ABSTRACT
Sedatives and hypnotics made from Chinese herbal medicine show great market prospects for minor side effects and zero potential addiction. In this study, the extraction conditions of Cordyceps militaris polysaccharide (CMP) and flavonoids (CMF) were, respectively, optimized by orthogonal experiments as follows: 30:1 ratio of water to plant material, three rounds of extraction at 90 °C and 3 h for each extraction; 40:1 ratio of 70% ethanol to plant material, reflux extraction at 90 °C for 4 h. Then, 200, 100 and 50 mg/kg of CMP and CMF were given intragastrically to mice for 30 days. The locomotor activity times of the mice were recorded on the 14th and 30th days. The effects of CMP and CMF on the sleep induced by pentobarbital sodium were observed on the 30th day. 5-Hydroxytryptamine (5-HT), acetylcholine (ACh), glutamate (Glu) and γ-aminobutyric acid (GABA) levels in the mouse brains were determined by enzyme-linked immunosorbent assay on the 30th day. The results showed that different doses of CMP and CMF could reduce the number of locomotor activities in mice and lower the Glu level (p < 0.05 in the 50 mg/kg CMP group and p < 0.01 in the other groups), elevate the 5-HT level and reduce the ACh level (p < 0.01 only in the 200 mg/kg CMP and CMF groups) in the mouse brains. Therefore, the elevated 5-HT levels and decreased ACh and Glu levels in the brains may be the main mechanisms through which CMP and CMF exert their sedative and hypnotic effects.
Introduction
With the rapid development and progression of modern society, people's pace of life is accelerating, stress is increasing and competition is becoming increasingly fierce. Consequently, humans are experiencing a rapidly increased psychological burden that seriously affects sleep quality. As a result, insomnia has become a common phenomenon. Insomnia affects the quality of life and the health state as well as causes a variety of physical and mental disorders in serious cases [Citation1,Citation2]. Studies have shown that insomnia is directly related to the increase in traffic accidents [Citation3], the decline in the survival rate [Citation4], and the increase in the rate of medical resource utilization [Citation5]. In order to increase people's awareness of insomnia, the International Foundation for Mental Health and Neuroscience (IFMHN) established March 21 as ‘World Sleep Day’.
Chemical drugs, such as benzodiazepines, are still primarily used for the treatment of insomnia worldwide. In recent years, the United States’ Food and Drug Administration and Australia's Therapeutic Goods Administration have repeatedly released warnings of the potential risks of sedatives and hypnotics, which may cause abnormal sleep (such as the behaviours related to dangerous complex sleep that may be related to zolpidem, including somnambulism and sleep-driving), as well as drug tolerance and addiction [Citation6]. In contrast, due to minor side effects and zero potential of addiction, traditional Chinese medicine sedatives and hypnotics are being increasingly used in clinics and have gained a certain market share, thus showing great market prospects. There are a variety of Chinese herbal medicines with sedative and hypnotic effects [Citation7–11], such as Cordyceps sinensis. C. sinensis is a valuable Chinese herbal medicine that was first recorded in New Compilation of Materia Medica by Wu Yiluo in the Qing Dynasty. It is capable of relieving consumptive disease, benefiting pneuma, relieving cough and facilitating expectoration. Chinese Materia Medica keeps record of C. sinensis and lists its anti-inflammatory properties, immune system benefits, sedative–hypnotic effects, anti-cancer effects, anti-aging effects and others. However, C. sinensis resources are limited and they are very expensive. In March 2009, the Ministry of Health, PRC, announced C. militaris (L.) link as a new food resource. C. militaris belongs to Ascomycotina, Clavicipitaceae and Cordyceps. Additionally, C. militaris and C. sinensis belong to the same genus, and many studies have shown that C. militaris has similar functions to those of C. sinensis [Citation12–15]. Presently, only a few reports have been made on the sedative and hypnotic effects of C. militaris, and the reports that do exist are not thorough [Citation16].
C. militaris contains many active ingredients. Along with polysaccharides, cordycepin and adenosine, there is a high content of flavonoids [Citation17] in C. militaris. Studies have shown that flavonoids are the main substantial basis of the sedative and hypnotic effects of many Chinese herbs, such as Semen Ziziphi Spinosae [Citation18] and Epimedium [Citation19]. The underlying mechanisms of the action of these Chinese herbs are associated with neurotransmitters, such as γ-aminobutyric acid (GABA), glutamate (Glu), acetylcholine (ACh) 5-hydroxytryptamine (5-HT). Among those, Glu and ACh are the main excitatory neurotransmitters and play important roles in insomnia. Higher levels of 5-HT and GABA will lead to a better quality of sleep due to their inhibition of the central nervous system [Citation20,Citation21].
In view of the above reasons, a systematic study on the extraction technology and the sedative–hypnotic effects of C. militaris polysaccharides (CMPs) and total flavonoids was carried out. The results from the study can provide a scientific basis for the further development and utilization of C. militaris.
Materials and methods
Materials
The fruiting body of fresh C. militaris was purchased from the Jilin Province Sericulture Science Research Institute. The fruiting body was washed with double distilled water, lyophilized, crushed and sifted with a 30-mesh sieve and then stored in an airtight container.
Animals
Male ICR mice weighing 18–20 g were purchased from the Changchun Yisi Experimental Animal Technology Co., Ltd. (Changchun, Jilin, China). The animal quality certificate number was SCXK (Jin)-2011-0004. All experimental procedures were approved by the Ethics Committee for the Use of Experimental Animals of Beihua University (Jilin, China).
Preparation of C. militaris polysaccharide (CMP)
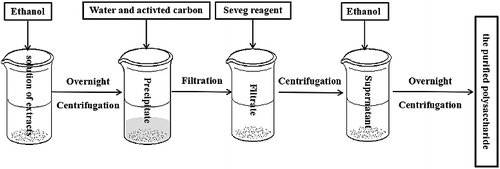
Extraction of CMP: Based on preliminary experiments, the extraction temperature (80, 90 and 100 °C), the extraction duration (1, 2 and 3 h), the solid–liquid ratio (1:10, 1:20 and 1:30 g/mL) and the extraction times (1, 2 and 3 times) were selected as the parameters. An orthogonal test design was used to determine the optimum conditions of the CMP extraction process. Then, 10 g of the extract was weighed and dissolved with distilled water to 100 mL and then mixed with 300 mL of ethanol. The mixed solution was left to stand overnight and then centrifuged at 1500g for 15 min (5430 R centrifuge, Eppendorf Company, Hamburg, Germany) to obtain a crude polysaccharide precipitate. The crude polysaccharide was dissolved in double distilled water and then decolourated with activated carbon and deproteinated with Seveg reagent [Citation22] in order to obtain the purified polysaccharide ().
Determination of the polysaccharide content: first, 10 mg of the purified polysaccharide was precisely weighed, dissolved and diluted with distilled water to 50 mL. Then, 2 mL of the solution and moderate amounts of distilled water were placed into a 10-mL volumetric flask to its scale line. Then, 0.5 mL of the diluted solution was precisely drawn and placed into a test tube, and 3.5 mL of concentrated sulphuric acid and 1 mL of 5% phenol were added into the tube. After that, the tube was shaken at 40 °C in a water bath for 30 min and was left to stand at room temperature for 15 min. The absorbance of the solution in the tube was determined at 490 nm (UV-2550 UV–visible spectrophotometer, Shimadzu Corporation, Tokyo, Japan). The polysaccharide content was determined based on a standard curve regression equation (A = 11.977 C + 0.2225, r = 0.9994, where A is the absorbance and C is the concentration). Then, the extraction rate of the purified CMP was calculated.
Preparation of total C. militaris flavonoids (CMF)
Based on preliminary experiments, the ethanol concentration (70%, 80% and 90%), solid–liquid ratio (1:20, 1:30 and 1:40 g/mL), extraction duration (2, 3 and 4 h) and extraction temperature (80, 85 and 90 °C) were selected as the parameters, and the optimal conditions for total CMF extraction were determined by an orthogonal test L9(34).
Determination of chromogenic method and ultraviolet (UV) detection wavelength of the total CMF: the sample and rutin standard solutions were prepared in accordance with AlCl3 and NaNO2–Al (NO3)3 [Citation23,Citation24] methods, respectively. Based on the full wave scanning of the reaction system mentioned above, the chromogenic method and detection wavelength of the total CMF were determined.
Sample preparation and total CMF content determination: the extract solution of the total CMF was filtered. Then, 0.1 mL of the filtered extract solution was precisely drawn and placed in a 10-mL volumetric flask, and then 0.1 mL of 0.1 mol/L AlCl3 solution was added into the flask. The solution was diluted with 60% ethanol to the scale, which was shaken well and left to stand for 10 min. The CMF concentration in the sample solution was determined based on a standard curve regression equation: A = 38.390 C + 0.0443, r = 0.9999 (where A is the absorbance and C is the concentration). Then, the extraction rate of total flavonoids in the C. militaris sample was calculated.
Sedative and hypnotic effects of CMP and CMF
CMP and total CMF 40 mg/mL solutions were prepared by adding distilled water and 60% ethanol aqueous solution to each, respectively.
Locomotor activity test: first, 84 ICR mice were randomly divided into seven groups: blank control group (distilled water, 0.1 mL/10 g), low-dose CMP group (CMP50, 50 mg/kg), medium-dose CMP group (CMP100, 100 mg/kg), high-dose CMP group (CMP200, 200 mg/kg), low-dose CMF group (CMF50, 50 mg/kg), medium-dose CMF group (CMF100, 100 mg/kg) and high-dose CMF group (CMF200, 200 mg/kg). All of the mice were kept at 8–22 °C and 40%–60% humidity and were fed a standard laboratory feed diet with water available ad libitum. All of the mice in each group were intragastrically administered successively for 30 days according to the doses described above. Fifty minutes after the administration, each mouse was put into a ZZ-6 mouse locomotor activity detector (Thai Union Technology Ltd, Chengdu, China) to adapt to the environment for 5 min, and then the number of their locomotor activities within 5 min was observed and recorded on the 14th and 30th days.
On the 30th day after the locomotor activity test, the mice were sacrificed by cervical dislocation for removing their whole brains. The brain tissue of each mouse was cut into pieces and mixed thoroughly. Then, 0.5 g of the brain tissues was dissolved in 4.5 mL of phosphate-buffered saline (pH 7.4), and the solution was homogenized for 2 min. The homogenate was centrifuged at 4 °C and 1500g for 20 min, and then the supernatant was placed into an EP tube for later use. According to the manufacturer's instructions (Dingguo Biotechnology Company, Shenyang, China), the Glu, ACh, 5-HT and GABA levels in the mice's brain tissues were determined by enzyme-linked immunosorbent assay (ELISA) using an Infinite M200 absorbance microplate recorder (TECAN Company, Hombrechtikon, Switzerland).
Effects of CMP and total CMF on the sleep induced by a subthreshold dose of pentobarbital [Citation25]: 96 ICR mice were randomly divided into eight groups: blank control group (0.1 mL/10 g distilled water), positive control group (4 mg/kg diazepam), CMP and total CMF-treated groups (the groups were the same as those used in the locomotor activity test). The mice were intragastrically given CMP and total CMF continuously for 30 days, and 30 min after the last administration, the mice were intraperitoneally injected with a subthreshold dose of pentobarbital sodium (37.5 mg/kg), and the number of sleeping mice was recorded to calculate the rate of sleep.
Effects of CMP and total CMF on the sleep induced by a threshold dose of pentobarbital sodium [Citation25]: the experimental method was the same as that described above. Thirty minutes after the last administration, the threshold dose of pentobarbital sodium (50 mg/kg) was given to mice by intraperitoneal injection. The loss of righting reflex for 1 min was taken as a sleep indicator; the duration from the injection of pentobarbital sodium to the mice's falling sleep was considered as the sleep latency and the duration from the loss to the recovery of the righting reflex, as the sleep duration.
Statistical methods
SPSS17.0 statistical software was used for the statistical analysis. The data are presented as mean values with standard deviation (±SD) (n = 12), and one-way analysis of variance (one-way ANVOA) was used for the analysis of the comparison between groups. P < 0.05 was considered to indicate a statistically significant difference.
Results and discussion
The optimum preparation process of CMP
There has been an increasing propensity to use herbal medicines to treat insomnia throughout the world in recent years. Some studies have focused on the extraction methods and sedative–hypnotic effects of herbal medicines [Citation26]. The mathematical models used in those studies, including theoretical models and statistical models, are effective statistical models for investigating the factors that affect the extraction rate of different components in raw medicinal materials and the optimization of extraction [Citation27]. Among the existing statistical models, orthogonal experiment design is a statistical model for multi-factor and multi-level influence factors with high quality and high efficiency. The extraction processes for many active components of traditional Chinese medicines have been determined by orthogonal experiments [Citation28,Citation29]. Therefore, the orthogonal experiment with three levels of four factors was selected for the optimization of the extraction processes of CMP and total CMF. The results from the orthogonal experiment showed that the influence of the extraction temperature on the extraction yield of CMP was the greatest, followed by the extraction duration and the extraction times, whereas that of the solid–liquid ratio was minimal. Through the comprehensive analysis, the optimum polysaccharide extraction conditions were determined to be as follows: distilled water equal to 30 times the weight of C. militaris (g/mL), three rounds of reflux extraction at 90 °C, and 3 h per extraction round. Then, the extract solutions were mixed, concentrated, precipitated with alcohol, decolorized by active carbon and deproteinated with Seveg reagent to obtain the purified CMP (). The process was repeated three times, and the average yield of the purified polysaccharide was 2.81% (Relative standard deviation (RSD) 1.4%), which demonstrates that the process is stable and feasible and can be used for the extraction and purification of CMP.
Optimum preparation process, colour development method and detection wavelength of the total CMF
In this study, the influence of different factors on the extraction rate of total CMF was ranked as follows: extraction temperature > extraction duration > solid–liquid ratio > ethanol concentration. Through the comprehensive analysis, the best combination of the four factors was identified as follows: a volume of 70% ethanol equal to 40 times the weight of C. militaris and reflux extraction at 90 °C for 4 h. The parallel experiment was repeated three times under the optimum extraction conditions, and the average extraction rate of total CMF was 3.70 mg/g (RSD, 2.1%), thereby indicating that the extraction method is stable and feasible.
Flavonoids are diverse in structure, and their functional groups vary as well. The physical and chemical properties of flavonoids with different structures are different, and the stability and capacity to form complexes with metal ions are different. The present study investigated the complexation of Al3+ from NaNO2–Al(NO3)3 or AlCl3 with flavonoids from C. militaris, which is a commonly used method [Citation30–32]. By comparing the full wavelength scan spectra of the samples treated with the two methods, there revealed to be more absorption peaks in the spectrum of the sample treated with NaNO2–Al (NO3)3, and they were out of order. Additionally, there was no obvious absorption peak at the conventional detection wavelength of 510 nm. However, both the sample and the standard substance solution treated with AlCl3 showed absorption peaks with good shapes and strong absorptions at 265 nm. The results showed that the AlCl3 method should be more stable and reliable than the NaNO2–Al (NO3)3 method for determining the total CMF content. The AlCl3 colour development method and a detection wavelength of 265 nm were selected for determination of the total CMF content.
Effects of CMP and total CMF on locomotor activities in mice
The locomotor activity of an animal can reflect the functional status of its central nervous system. With the locomotor activity test, the excitatory or inhibitory effects of drugs on the central nervous system can be visually verified. Thus, it is important to evaluate central nervous system drugs by the locomotor activity test.
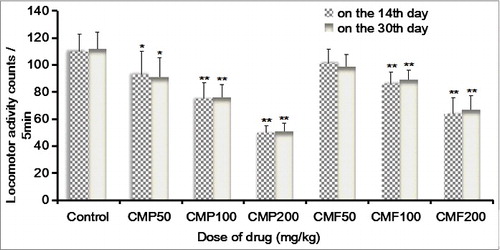
As shown in , the results obtained for the two time points were approximately coincident. The number of mice locomotor activities in the CMP50 group (P < 0.05), CMP100 group (P < 0.01) and CMP200 group (P < 0.01) was significantly reduced as compared with that in the control group. The number of locomotor activities in the CMF100 and CMF200 groups were significantly reduced (P < 0.01). These results indicate that both the polysaccharide and the total flavonoids extracted from C. militaris may have a strong inhibitory effect on the central nervous system.
Effects of CMP and total CMF on sleep in mice
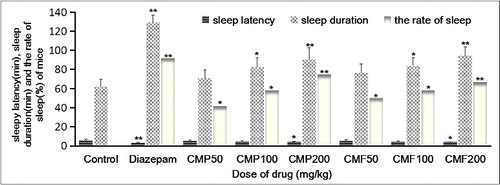
Pentobarbital sodium is a hypnotic, and its administration to mice together with tested drugs can be used for observations on the effect of the tested drugs on the sleep, in which the increase of the sleep rate, the increase of the sleep duration and the shortening of the sleep latency in the drug-treated groups in contrast to those in the control group indicate that the drugs may have a certain hypnotic effect. As shown in , compared with the control group, the different doses of CMP and total CMF could promote the sleep of mice induced by the subthreshold dose of pentobarbital sodium, in which total CMF200 and CMP200 could significantly increase the rate of sleep (P < 0.01). At the same time, CMF200 and CMP200 could significantly shorten the sleep latency (P < 0.05) and prolong the sleep duration (P < 0.01) induced by the threshold dose of pentobarbital sodium in mice. The results demonstrated that CMP and total CMF could be considered to improve the sleep of mice, although their effects were lower than that of diazepam.
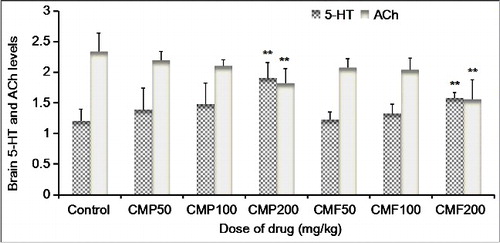
Effects of CMP and total CMF on Glu, ACh, 5-HT and GAGA levels in the brain tissues of mice
The locomotor activity and sleep are regulated by certain neurochemicals and physiological mechanisms in animals. Glu and GABA are closely related to the excitability and inhibition of the central nervous system, respectively, and their balance is an essential precondition for maintaining normal sleep. Furthermore, Glu is the main excitatory neurotransmitter, and it is widely distributed in the central nervous system and plays an important role in insomnia [Citation33]. An increased Glu level in the brain tissue may be one cause of insomnia. Additionally, GABA is an important inhibitory neurotransmitter and exerts postsynaptic inhibition on the central nervous system. Studies have shown that GABA levels in the brain during sleep are elevated by 15% as compared to levels while waking up [Citation20].
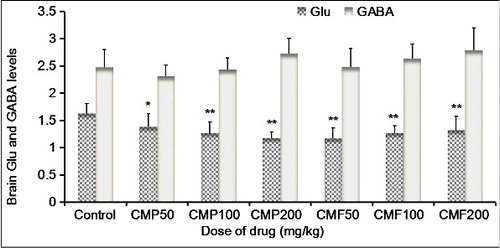
This study demonstrated that different doses of CMP can significantly reduce Glu levels in the brain tissues of mice (), and the Glu-lowering effect was enhanced dose dependently, in which the effect reached a significant level in the 50 mg/kg group (P < 0.05) and a very significant level in both the 100 and 200 mg/kg groups (P < 0.01). The different doses of the total CMF can significantly reduce Glu levels in the brain tissues of the mice (P < 0.01); however, the effect exhibited a declining trend as the CMF doses increased. In contrast, the effects of CMP and total CMF on GABA () were not so obvious; that is, 200 mg/kg CMP and 100 and 200 mg/kg CMF showed an increasing trend in GABA levels in the brain tissues of mice, without statistical significance (P > 0.05). The results in this study indicate that CMP and total CMF may exert their sedative and hypnotic effects by promoting other metabolic pathways in the body, such as the formation of glutamine and α-ketoglutaric acid through metabolism, protein synthesis or generation of glucose via gluconeogenesis, which reduce the content of Glu in the central nervous system.
As an important part of the ascending activating system, 5-HT plays an important role in maintaining arousal and alertness. 5-HT, also known as serotonin, is the precursor of melatonin, a central inhibitory neurotransmitter. When the level of 5-HT increases, the content of melatonin in the central nervous system also increases, thereby leading to a better quality of sleep [Citation34]. Additionally, ACh, the first neurotransmitter found, plays an important role in both the central and peripheral nervous system and is also an important neurotransmitter that maintains the awakening in the body [Citation21]. The change in the level of ACh in the brain can affect the state of consciousness or the state of sleep in mice.
Both CMP and total CMF can increase the level of 5-HT in the brains of mice and lower the excitability of the central nervous system, which then promotes sleep in mice. Additionally, this effect could be enhanced with an increase in their doses and was very significant when the CMP and total CMF doses increased to 200 mg/kg (P < 0.01) ().
This study revealed some effects of the studied extracts from C. militaris on the ACh level in the brain of mice (). The different doses of CMP could reduce the ACh level in the brains of mice: the effect increased with an increase in the dose of CMP, and the ACh-lowering effect showed a statistical significance at a CMP dose of 200 mg/kg (P < 0.01). The ACh-lowering effect of total CMF in the brains of mice also showed an enhanced trend with an increase in dose, and the effect produced by 200 mg/kg of total CMF was significant (P < 0.01). These results indicate that both CMP and total CMF can reduce the ACh level in the brains of mice and have certain sedative and hypnotic effects.
Conclusions
CMP and total CMF could significantly reduce the locomotor activity, promote the sleep induced by subthreshold dose of pentobarbital sodium and shorten the sleep latency and prolong the sleep duration induced by a threshold dose of pentobarbital sodium of mice. The effects of the high doses of CMP and total CMF were more significant. CMP and total CMF could lower the levels of Glu and ACh and elevate the level of 5-HT in the brain to cause a sedative-hypnotic effect in mice. This study may provide a scientific basis for the further development and application of C. militaris in the field of sleep improvement.
Acknowledgments
The authors are grateful to the National and Local United Engineering Laboratory R&D Center of research and development on the active peptide of medicinal plants and animals in the Chang Bai Mountain for the technical support.
Disclosure statement
The authors declare no conflict of interest.
Additional information
Funding
References
- Mellinger GD, Balter MB, Uhlenhuth EH. Insomnia and its treatment: prevalence and correlates. Arch Gen Psychiatry. 1985;42(3):225–232.
- Feng D, Ji L, Xu L. The influence of social support, lifestyle and functional disability on psychological distress in rural China: structural equation modeling. Aust J Rural Health. 2013;21(1):13–19.
- Benca RM. Consequences of insomnia and its therapies. J Clin Psychiatry. 2001;62:33–38.
- Hublin C, Partinen M, Koskenvuo M, et al. Heritability and mortality risk of insomnia-related symptoms: a genetic epidemiologic study in a population-based twin cohort. Sleep. 2011;34(7):957–964.
- Bramoweth AD, Taylor DJ. Chronic insomnia and health care utilization in young adults. Behav Sleep Med. 2012;10(2):106–121.
- Hoque R, Chesson AL Jr. Zolpidem-induced sleepwalking, sleep related eating disorder, and sleep-driving: fluorine-18-flourodeoxyglucose positron emission tomography analysis, and a literature review of other unexpected clinical effects of Zolpidem. J Clin Sleep Med. 2009;5(5):471–476.
- Lee HJ, Lee SY, Jang D, et al. Sedative effect of Sophora flavescens and Matrine. Biomol Ther. 2017; 1–7
- Hu JY, Bai XL, Lei L, et al. The hypnotic effect of lignans from Schisandra sphenanthera and effects on brain monoamine neurotransmitters. Pharmacol Clin Chin Mater Med. 2016;32(2):110–113.
- Paudel KR, Panth N. Phytochemical Profile and Biological Activity of Nelumbo nucifera. Evid Based Complement Alternat Med. [Internet]. 2015 [cited 2015 Dec 30]; 2015:789124. Available from: http://dx.doi.org/10.1155/2015/789124.
- Ma XY, Li RH, Jia TZ. Research on pharmacological activity and extracting process of total terpenoids in platycladi seed. Pract Pharm Clin Remedies. 2017;20(1):65–68.
- Li YH, Li ZH, Fu XJ, et al. Hypnotic and sedative effect of L-linalool and two kinds of blended essential oil. J Fujian Agr Forestry Univ. 2016;45(1):65–69.
- Park JM, Lee JS, Lee KR, et al. Cordyceps militaris extract protects human dermal fibroblasts against oxidative stress-induced apoptosis and premature senescence. Nutrients. 2014;6 (9):3711–3726.
- Bizarro A, Ferreira IC, Soković M, et al. Cordyceps militaris (L.) link fruiting body reduces the growth of a non-small cell lung cancer cell line by increasing cellular levels of p53 and p21. Molecules. 2015;20 (8):13927–13940.
- Das SK, Masuda M, Sakurai A, et al. Medicinal uses of the mushroom Cordyceps militaris: current state and prospects. Fitoterapia. 2010;81(8):961–968.
- Shrestha B, Zhang WM, Zhang YJ, et al. The medicinal fungus Cordyceps militaris: research and development. Mycol Prog. 2012;11(3):599–614.
- Liu J, Yang X, Chen Z, et al. The calmative and sex hormone-like effect of Cordyceps militaris (L) Link (Cantherea pernyi). J N Bethune Univ Med Sci. 1994;20(1):14–16.
- Jiang Y, Wong JH, Fu M, et al. Isolation of adenosine, iso-sinensetin and dimethylguanosine with antioxidant and HIV-1 protease inhibiting activities from fruiting bodies of Cordyceps militaris. Phytomedicine. 2011;18(2-3):189–193.
- Niu CY, Wu CS, Sheng YX, et al. Identification and characterization of flavonoids from semen zizyphi spinosae by high-performance liquid chromatography/ linear ion trap FTICR hybrid mass spectrometry. J Asian Nat Prod Res. 2010;12(4):300–312.
- Wen DC, Li YB, Hu XY, et al. Effect of ASF (a compound of Traditional Chinese Medicine) on behavioral sensitization induced by ethanol and conditioned place preference in mice. Evid Based Complement Altemat Med. [Internet]. 2014 [cited 2014 Oct 29];2014:304718. Available from: https://www.hindawi.com/journals/ecam/2014/304718/
- Sherin JE, Elmpuist JK, Torrealba F, et al. Innervation of histaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat. J Neurosci. 1998;18(12):4705–4721.
- Xu YB, Xu ZL, Li MY, et al. Nature and flavor pharmacology of Fuling and its components for learning and memory improvement as well as sedative and hypnotic effects. Chin Tradit Herb Drugs. 2014;45(11):1577–1584.
- Navarini L, Gilli R, Gombac V, et al. Polysaccharides from hot water extracts of roasted Coffea arabica beans: isolation and characterization. Carbohydr Polym. 1999;40(1):71–81.
- Liu CQ, Han C, Li DJ, et al. Microwave -assisted extraction of flavonoids from Cordyceps militaris. Jiangsu J of Agr Sci. 2007;23(4):356–359.
- Bao YF, Li JY, Zheng LF, et al. Antioxidant activities of cold-nature Tibetan herbs are signifcantly greater than hot-nature ones and are associated with their levels of total phenolic components. Chin J Nat Med. 2015;13(8):609–617.
- Wang XC, Dong JW, Zhao XY, et al. Evaluation of the sedative and hypnotic effects of H057. Chin Pharmacol Bull. 2016;32(5):638–642.
- Andalib S, Vaseghi B, Vaseghi A, et al. Sedative and hypnotic effects of Iranian traditional medicinal herbs used for treatment of insomnia. Excli J. 2011;10:192–197.
- Tao Y, Wu D, Zhang QA, et al. Ultrasound-assisted extraction of phenolics from winelees: modeling, optimization and stability of extracts during storage. Ultrason Sonochem. 2014;21(2):706–715.
- Ji LJ, Si YF, Liu HF, et al. Application of orthogonal experimental design in synthesis of mesoporous bioactive glass. Microporous Mesoporous Mater. 2014;184:122–126.
- Wang HJ, Pan MC, Chang CK, et al. Optimization of ultrasonic-assisted extraction of cordycepin from Cordyceps militaris using orthogonal experimental design. Molecules. 2014;19(12):20808–20820.
- Shao JF, Wen ZZ, Zhu HW. The detection of flavonoids glycosides from ginkgo biloba based on spectrofluorimetry. Guang Pu Xue Yu Guang Pu Fen Xi. 2013;33(11):3055–3060.
- Zheng YY, Li C, Feng SL, et al. Study on the content determination of total flavonoids in Olea europaea L. leaves. Guang Pu Xue Yu Guang Pu Fen Xi. 2011;31(2):547–550.
- He Y, He Z, He F, et al. Determination of quercetin, plumbagin and total flavonoids in Drosera peltata Smith var. glabrata Y.Z. Ruan. Pharmacogn Mag. 2012;8(32):263–267.
- Yamane H, Tomonaga S, Suenaga R, et al. Intracerebroventricular injection of glutathione and its derivative induces sedative and hypnotic effects under an acute stress in neonatal chicks. Neurosci Lett. 2007;418(1):87–91.
- Yi PL, Lin CP, Tsai CH, et al. The involvement of serotonin receptors in suanzaorentang-induced sleep alteration. J Biomed Sci. 2007;14(6):829–840.