ABSTRACT
Kawasaki disease (KD) is a childhood vasculitis syndrome and the coronary arteries are the main target of the vascular damage. The outcome is formation of coronary lesions (CL) that develop in 20%–25% of untreated children. KD is the leading cause of myocardial infarction in infancy. Its consequences among young people are acute coronary syndrome and susceptibility to early atherosclerosis, if the disease were to remain unrecognized. We investigated the cardiac manifestations during the acute phase of the disease, the values of the fever, C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) and the therapeutic factors that created increased coronary risk in our cohort of patients. The study is retrospective (1993–2014). In the cohort (n = 107), 30.8% had coronary lesions, including 19.6% coronary aneurysms and 11.2% significant dilatations. We found association between myocarditis, papillary and left ventricular dysfunction in the acute phase of KD and the risk of coronary aneurysms (p < 0.001). The expressively elevated CRP levels and the persistent fever during the early subacute phase correlated significantly with coronary risk (p < 0.002). The treatment with intravenous immunoglobulin (IVIG) reduced the risk of coronary aneurysms 4.8 times (p < 0.002). A follow-up was performed in 53 children with coronary lesions during the first year of the disease. A longitudinal follow-up was performed in 38 patients. Their results indicate that cardiac monitoring is obligatory for all patients who have experienced KD.
Introduction
Kawasaki disease is a vasculitis syndrome and represents 23% of all forms of vasculitis in Caucasian children [Citation1]. According to the revised 2012 nomenclature of childhood vasculitis syndromes, it is defined as arteritis, affecting mainly medium and small arteries [Citation2]. The main target of the vascular damage are the coronary arteries and this makes it a unique, life-threatening disease. The myocardium and the heart valves are affected, too. The outcome of the vascular inflammation in its acute stage is the coronary endothelial lesion, later forming coronary ectasia and aneurysms. Coronary aneurysms develop in 20%–25% of untreated children, causing coronary artery disease. They are the main cause of morbidity and mortality in KD patients [Citation3,Citation4]. Patients with coronary lesions are at risk of complications: ruptured aneurysms, thrombosis and stenosis, risk of myocardial infarction, congestive heart failure and sudden death [Citation5,Citation6]. KD is the leading cause of myocardial infarction in infancy. Its consequences among young people are acute coronary syndrome and susceptibility to early atherosclerosis, if the disease were to remain unrecognized [Citation7].
The objective of the present study was to discuss the clinical experience with KD of the Pediatric Rheumatology Clinic at the University Pediatric Hospital, Sofia, Bulgaria [Citation8], and to assess the diagnostic and therapeutic factors that influence the risk of coronary complications: the values of CRP, ESR and fever in the acute and subacute phase of the disease, the cardiac manifestations in the acute phase of KD and the provided treatment. We discuss the results from the follow-up during the first year after the disease onset and from the longitudinal follow-up.
Subjects and methods
Selection of patients
The study was retrospective. The data of 107 patients diagnosed with KD over a period of 21 years (1993–2014) were analysed: 65 boys and 42 girls from 2 months to 13 years of age [Citation8]. The male-to-female ratio was 1.5:1. A follow-up was performed in 53 children with coronary lesions (CL) during the first year of the disease. Longitudinal follow-up from 1.5 to 17 years after the disease onset (mean 6 years) was provided in 38 children from 3 to 17 years of age (mean 8.6 years). Informed consent forms were obtained from the parents. The present study was approved by the Ethics Committee at the Medical University of Sofia, Bulgaria.
Diagnostic methods
The diagnosis of KD typical, incomplete and atypical course was assessed according to the American Heart Association (AHA) criteria [Citation4]. Echocardiography was performed by paediatric cardiologists according to the Newburger and Takahashi criteria for evaluation of the coronary lesions.
Statistical analysis
The clinical and patient data are expressed as median values with a range or mean values with standard deviation (±SD). The distribution of quantitative variables was evaluated with Kolmogorov–Smirnov and Shapiro–Wilk tests. For comparison of different groups, the unpaired T-test or Mann–Whitney U-test was applied when appropriate. The results were regarded as statistically significant when p < 0.05.
Results and discussion
Cardiovascular manifestations of Kawasaki disease
The release of cytokines in the acute febrile phase of the Kawasaki disease causes coronaritis with the consequences of formation of significant dilations and aneurysms [Citation8]. Such were observed by echocardiography in 25% of the patients with no intravenous immunoglobulin (IVIG) treatment [Citation4].
Coronary aneurysms and significant dilations were observed in 30.8% (n = 33) of our patients. Aneurysms affecting the left coronary artery, left anterior descending artery, the circumflex artery and right coronary artery were observed in 19.6% (n = 21). We detected a giant 8-mm aneurysm, seven medium-sized (5–8 mm), 21 small (<5 mm), four saccular ones and the rest were fusiform aneurysms. Six children had aneurysms of both coronary arteries and three had multiple coronary aneurysms. Significant dilatations were seen in 11.2% (n = 12 children). Twenty children had insignificant reversible dilatations and were, therefore, not included in the group with coronary lesions.
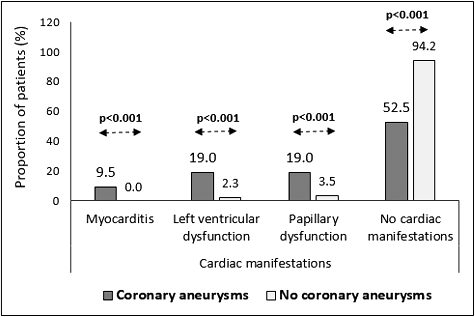
During the acute phase of the disease, cardiac manifestations such as myocarditis, heart failure, pericardial effusion, valvular insufficiency and electrocardiography (ECG) changes are observed in up to 30% of the patients and are present in all clinical forms of KD [Citation9,Citation10]. They are not always associated with the risk of coronary aneurysms [Citation11], but myocarditis with cardiomegaly is considered as a risk factor [Citation12]. In our cohort of patients, 29 children (27%) had pericarditis. The pericardial effusions were small and hemodynamically insignificant. Two patients (1.86%) had signs of severe myocarditis and intensive medical care was provided. Six children (5.6%) had left ventricular dysfunction and seven patients (6.5%) had mitral regurgitation-type papillary dysfunction which was hemodynamically insignificant. There was a significant association between myocarditis, papillary and left ventricular dysfunction in the acute phase of KD and the risk of coronary aneurysms (p < 0.001) ().
The degree of the fever and the severity of the coronary changes are a reflection of the role of cytokines in KD. In the patients without coronary complications, the mean temperature (t°) in the acute KD phase reached 39 °C and the children with CL had the same values. We did not find association between the t° values in the acute phase of KD and coronary involvement (p = 0.428). The persistence of fever after the 10th day of the disease onset indicates prolonged cytokine impact and has a significant association with the coronary aneurysm formation [Citation13–15]. In our cohort of patients, the persistent fever during the early subacute phase was also associated significantly with the coronary risk (p < 0.002).
The most common laboratory parameters studied in KD patients are C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR). These acute-phase inflammatory markers outline the inflammatory background of the disease and are increased because of the influence of various cytokines which also damage the coronary endothelium. In the scoring systems, CRP is considered a predictive sign for development of coronary lesions [Citation16]. The studies of Koyanagi et al. [Citation17] indicated increased coronary risk when CRP values are above 100 mg/L. In our patients with coronary lesions, CRP reached a mean value of 147.83 mg/dL, whereas in the group without coronary involvement, the mean value was 96.57 mg/dL (p = 0.038). Our data also support the fact that significantly elevated CRP levels predict coronary risk. The differences in the ESR between these two groups were not significant (p > 0.05).
Treatment of patients with Kawasaki disease
Coronary aneurysms are the main cause of morbidity and mortality in KD [Citation3,Citation18]. They are formed in the early subacute phase of the disease. IVIG infusion should be performed in the acute phase, up to the 10th day of the fever onset, with the goal of preventing coronary aneurysms. All studies conclude that coronary aneurysms develop in 20%–25% of the untreated children [Citation3,Citation4,Citation12]. Treatment with IVIG and a high dose of aspirin, reduces the coronary aneurysm formation to 5% (three to five times) [Citation4,Citation19,Citation20].
The treatment of children with KD was performed initially according to the guidelines of Dajani et al. [Citation21], followed by the American Heart Association (AHA) protocol [Citation22]. With the assessment of KD diagnosis, in all patients, the treatment was initiated immediately with aspirin (80–100 mg/kg/24 h). Patients with high risk of coronary artery lesions (CAL) received also IVIG infusion (2 g/kg), between the 8th and 12th days of the illness (in the early 1990 s, 400 mg/kg/24 h, for four consecutive days). After fever cessation, in 48–72 h, the aspirin dose was reduced to 3–5 mg/kg/24 h and was maintained for at least six weeks or until the patient had no more evidence of coronary changes.
IVIG is indicated for every child diagnosed with KD [Citation22]. Due to the high incidence of the disease in Japan and the high cost of the infusion in some centres there, IVIG is still applied according to the Harada score, which indicates increased coronary risk [Citation10]. Scoring systems predicting high coronary risk have been created and evaluated in Europe and the United States. But they are considered unsuitable for the Caucasian population [Citation23]. IVIG is an expensive treatment and the expenses in Bulgaria (government-funded hospitals) are covered only for patients with clinical and laboratory evidence of increased coronary risk. In our studies, we followed the criteria approved for the European population [Citation18].
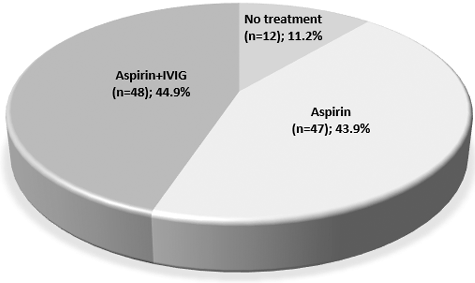
Ninety-five children (88.8%) hospitalized in the acute febrile phase were treated with high-dose aspirin. Forty-eight of them (44.9%) also received IVIG, which is a low rate compared to all patients with KD treated with IVIG in developed countries. Twelve patients (11.2%) hospitalized in the late subacute and convalescent phase received only low-dose aspirin treatment until the regression of the CL. Cases without CL received low-dose aspirin until the normalization of thrombocytosis for at least six weeks ().
The number of coronary aneurysms in our cohort of patients was relatively high (19.6%), similar to those in the group without IVIG treatment, where the incidence of coronary aneurysms was between 20% and 25% [Citation24]. The infusions with IVIG in Bulgaria started in 1998, but only in a limited number of patients. Many patients were diagnosed late and some of them came with already formed CL (18 of 21 children with coronary aneurysms were hospitalized in the convalescent stage of the disease) and were, therefore, indicated only for antiplatelet treatment and monitoring.
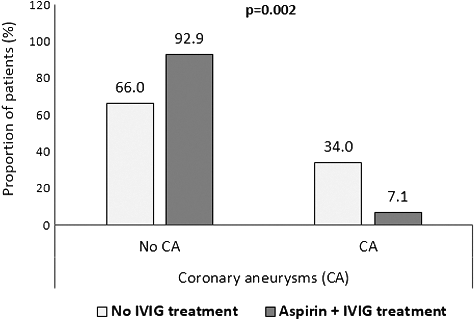
In our cohort of patients, IVIG treatment significantly reduced (by 4.8-fold) the risk of coronary aneurysms (p < 0.002) (). IVIG preparations are considered one of the safest biological products [Citation25]. Adverse reactions to treatment with IVIG were observed in 13 patients (27%). Seven patients experienced drug fever, one patient suffered an allergic reaction (bronchial obstruction and exantema), and hemolytic anaemia (Coombs+) was assessed in five patients.
Figure 3. Comparative analysis of patients with and without IVIG treatment and risk of coronary aneurysms.
According to a number of studies, IVIG resistance can be detected in up to 20% of the patients [Citation10,Citation18,Citation26,Citation27]. Resistance to IVIG could be genotype-dependent or dose-dependent, with an effect after the second IVIG infusion [Citation15,Citation28]. In our study, three patients (6.8%) were IVIG resistant. In these cases, the Kobayashi score of ≥5 points was predictive of IVIG resistance. One patient responded to the second IVIG infusion and two children had a second IVIG infusion plus oral corticosteroid treatment [Citation29]. All coronary aneurysms regressed within a year.
Follow-up
The condition of 53 children with CL was followed during the first year of the disease onset: 21 with coronary aneurysms, 12 with significant dilatations and 20 with no significant dilatations. Regression of coronary aneurysms was observed in 42.8%. Three aneurysms regressed after the second IVIG infusion and six patients had spontaneous regression of small aneurysms. Most of the significant dilatations (91.7%) were transient (five ones after IVIG infusion; six ones had spontaneous normalization). All insignificant dilatations were transient: 12 ones after IVIG infusion and 8 ones had spontaneous normalization.
‘Regression’ of coronary aneurysms often occurs within 1–2 years after onset and typically occurs in the case of small or medium aneurysms. It has been reported that patients may develop coronary stenosis at the site of regressed coronary aneurysms, a decrease in diastolic function or abnormal vascular endothelial function after a long period of time [Citation30–32]. Therefore, patients should be followed up even after regression of coronary aneurysms. Patients who have experienced KD without coronary lesions should be followed up as well because of suspicion of subclinical vasculitis, suggested by detection of circulating endothelial cells without underlying systemic inflammation or changes in peripheral blood flow [Citation7].
Longitudinal follow-up from 1.5 to 17 years after the disease onset (mean 6 years) was provided for 38 children from 3 to 17 years of age (mean 8.6 years). In these patients, in the acute phase of the disease, the following were recorded: coronary aneurysms in 11 children, significant dilatations in 3 children, no significant dilatations in 11 children and no coronary lesions in 13 children. Nineteen children (three ones with coronary aneurysms) were treated with IVIG infusions plus aspirin.
The follow-up results showed that none of the patients had any residual CL on the echocardiography follow-up. None of the patients had any complains of reduced functional capacity. Reduced ejection fraction between 61% and 64% was found in 10 patients (26%). Three of them had regressed coronary aneurysms, four ones had transient insignificant dilatations, and three ones had no CL during the acute phase KD. These patients had only been treated with high-dose aspirin and none of them had IVIG infusion. We suppose that the reduced ejection fraction is due to subclinical myocardial fibrosis. According to some authors, myocardial fibrosis is related to previous ischemia in the area perfused by the coronary artery where an aneurysm has been present or as a sequel of myocarditis in the acute phase of the disease [Citation33,Citation34].
Further cardiology studies of the myocardial function are required for the patients with reduced ejection fraction to determine whether it is a significant symptom related to the experienced KD and the lack of IVIG treatment. The data from the longitudinal study are not subject to statistical analysis due to the small number of patients. However, the data prove the need for life-long cardiac monitoring of all children who have experienced KD, regardless of the presence or absence of cardiac lesions.
Conclusions
This retrospective study showed that a high coronary risk was observed in patients with cardiac symptoms during the acute phase of KD, in patients with persistent fever during the subacute phase of the disease, in patients with high CRP and in IVIG-resistant patients. The high incidence of the observed coronary lesions was associated with the delay in the diagnosis and the lack of proper treatment with IVIG infusions. The IVIG infusions had a statistically significant clinical effect on the reduction of the incidence of coronary lesions. The longitudinal study indicated that cardiology follow-up has to be done in all patients who have experienced Kawasaki disease, regardless of whether they have persistent, regressed or no coronary lesions at all during the acute phase of the disease.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Jamieson N, Singh-Grewal D. Kawasaki disease: a clinician's update. Int J Pediatrics. 2013 [cited 2017 May 8];2013:645391. DOI: 10.1155/2013/645391
- Ozen S, Ruperto N, Dillon MJ, et al. EULAR/PReS endorsed consensus criteria for the classification of childhood vasculitides. Ann Rheum Dis. 2006;65(7):936–941.
- Burns J, Glode M. Kawasaki syndrome. Lancet. 2004;364(9433):533–544.
- Newburger JW. Kawasaki disease. Curr Treat Options Cardiovasc Med. 2000;2:227–236.
- Okura N, Okuda T, Shiotani S, et al. Sudden death as a late sequel of Kawasaki disease: postmortem CT demonstration of coronary artery aneurysm. Forensic Sci Int. 2013;225(1–3):85–88.
- Liberthson RR. Sudden death from cardiac causes in children and young adults. N Engl J Med. 1996;334(16):1039–1044.
- Shah V, Christov G, Mukasa T, et al. Cardiovascular status after Kawasaki disease in the UK. Heart. 2015;101(20):1646–1655.
- Telcharova-Mihaylovska A, Nikolova I, Marinov R, et al. Kawasaki disease – experience of Pediatric University Hospital, Sofia, Bulgaria, 1993–2014. Part I: clinical manifestations. Biotechnol Biotechnol Equip. Forthcoming 2017; Available from: https://doi.org/10.1080/13102818.2017.1316683
- Momenah Т, Sanatani S, Potts J, et al. Kawasaki disease in the older child. Pediatrics. 1998 [cited 2017 May 8];102(1):e7. Available from:http://www.pediatrics.org/cgi/content/full/102/1/e7
- JCS Joint Working Group. Guidelines for diagnosis and management of cardiovascular sequelae in Kawasaki disease (JCS 2013). Circul J. 2014;78(10):2521–2562.
- Scheinfeld NS, Jones EL. Kawasaki disease [Internet]. New York: Medscape; c 1994–2017. [updated 2016 Oct 25; cited 2017 Jan 17]. Available from: http://emedicine.medscape.com/article/965367-overview
- Son MB, Sundel R. Kawasaki Disease. In: Petty R, Lindslay C, Laxter R, Wadderburn L, editors. Textbook of pediatric rheumatology. Philadelphia (PA): Elsevier; 2016. p. 467–483.
- Baker A, Newburger J. Kawasaki disease. Circulation. 2008;118:e110–e112.
- Barclay L, Vega C. American heart association revises guidelines on Kawasaki disease. Medscape. 2004 Oct 25; [cited 2017 May 17]. Available from:http://www.medscape.org/viewarticle/491960
- Scuccimarri R. Kawasaki disease. Ped Clin North Am. 2012;59(2):425–445.
- Sleeper LA, Minich L, McCrindle B, et al. Valuation of Kawasaki disease risk scoring systems for intervenous immunoglobulin resistance. J Pediatr. 2011;158(5):831–835.
- Koyanagi H, Yanagawa H, Nakamura Y, et al. Serum C-reactive protein levels in patients with Kawasaki disease: from the results of nation-wide surveys of Kawasaki disease in Japan. Acta Paediatr. 1997;86(6):613–619.
- Eleftheriou D, Levin M, Shingadia D, et al. Management of Kawasaki disease. Arch Dis Child. 2014;99:74–83.
- Nimmerjahn F, Ravetch JV. The anti-inflammatory activity of IgG: the intravenous IgG paradox. J Exp Med. 2007;204:11–15.
- Wood LE, Tulloh RM. Diagnosis and management of Kawasaki disease in children. Pediatrics Child Health. 2007;18(2):70–74.
- Dajani AS, Taubert KA, Takahashi M, et al. Guidelines for long-term management of patients with Kawasaki disease. Report from the committee on rheumatic fever, endocarditis, and Kawasaki disease, council on cardiovascular disease in the young, American Heart Association. Circulation. 1994;89:916–922.
- Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the committee on rheumatic fever, endocarditis, and Kawasaki disease, council on cardiovascular disease in the young, american heart association. Pediatrics. 2004;114(6):1708–1733.
- Yellen ES, Gauvreau K, Takahashi M, et al. Performance of 2004 American Heart Association recommendations for treatment of Kawasaki disease. Pediatrics. 2010;125(2):234–241.
- Lin MT, Sun LC, Wu ET, et al. Acute and late coronary outcomes in 1073 patients with Kawasaki disease with and without intravenous γ-immunoglobulin therapy. Arch Dis Child. 2015;100(6):542–547.
- Duhem C, Dicato MA, Ries F. Side-effects of intravenous immune globulines. Clin Exp Immunol. 1994;97(Suppl 1):79–83.
- Michihata N, Matsui H, Fushimi K, et al. Guideline-concordant treatment of Kawasaki disease with immunoglobulin and aspirin and the incidence of coronary artery aneurysm. Clin Pediatr (Phila). 2015;54:1076–1080.
- Vierucci F, Azzarelli A, Gualtierotti R, et al. Changing management of Kawasaki disease. J Compr Ped [Internet]. 2014 [cited 2017 May 8];5:e19519. Available from:https://doi.org/10.17795/compreped-19519
- Shrestha S, Wiener HW, Olson AK, et al. Functional FCGR2B gene variants influence intravenous immunoglobulin response in patients with Kawasaki disease. J Allergy Clin Immunol. 2011;128(3):677–680.
- Telcharova-Mihaylovska A, Stefanov S, Nikolova I. Kawasaki disease and acute haemolytic anaemia after two IVIG infusions. Biotechnol Biotechnolog Equip. 2016;30(3):448–452.
- Suzuki A, Yamagishi M, Kimura K, et al. Functional behavior and morphology of the coronary artery wall in patients with Kawasaki disease assessed by intravascular ultrasound. J Am Coll Cardiol. 1996;27:291–296.
- Mitani Y, Okuda Y, Shimpo H, et al. Impaired endothelial function in epicardial coronary arteries after Kawasaki disease. Circulation. 1997;96:454–461.
- Sugimura T, Kato H, Inoue O, et al. Intravascular ultrasound of coronary arteries in children: Assessment of the wall morphology and the lumen after Kawasaki disease. Circulation. 1994;89:258–265.
- Xie L, Wang R, Huang M, et al. Quantitative evaluation of myocardial fibrosis by cardiac integrated backscatter analysis in Kawasaki disease. Cardiovasc Ultrasound. 2015 [cited 2017 May 17];14:3. DOI: 10.1186/s12947-016-0046-7
- Yonesaka S, Nakada T, Sunagawa Y, et al. Endomyocardial biopsy in children with Kawasaki disease. Acta Paediatr Jpn. 1989;31:706–711.