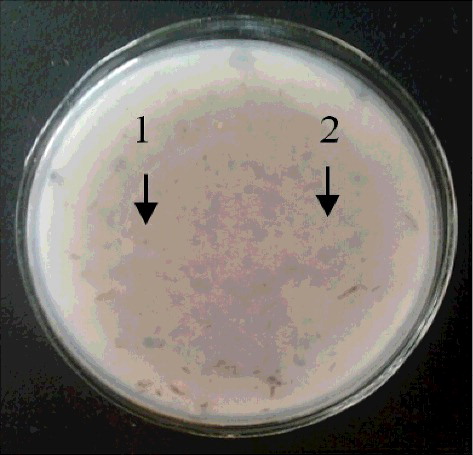
Following homologous recombination of yodF to generate deletion strains, the two strains examined were found to have no inhibitory effect on grey mould () because yodF was disrupted.
B. subtilis yodF deletion mutant functional recovery
To extract the knockout insert from the pB-y plasmid for use as a template, primers P1 and P2 were utilized to generate a 1.5-kb PCR fragment that was purified and used to transform B. subtilis BSD-2 by protoplast transformation. Following screening, eight resistant strains were identified and genomic DNA was extracted for PCR testing. Only three bacterial strains generated the predicted 1.5-kb yodF product (Supplementary Figure S6), confirming that homologous recombination had occurred in these strains.
Effect of recovery strains on grey mould
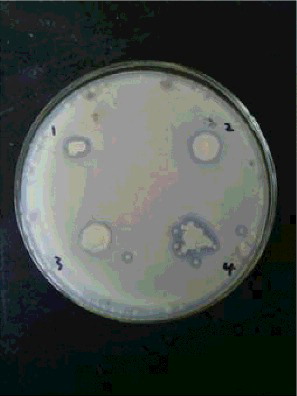
Following exposure to the mutant strains containing deletions, the recovery strains showed a restored ability to inhibit grey mould ().
In a previous study examining B. subtilis PY79, Tn10 transposon mutants were screened for three independent bacitracin deletions, with the mutation caused by the insertion of yvfI [Citation15]. Of the six bases of the transposon mini-Tn10 consensus sequence [Citation16], nearly every 4-kb segment of the B. subtilis genome should contain a recognition sequence [Citation17]. By utilizing random transposon insertions, genetic changes and biological phenotypic trait changes can be evaluated to provide functional insight into specific genes. The mini-Tn10 transposon system was used to identify genes in B. amyloliquefaciens to reduce biofilm formation [Citation18]. Furthermore, another study utilized a mini-Tn10 random insertion system to generate a mutant library, enabling the identification of B. amyloliquefaciens spores unable to synthesize subtilosin A [Citation19]. Finally, another study [Citation20] utilized the pHV1249 plasmid carrying the mini-Tn10 transposon in B. subtilis to characterize the surfactin defence-related gene yerP.
The generation of mutants with an inhibitory effect was enhanced by utilizing the chromosome -walking method to locate target genes followed by flanking sequence analysis. The insertion mutant strains 1–9 and 1–18 were found to have homology to ybfB, which encodes an acid transporter. Following transposon insertion, the ybfB function was altered, showing an enhanced antimicrobial/antifungal effect in the presence of grey mould. Acid transport proteins transport short-chain fatty acids such as succinic acid, fumaric acid and malic acid between cells, thereby regulating the intracellular pH and affecting the metabolic homeostasis through processes such as the Krebs cycle. The 1–24 strain fell between ybcL and ybcM. Overall, little is known regarding the relevance of these genes and how they relate to antifungal activities, and thus further studies are required.
The mutant strains 1–19 and 2–59 showed sequence homology to yodF, but because of the different insertion sites, the 1–19 strain generated a negative mutant that was unable to inhibit grey mould, while the 2–59 strain generated a positive mutant with an enhanced inhibitory ability. Previous studies examining B. subtilis showed that the TnrA box associated with yodF acts as a negative regulator by associating with a repressor gene, ultimately blocking nitrogen use [Citation21]. Inside the cell, the synthesis of glutamate from alpha-ketoglutaric acid is catalysed by glutamate synthase, with glutamate and glutamine synthesis inhibited by TnrA. When cells are grown under nitrogen-limited conditions, TnrA is induced, inhibiting the gene expression of yodF, which encodes a Na+ metabolite permease protein [Citation22], also known as a carrier, that functions as a major membrane transport protein mainly regulating the Na+ and NH4+ permeability. In E. coli, tryptophan intracellular transport is mainly carried out by enzymes, with the Na+ permeability of the enzyme responsible for the amino acid metabolite transporters involved in nitrogen metabolism. However, how this gene relates to antifungal properties requires further analysis.
In recent years, the physiological ecology, bio-control mechanism, and other aspects of Bacillus have been widely examined. The area of microbial bio-control research is no longer confined to the discovery of new bio-control strains, but focused on bio-control tools by constructing mutant libraries to isolate genes and characterize their functions. Gene knockout technology has enabled substantial gains in genetic engineering. Collectively, these technologies have aided the identification of genes such as those identified in the present study, which may have significant economic effects.
Conclusion
In summary, the analysis of the data obtained from this study demonstrated that, among the mutant library of B. subtilis BSD-2, four mutants showed enhanced inhibitory effects in the presence of grey mould, and one mutant lost its inhibitory effect. The gene of the negative 1–19 mutant strain showed 98% sequence homology to the yodF gene, which encodes a Na+/metabolite permease. The knockout experiments showed that the yodF gene is essential to B. subtilis BSD-2 for its inhibitory ability to grey mould.
tbeq_a_1329633_sm2304.pdf
Download PDF (605 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Inaoka T, Ochi K. Scandium stimulates the production of amylase and bacilysin in Bacillus subtilis. Appl Environ Microb. 2011;77:8181–8183.
- Wei YH, Wang LC, Chen WC. Production and characterization of fengycin by indigenous Bacillus subtilis F29-3 originating from apotatofarm. Int J Mol Sci. 2010;11:4526–4538.
- Yin JF, Zhang WH, Li JQ, et al. Pepper blight of bio-control bacteria screening and its antibacterial mechanism. Acta Phytopathol Sinica. 2007;37(1):88–94.
- Chen XL, Wang GH, Jin J, et al. Biocontrol effect of Paenibacillus polymyxa BRF-1 and Bacillus subtilis BRF-2 on fusarium wilt disease of cucumber and tomato. Chin J Eco-Agric. 2008;16:446–450.
- Hu RP, Zhang D, Zhang LP, et al. Purification and identification of an antimicrobial peptides from Bacillus subtilis BSD-2. Acta Agric Boreali-Sinica. 2011;6:201–206.
- Petit MA. Tn10-derived transposons active in Bacillus subtilis. J Bacterioal. 1990;172:6736–6740.
- Yazgan A, Ozeengiz G, Marahiel MA. Tn10 insertional mutations of Bacillus subtilis that block the biosynthesis of basilysin. Biochim Biophys Acta. 2001;1518:87–94.
- Sanchez A, Olmos J. Bacillus subtilis transcriptional regulators interaetion. Biotechnol Lett. 2004;26:403–407.
- Chang, Stanley NC. High frequency transforation of Bacillus subtilis protoplasts by plasmid NA. Mol Genet Genomics. 1979;168:111–115.
- Tsuge K, Ano T, Hirai M, et al. The genes degQ, pps, and lpa-8(sfp) are responsible for conversion of Bacillus subtilis 168 to plipastatin production. Antimicrob Agents Chemothe. r. 1999;43:2183–2192.
- Li RF, Xue WW, Huang L, et al. Competent preparation and plasmid transformation of Bacillus subtilis. Biotechnol Bull. 2011;5:227–230.
- Gao J, Li YB, Ke XW, et al. Development of gengtic transformation system of Valsa mali of apple mediated by PEG. Acta Microbiol Sinica. 2011;9:1194–1199.
- Xu MQ, Jiang J, Sun MH, et al. Construction of Clonostachys rosea 67-1 genetic transformation system by restriction enzyme-mediated integration (REMI). Chin J Biol Control. 2013;2:263–269.
- Guo QG, Li SZ, Lu XY, et al. Mapping and cloning function genes from antagonistic bacterium NCD-2 against Verticillium dahliae. Acta Agric Boreali-Sinica. 2007;22:190–194.
- Krolu TE, Kurt-Gür G, Unlü EC, et al. The novel gene yvfI in Bacillus subtilis is essential for bacilysin biosynthesis. Antonie Van Leeuwenhoek. 2008;94(3):471–479.
- Bender J, Kleckner N. IS10 transposase mutations that specifically alter target site recognition. Embo J. 1992;11:741–750.
- Halling SM, Kleckner N. Asymmetrical six-base-pair target site sequence determines Tn10 insertion specificity. Cell. 1982;28:155–163.
- Gao WH, Hao JA, Xia SY, et al. Using mini-Tn10 transposon system to research the genes involved in biofilmformation in Bacillus. Microbiol China. 2009;36:345–349.
- Dai S. Isolation and regulation mechanisms study of the antibiotics in B. amyloliquefaciens [master's thesis]. Tianjin: NanKai University; 2011.
- Tsuge K, Yuichiro O, Makoto S. Gene yerP, involved in surfactin self-resistance in Bacillus subtilis. Antimicrob Agents Chemother. 2001;45:3566–3573.
- Yoshida K, Yamaguchi H, Kinehara M, et al. Identification of additional TnrA-regulated genes of Bacillus subtilis associated with a TnrA box. Mol Microbiol. 2003;49:157–165.
- Zhang Y. Transcriptome analysis of Bacillus subtilis responding to valine, glutamate and glutamine [master's thesis]. Shanghai: East China University of Science and Technology; 2010.