ABSTRACT
Wild olives or oleaster (Olea europaea ssp. oleaster) are found naturally in the Mediterranean basin. The changes in climate and environmental conditions have been gradually accelerating in the last decades. Since oleasters are more resistant to diseases, pests and unfavorable environmental conditions, they have become a very important source for resistance breeding studies that would be required in the future. Twelve inter simple sequence repeat (ISSR) and 12 random amplified polymorphic DNA (RAPD) markers were used; the effectiveness of these markers was studied and different parameters such as effective multiplex ratio, marker index, resolving power, and polymorphic information content, Shannon information index (I), effective allele number (ne), and Nei's genetic distance (h) were used in the determination of genetic varieties of 18 oleasters grown in Turkey. When both marker systems were analyzed in this study, it was observed that MI, RP and EMR parameters in ISSR gave higher values than RAPD while ne, I, and h were lower in ISSRs. Neighbour-joining dendrograms were developed by using Nei's genetic distance matrix, and principal coordinate analysis was performed.
KEYWORDS:
Introduction
Olea europaea ssp. oleaster wild olives or oleasters are found naturally in Anatolia. Olea europaea ssp. sativa, or cultivated olives, are obtained by grafting oleasters. While wild olive takes tap-roots, cultivated olives mostly take hairy roots. Oleasters grow in a bush form and their tolerance against negative ecologic conditions is higher than that of the cultivated olives. Cultivated olives grow generally in a tree form and may also form small trees. The cultivated olive has large leaves, fruits and high oil content. Both wild olives (Olea europaea ssp. oleaster) and cultivated olives (Olea europaea ssp. sativa) are found commonly along Aegean and Mediterranean shores in Turkey [Citation1].
Wild olives are divided into two types as ‘White Wild’ and ‘Black Wild’. White Wild olives grow stronger; their leaves are larger and their colour lighter than Black Wild olives and the White Wild is closer to the cultivated olives in terms of its features. Therefore, they are used as rootstock in cultivated olive production. Black Wild olives grow very slowly and their leaves are very small and of dark colour; they are a shrub rootstock and grow into a small tree [Citation1,Citation2].
Determination of genetic structure of plants with economic importance, such as the olives, is important in terms of cultivation and improvement studies; determination of genetic similarities and distances in both wild and cultivated forms is valuable in terms of identifying genetic variability [Citation3]. Molecular marker methods are based on the principle of determining polymorphic regions in DNA molecules [Citation4]. Molecular markers are being used commonly for determining genetic polymorphisms among plants, genetic identification, hybrid plant identification in hybridization improvement, genetic mapping and marker-assisted selection (MAS) [Citation5]. Today, randomly amplified polymorphic DNA (RAPD) and inter simple sequence repeats (ISSR) are frequently used as molecular markers for the determination of genetic characterization of olives [Citation6–8].
RAPD is a polymerase chain reaction (PCR)-based method. RAPD-PCR is easy to use and features random primers with a length of 9–10 bases [Citation9,Citation10]. The number of studies in which RAPD method is used in the determination of genetic polymorphism in olives and relationships or distances among varieties has increased gradually in the recent years [Citation11].
Development of ISSR technique has allowed it to be used rapidly in the study of genetic variability in organisms. ISSR is based on the multiplication through PCR of the DNA fragment among two reverse positioned, simple sequential repetition regions of expandable distances [Citation12]. ISSR markers are used frequently for the determination of genetic variability of olive types and variation in olive clones, just like RAPD markers [Citation13–15].
Both PCR-based methods have been used to examine wild olive populations in different studies because of their genetic variations related to adaptations to environmental conditions. The ISSR and RAPD have also been found useful molecular markers for evaluation and discrimination of wild olives, their evolutionary backgrounds and distinguishing feral and wild populations [Citation16,Citation17]. Molecular markers such as ISSR, SSR, and RAPD could be used for discriminating homonyms and synonyms, and identification of unknown samples [Citation18].
The purpose of this study was to evaluate relatively as to which of ISSR and RAPD markers are more effective for revealing the genetic diversity in wild olives grown in Turkey. Thus, identified genetic differences may facilitate effective selection in the gene pool, allowing to grow resistant and healthy varieties and selecting the correct rootstock.
Materials and methods
DNA extraction
Eighteen wild olives were obtained from Mugla, Manisa, Izmir Provinces. Leaf samples were taken from three trees close to each other in each region. They were grouped from Wild 1 to Wild 6 ().
Table 1. Origin of oleaster samples.
DNAs of all samples were isolated by the Doyle and Doyle method [Citation19,Citation20] with minor modifications. Plant tissues were powderized with liquid nitrogen in a mortar. Ground tissues were immediately transferred to 1.5-mL Eppendorf tubes, 700 µL preheated cetyl trimethylammonium bromide extraction buffer (CTAB) (2% CTAB, 20 mmol/L ethylenediaminetetraacetic acid (EDTA), 1.4 mol/L NaCl, 100 mmol/L Tris-HCl pH 8.0, 2% mercaptoethanol) added and mixed several times by gentle inversions. Samples with CTAB buffer were incubated for 30 min in 65 °C. Tubes were mixed by inversions every 5 min. Tubes were removed from the water bath, left to cool down and then added to 700 µL of cold chloroform:isoamyl alcohol (24:1). Tubes were spun for 10 min at 10,000 rpm/min in a refrigerated centrifuge (Nuve 1200 R, Izmir, Turkey). Supernatants were poured into new tubes; 600 µL of cold chloroform:isoamyl alcohol (24:1) was added and mixed by gentle inversions for 5 min. Samples were spun for 10 min at 10,000 rpm/min again and supernatants were transferred to fresh tubes including 10 M ammonium acetate and 3 mol/L sodium acetate; 500 µL cold isopropanol was added and mixed by shaking very gently for DNA precipitation. Precipitated DNA was removed with pipette and washed with 70% ethanol. DNA samples were dried and re-suspended in EDTA and RNAase was added [Citation19,Citation20].
DNA quantification
Following the isolation of DNAs of spectrophotometric method was used in the determination of DNA quantities. Spectrophotometric analyses were performed in Thermo Unicam Helios Gamma spectrophotometer (Thermo Fisher Scientific, Waltham, MA, ABD). The DNA quality and quantity was calculated by their optical density values at 260 and 280 nm. Optical density ratios were evaluated and good quality DNA samples were used in PCR [Citation6].
The PCR and gel electrophoresis
Three samples each from the groups were bulked in an Eppendorf tube and kept in deep-freezer before the PCR process. Then, PCRs of samples were performed both through RAPD and ISSR primers. RAPD amplification reactions were carried out in 25 µL volume containing 25 ng DNA, 1 µM primer, 2.42 µL PCR buffer, 0.44 µL deoxynucleotide triphosphate (dNTP) stock, and 0.13 µL Taq DNA polymerase. The amplification reactions were carried out following these steps: initial denaturationat 94 °C for 1 min; followed by 35 cycles at 94 °C for 20 s, annealing at 35 °C for 20 s, extension at 72 °C for 20 s, and final extension at 72 °C for 5 min [Citation21].
ISSR amplification reactions were carried out in 25 µL volume containing 25 ng DNA, 4 µL primer, 2.5 µL PCR buffer, 2 µL dNTP stock, and 0.5 µL Taq DNA polymerase. Annealing temperatures were determined based on the melting temperatures of ISSR primers. The amplification reactions of ISSR were carried out following these steps: initial denaturation at 94 °C for 5 min; followed by 45 cycles at 94 °C for 30 s, 52 °C for 45 s., and 72 °C for 2 min, and final extension at 72 °C for 5 min. and show the properties of RAPD and ISSR primers [Citation22]. Tubes with PCR reagents without genomic DNA were used as controls. The PCRs were also carried out as duplicates for controlling the reproducibility of primers, only scorable bands were used in data analysis.
Table 2. RAPD decanucleotide primers.
Table 3. ISSR primers.
Agarose-gel-electrophoresis was performed for segregation of DNA fragments that were reproduced following the PCR. Electrophoresis was performed in 1 x Tris-borate-EDTA (TBE) buffer with 1.5% agarose gel for RAPD products and 1.2% for ISSR products. Ethidium bromide was used for DNA staining, and added to gel.
Data analysis
Gel images were evaluated and a data matrix was developed using ‘1’ for the existence and ‘0’ for the absence of RAPD and ISSR bands. Each band was evaluated and processed as an independent locus in the samples. BioOneD++ (Vilber Lourmat, France) software was used in the determination of molecular weights as base pairs while evaluating the bands. The productivity of ISSR and RAPD marker systems were compared relatively in order to determine genetic diversity within the wild olive genotypes.
A binary data matrix was developed from ISSR and RAPD electrophoresis results. Nei's genetic distance matrix was calculated through Popgene 1.32 and neighbour-joining trees using Nei's genetic distance matrix were revealed through MEGA6 [Citation23,Citation24]. The genetic distance matrices were developed from amplified products of RAPD and ISSR markers. The Nei's genetic matrices were used in the development of RAPD and ISSR neighbour joining dendrograms in Popgene 1.32 [Citation23].
A combined dendrogram was constructed by the data obtained from RAPD and ISSR electrophoretic results with MEGA6 program [Citation24].
The performances of RAPD and ISSR markers were determined by using various parameters. These were effective multiplex ratio (EMR, number of polymorphic locus in germplasm), polymorphic information content (PIC), marker index (MI) and resolving power (RP) [Citation25–Citation28]. EMR is an important comparison measure. MI is used to evaluate the marker system [Citation29], RP is used to segregate the genotypes or determine the segregation capacity of primers [Citation26], and PIC value is used to calculate the polymorphism content of each locus [Citation30]. Effective allele numbers (ne), Shannon Information Index (I), Nei's genetic distance (1973) (h) were also determined by POPGEN 1.32.
The matrix for PCA (principal coordinate analysis) was calculated with minimum similarity option in FAMD (Fingerprint Analysis with Missing Data) 1.31 software, then PCA was performed [Citation31].
Results and discussion
Comparing parameters of RAPD and ISSR primers, it was determined that the highest PIC in RAPD was in the OP-I17 primer (0.35), and the lowest PIC value was in the OP-I16 primer (0.28). The average PIC value for each primer was found as (0.31). The PIC value varied between 0.28 and 0.35. The highest PIC value (0.32) in ISSR was obtained with primer UBC889 and the lowest PIC value (0.28), with primers UBC834 and UBC855. The average PIC value for each primer was found as (0.30). The PIC value varied between 0.28 and 0.32.
Highest EMR value in RAPD was obtained from primer OP-I14 and lowest EMR value was obtained from primer OP-Q17. Highest EMR value in ISSR was obtained from primer UBC818 and lowest EMR value was obtained from primer UBC889. The EMR average of ISSR markers (12.17) was higher than the EMR average of RAPD markers (11.17).
In RAPD, highest MI value was obtained from primer OP-I14 (6.11), highest MI value was obtained from primer OP-Q17 (1.00). In ISSR, highest MI value was obtained from primer UBC818 (5.94) and lowest MI value was obtained from primer UBC889 (1.28). MI average of ISSR markers (3.66) was higher than the MI average of RAPD markers (3.45).
The fact that both EMR and MI were high in ISSR shows that the ISSR marker system is practical for molecular characterization of plants and determination of genetic relationships [Citation32].
The highest RP value in RAPD was obtained from primer OP-I14 (8.00), and the lowest RP value, from primer OP-Q17 (1.33). The highest RP value in ISSR was obtained from primer UBC818 (7.67), and the lowest RP value was obtained from primer UBC889 (1.67). The primer that provided most bands among RAPD primers was OP-I14 with 20 bands and OP-Q17 primer provided the least bands with 3. A total of 67 bands were obtained from RAPD primers. Average number of band per individual was 3.72 (total number of bands/number of individuals). The number of bands per primers was 11.17 (total number of bands/number of primers providing valuable bands). Among ISSR primers, the one providing most bands was primer UBC818 with 19 bands and the one providing least bands was primer UBC889 with 4 bands. Totally, 73 bands were obtained from ISSR primers. The average number of bands per individual was 4.05 (total number of bands/number of individuals). The number of bands per primers providing valuable bands was 12.17 (total number of bands/number of primers providing valuable bands) ( and ).
Table 4. Marker parameter values for RAPD primers providing valuable bands.
Table 5. Marker parameter values for ISSR primers providing valuable bands.
The primers that did not provide any valuable bands in RAPD were OP-C11, OP-C12, OP-C13, OP-K1, OP-K2 and OP-K3, and the primers with which no bands were obtained in ISSR were UBC810, UBC811, UBC812, UBC823, UBC825 and UBC826. The obtained results of RAPD and ISSR primers are summarized in .
Table 6. Comparison of RAPD and ISSR markers for evaluated parameters in wild olives.
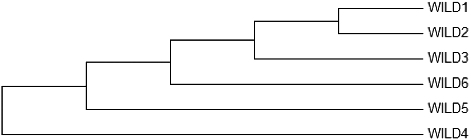
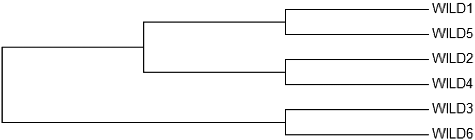
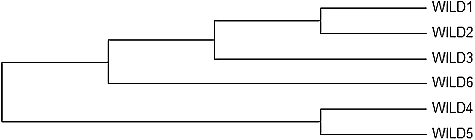
The genetic distance matrices were developed from amplified products of RAPD and ISSR markers ( and ). The Nei's genetic matrices were used in the development of RAPD and ISSR neighbour-joining dendrograms in Popgene 1.32 [Citation23]. and show the neighbour-joining dendrograms constructed from RAPD and ISSR data, respectively.
Table 7. Genetic distance matrix obtained from RAPD primers providing valuable bands in wild olives.
Table 8. Genetic distance matrix obtained from ISSR primers providing valuable bands in wild olives.
The data obtained from RAPD primers showed that genetic distance values were between 0.0458 (Wild 1 and Wild 2) and 0.7082 (Wild 3 and Wild 4). Thus, the samples closest to each other in accordance with the genetic distance values were Wild 1 and Wild 2; and the samples farthest to each other in accordance with the genetic distance values were Wild 3 and Wild 4).
Based on the ISSR data, genetic distance values were between 0.2831 (Wild 2 and Wild 4) and 0.5528 (Wild 3 and Wild 5) and 0.5528 (Wild 5 and Wild 6). Thus, the samples closest to each other in accordance with the genetic distance values were Wild 2 and Wild 4; samples farthest to each others in accordance with the genetic distance values were Wild 3 and Wild 5, Wild 5 and Wild 6.
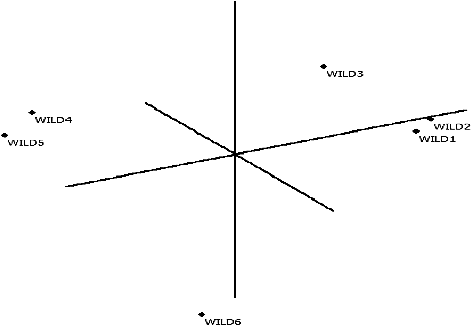
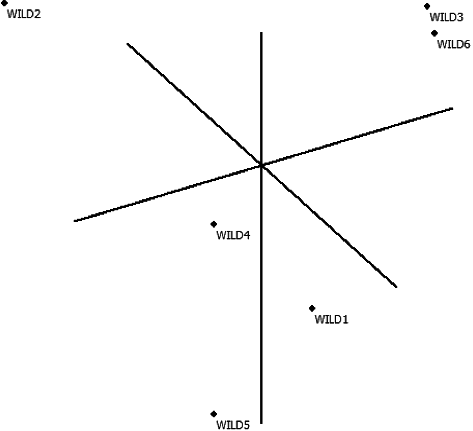
Neighbour-joining dendrogram and PCA of RAPD primers as formed per Nei's genetic distance matrix showed that Wild 1 and Wild 3 made a set together, i.e. they were the samples closest to each other. When the neighbour-joining dendrogram and PCA of ISSR were examined, it was observed that the samples farthest from each other were (Wild 3 and Wild 5) (Wild 5 and Wild 6). The results from the dendrogram and PCA were consistent. The results obtained for PCA RAPD and ISSR by calculating the matrix using minimum similarity option through FAMD 1.31 program are provided in and [Citation31].
The combined dendrogram from both marker data is shown in . There were two main clusters in the dendrogram. Wild 1 and Wild 2, both from the same district (Milas), formed a cluster as Wild 3 (Akhisar Sabancılar) and Wild 6 (Akhisar Isikkoy) contributed the major cluster. Wild 4 (Dikili Bademli) and Wild 5 (Soma Karacakas) set another main cluster. There is 75 km between Dikili and Soma, so it was suggested that an interchanging of genes could have occurred between these samples.
Noormuhammadi et al.,evaluated the intra-specific genetic diversity (Olea europaea ssp. cuspidata) in Iran by RAPD and ISSR markers. It was determined that the mean polymorphism in ISSR data was higher than that in RAPD markers. They also described population-spesific RAPD bands as there were not significant ISSR bands. The high genetic diversity among Olea europaea ssp. cuspidata populations, and the usefulness of RAPD and ISSR markers for identifying genetic variations has been shown in wild olive trees [Citation16].
In this study, the genetic variability of wild olives obtained from Mugla, Manisa and Izmir provinces were studied by using RAPD and ISSR marker methods. Both marker systems have specific advantages and disadvantages. In RAPD method the bands formed as a random primer finds and binds to the region that it is complementary to in the target genomic DNA are evaluated. The existence and absence of bands determine polymorphism. The advantage of RAPD markers is that they have ease of use, provide rapid results and allow revealing genetic similarities or distances as the number of polymorphic loci determined is high [Citation33]. A disadvantage of RAPD markers is that the marker may not be found mostly outside its population of production.
In the ISSR method, the repetitive bases of GA CT AG AC TC and CA used as primers. ISSR markers are frequently used in determining genomic fingerprint, genetic expansion, phylogenic analysis and MAS, genetic variability in natural populations, in the segregation of close relatives, and marking the regions rich in genes [Citation4]. If the chain repeated in a reaction remains the same, the number of target DNA chains strengthened in a single PCR reaction is increased by using different combinations of fixing DNAs as primer in the same reaction. This leads to increased number of bands or markers that may be produced on a single gel and provides a significant advantage when compared to the numbers that other DNA marker methods can produce [Citation34]. The disadvantage of ISSR markers is that the reproducibility could be low as it is in RAPD markers and the possibility of detecting similarly sized but non-homologues fragments [Citation35]. However, both are quite commonly used because of allowing the selection of some agricultural characters.
Based on the results we obtained from RAPD, most similar samples according to genetic distance values were Milas Dipcik and Milas Pinarcik wild olives. Since these samples were obtained from the same region, this result shows that RAPD markers are suitable for the determination of genetic similarities or distances among the wild olives [Citation36].
The results from our study support the use of ISSR analysis as a suitable method for the determination of genetic variability in natural populations [Citation37]. Yet, it was determined through the ISSR method that our samples of Milas Pinarcik and Dikili Bademli wild olives are close to each other genetically, although they are localized in distant regions. Accordingly, it was determined before that there is gene interchanging through regions among these wild olives [Citation36].
The fact that wild gene sources containing resistance sources came to the forefront in recent years led to identifying the plants of different genetic structures by using molecular markers. Because of the rapid assay time, minimal DNA requirement, rapid detection of DNA and their low costs, RAPD and ISSR methods were started to widespread use for determining the genetic variation in wild populations [Citation38,Citation39].
The changes in climate and environmental conditions in recent years have been gradually speeding up. Average temperatures increase and changes occur in the quantity and pattern of precipitations. The changes observed in heat waves, drought and flood frequencies and severity lead to the prediction that agricultural production will be affected negatively in future years. Likewise, increasing salinity in fertile soils causes important problems in arid and semi-arid regions. Developing plants resistant to salinity, drought and cold stress would be a step forward towards being prepared against the possible changes in agricultural production [Citation40]. Wild types containing the resistance genes have become a very important source for resistance improvement studies that would be required in the future. In this regard, use of marker aided selection for transferring genes from wild gene sources facilitates the improvement studies.
Conclusions
The study covered 18 wild olive samples and genetic variation was determined on DNA level through RAPD and ISSR methods. Valuable bands obtained as a result of RAPD and ISSR were used in the development of Nei's genetic distance matrices, neighbour-joining dendrograms and PCAs. It was determined that samples closest to each other based on genetic distance value were Wild 1 and Wild 2 according to the RAPD result and Wild 2 and Wild 4 according to the ISSR result. It is evident that various parameters are required to examine these two markers systems. In this respect and based on the parameters considered in our study, the RP value obtained from ISSR (4.61) was higher that the value obtained from RAPD (4.56) and the MI value (3.66) was higher than the value (3.45) obtained in RAPD. On the contrary, the PIC value (0.31), I (0.48), ne (1.46) and h (0.47) values, obtained from RAPD, were higher than the PIC value (0.30), I (0.30), ne (1.44) and h (0.30) values we obtained from ISSR. All these results show that both marker systems are equally effective in the determination of genetic variability. It is evident that the use of both markers is suitable in the determination of the genetic variation of wild olive trees. This study, which was performed by using RAPD and ISSR markers in Turkish wild olives, would contribute to further research on genetic variability in wild olives and to MAS studies.
Disclosure statement
The authors declare there is no conflict of interest for their study.
Additional information
Funding
References
- Efe R, Soykan A, Cürebal İ, et al. Dünyada, Türkiye'de, edremit körfezi çevresinde zeytin ve zeytinyağı [ Olive and olive oil in world, Turkey and Edremit bay area]. Edremit: Edremit Municipality Culture Publishings; 2013. p. 7–122. Turkish.
- Mendilcioğlu K. Subtropik iklim meyveleri [ Subtropic climate fruits]. Izmir: Ege University Agriculture Faculty Publishings; 1999. p. 1–8. Turkish.
- Parlak S, Coşkun F. Marmara bölgesinde yetiştirilen bazı zeytin kültivarlarının moleküler sistematik analizi [ Molecular systematic analysis of some olive cultivars (Olea europaea L.) grown in Marmara region] [master's thesis]. Balikesir: Balıkesir University; 2007. Turkish.
- Altınkut Uncuoğlu A. Moleküler markırlar ve haritalama, modern biyoteknoloji ve uygulamaları [ Molecular markers and mapping: applications of modern biotechnology]. Vol. 80. Erciyes: Erciyes University Publishing; 2010. p. 574–584. Turkish.
- Jiang GL. Plant breeding from laboratories to fields. In: Andersen SB, editor. Molecular markers and marker-assisted breeding in plants. IntechOpen; 2013. p. 45–83.
- Wu SB, Collins G, Sedgley M. A molecular linkage map of olive (Olea europaea L.) based on RAPD, microsatellite, and SCAR markers. Rijeka, Genome. 2004;47(1):26–35.
- Asadiar LS, Rahmani F, Siami A. Assessment of genetic diversity in the Russian olive (Elaeagnus angustifolia) based on ISSR genetic markers. Rev Cienc Agron. 2013;44(2):310–316.
- Golding S, Doveri S, Diaz A, et al. SNP-based markers for discriminating olive (Olea europaea L.) cultivars. Genome. 2006;49(9):1193–1205.
- Welsh J, McClelland M. Fingerprinting genomes using PCR with arbitrary primers. Nucl Acids Res. 1990;18(24):7213–7218.
- Williams JGK, Kubelik AR, Livak KJ, et al. DNA Polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucl Acids Res. 1990;18:6531–6535.
- Trujillo I, de la Rosa R, Rallo L, et al. Selection of RAPD markers for olive (Olea europaea L.) cultivars identification. Acta Hortic. 1999;474:495–498.
- Zietkiewicz E, Rafalski A, Labuda D. Genome fingerprinting by simple sequence repeat (SSR)-anchored polymerase chain reaction amplification. Genomics. 1994;20(2):176–183.
- Gomes S, Martins-Lopes P, Lima-Brito J, et al. Evidence of clonal variation in olive ‘Verdeal-Transmontana’ cultivar using RAPD, ISSR and SSR markers. J Hortic Sci Biotechnol. 2008;83(4):395–400.
- Leva AR, Petruccelli R. Monitoring of cultivar ıdentity in micropropagated olive plants using RAPD and ISSR markers. Biol Plant. 2012;56(2):373–376.
- Martins-Lopes P, Gomes S, Lima-Brito J, et al. Assessment of clonal genetic variability in Olea europaea L. ‘Cobrancosa’ by molecular markers. Sci Hortic. 2009;123(1):82–89.
- Noormohammadi Z, Samadi-Molayousefi H, Sheidai, M. Intra-specific genetic diversity in wild olives (Olea europaea ssp cuspidata) in hormozgan province, Iran. Genet Mol Res. 2012;11(1):707–716.
- Belaj A, Munoz-Diez C, Baldoni L, et al. Genetic diversity and population structure of wild olives from the north-western mediterranean assessed by SSR markers. Ann Bot. 2007;100(3):449–458.
- Zhan MM, Cheng ZZ, Su GC, et al. Genetic relationships analysis of olive cultivars grown in China. Genet Mol Res. 2015;14(2):5958–5969.
- Doyle JJ, Doyle JL. A Rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull. 1987;19:11–15.
- Doyle JJ, Doyle JL. Isolation of plant DNA from fresh tissue. Focus. 1990;12:13–15.
- Ergulen E, Ozkaya MT, Ulger S, et al. Identification of some turkish olive cultivars by using RAPD-PCR technique. Acta Hortic. 2002;(586):91–95.
- Martins-Lopes P, Lima-Brito J, Gomes S, et al. RAPD and ISSR molecular markers in Olea europaea L. genetic variability and molecular cultivar identification. Genet Res Crop Evol. 2007;54(1):117–128.
- Yeh FC, Yang RC, Boyle TBJ, et al. POPGENE: The user-friendly shareware for population genetic analysis [Freeware]. Version 1.32. Alberta: Molecular Biology and Biotechnology Centre, University of Alberta; 1997.
- Tamura K, Stecher G, Peterson P, et al. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729.
- Kayış SA, Erdogan EH, Emin, P. Comparison of Effectiveness of ISSR and RAPD markers in genetic characterization of seized marijuana (Cannabis sativa L.) in Turkey. African J Agric Res. 2010;5(21):2925–2293.
- Prevost A, Wilkinson, MJ. A new system of comparing PCR primers applied to ISSR fingerprinting of potato cultivars. Theo Appl Genet. 1999;98:107–112.
- Anderson JA, Churchill GA, Autrique JE, et al. Optimizing parental selection for genetic-linkage maps. Genome. 1993;36(1):181–186.
- Milbourne D, Meyer R, Bradshaw JE, et al: Comparison of PCR-based marker systems for the analysis of genetic relationships in cultivated potato. Mol Breed. 1997;3(2):127–136.
- Powell W, Morgante M, Andre C, et al. The comparison of RFLP, RAPD, AFLP and SSR (microsatellite) markers for germplasm analysis. Mol Breed. 1996;2:225–238.
- Roldan-Ruiz I, Dendauw J, Vanbockstaele E, et al. AFLP markers reveal high polymorphic rates in ryegrasses (Lolium spp.). Mol Breed. 2000;6:125–134.
- Schlüter PM, Harris SA. Analysis of multilocus fingerprinting data sets containing missing data. Mol Ecol Notes. 2006;6:569–572.
- Singh A, Dikshit HK, Jain NM, et al. Efficiency of SSR, ISSR, and RAPD Markers in molecular characterization of mungbean and other Vigna species. Indian J Biotechnol. 2014;13:81–88.
- Ivgin R, Bilgen G. Estimation of Genetic distance in meat and layer pure lines using randomly amlified polymorphic DNA. Turk J Vet Anim Sci. 2002;26:1116–1120.
- Fang DQ, Roose ML, Krueger RR, et al. Fingerprinting trifoliate orange germplazm accessions with ısozymes, RFLPs, and inter-simple sequence repeats. Theo Appl Genet. 1997;95:211–219.
- Kesawat MS, Das BK. Molecular markers: it's application in crop improvement. J Crop Sci Biotech. 2009;12(4):169–181.
- Wiesman Z, Avidan N, Lavee S, et al. Molecular characterization of common olive varieties in Israel and the West Bank using randomly amplified polymorphic DNA (RAPD) markers. J Am Soc Hortic Sci. 1998;123:837–841.
- Qian W, Ge S, Hong DY. Genetic variation within and among populations of wild rice Oryza granulata from China detected by RAPD and ISSR markers. Theor Appl Genet. 2001;102:440–449.
- Gülşen O, Mutlu N. Bitki biliminde kullanılan genetik markırlar ve kullanım alanları [Genetic markers used in plant sciences and their utilization]. Alatarım. 2005;4(2):27–37. Turkish.
- Baird WV, Ballard RE, Rajapakse, S, et al. Progress in Prunus mapping and application of molecular markers to germplasm improvement. Hortic Sci. 1996;7:1099–1106.
- ISAA [Internet]. International Service for the Acquisition of Agri-biotech Applications; c 2017. Climate change and its effect in agriculture; [cited 2017 Jan 26]. Available from: https://www.isaaa.org/resources/publications/pocketk/43/default.asp.