ABSTRACT
In this study, plant (leaf, branch and bark) and soil samples of nettle tree were collected from 40 different localities in Istanbul, Turkey, to investigate the heavy metal pollution levels as well as to understand the employability of this plant species in pollution monitoring. Besides, the importance of pollution sources and their distance to the plant species were emphasized, and assessment of the air and soil related contamination was performed. The heavy metal concentrations in the samples were measured using atomic absorption spectrophotometry. The average highest values of Pb (14.90 ± 2.96 μg/g), Cd (0.65 ± 0.13 μg/g), Cu (19.94 ± 1.17 μg/g) and Zn (42.53 ± 3.08 μg/g) were found in unwashed leaf samples taken from the roadside. However, the average lowest values of Cd (0.30 ± 0.06 μg/g) and Cu (5.99 ± 0.21 μg/g) were in washed leaf samples, whereas the lowest levels of Pb (1.19 ± 0.12 μg/g) and Zn (14.34 ± 0.71 μg/g) were in branches. In addition, there was also a direct correlation between heavy metal accumulation, traffic density and closeness to roadside. It was demonstrated that Celtis australis could be a useful plant species in the biomonitoring of environmental pollution with these four heavy metals. Moreover, the results also indicated that nettle tree barks might be employed in long-term measurements of heavy metal accumulation.
Introduction
With rapid urbanization and industrial development particularly by the beginning of the twentieth century, heavy metal pollution in the biosphere has now become one of the most serious environmental concerns due to its severe long-term implications on human health and the environment [Citation1–3]. Thus, control and monitoring of airborne pollution around the world are a significant part of the environmental planning and control programmes [Citation4–6]. Cd and Pb are two serious environmental pollutants, especially in areas affected by anthropogenic pressure, and their presence, even in very small amounts, in the air, soil and water, can be harmful to many organisms [Citation7,Citation8]. Additionally, the number of motorized vehicles has increased enormously in the last decade (more than 2.5 million), which has further aggravated the pollution problem. Cd and Pb have high mobility and are accepted as carcinogenic elements; hence, their concentrations should be as closest to zero as possible [Citation9–12]. The accumulation of heavy metals in plants depends on various factors, such as distance from the pollution source, physical properties of heavy metals (e.g. mobility), emission intensity (e.g. traffic density), climatic factors (e.g. wind direction and force, precipitation and air circulation), soil and season types, aerosol accumulation and topographical structures [Citation3,Citation8,Citation13]. Besides, heavy metals such as Cu and Zn are essential for human metabolism [Citation3] but, at higher concentrations, can also lead to poisoning [Citation14].
Plants growing in contaminated areas mainly demonstrate visible symptoms on plant surfaces because of the heavy-metal accumulation in plant parts [Citation15]. However, some plants, or hyperaccumulators, are able to accumulate higher amounts of heavy metals in various plant tissues without toxicity symptoms; thereby, these plants cannot provide acceptable quantitative information on environmental pollution when compared with biomonitors [Citation16–18]. Thus, the selection of biomonitor plants is a very important initial step in research programmes aiming to decontaminate the polluted areas [Citation12,Citation14,Citation17,Citation19]. As stated by Wittig [Citation20], a good biomonitor plant should have a wide geographical range, should be able to differentiate air- and soil-borne pollutants, and could be easily sampled and identified.
The aim of this study was to investigate the Pb, Cd, Cu and Zn pollution levels in Istanbul, Turkey, by using Celtis australis, which is a member of the Ulmaceae family showing a broad geographical distribution, widely represented in South Europe and North Africa as well as in the study area [Citation21,Citation22]. For this aim, the heavy metal concentrations were determined by atomic absorption spectrophotometry (AAS) in C. australis and soil samples collected from different sites with different pollution levels, such as urban, suburban, highway and industrial areas, and a rural site (control).
Materials and methods
Study area and plant specifications
Istanbul is located in the northwestern part of Turkey, in Marmara Region, covering an area of 5343 km2 [Citation23]. It is the largest city of Turkey, with 13 million metropolitan population and a nearly 4.5% annual population growth rate. It has significant industrial activity and heavy traffic load. According to the road motor vehicle statistics, as of 2016, there are 3.651.166 motor vehicles in Istanbul, exclusive of tractors, motorcycles and other special purpose vehicles [Citation24]. Moreover, unplanned urbanization, gradual decrease of green areas and construction of high buildings have also negatively affected the air circulation, and the city is exposed to high air pollution especially in the cold months [Citation25].
According to the Köppen climate classification, Istanbul has Mediterranean climate, becoming more oceanic towards the North [Citation26]. High temperature and less precipitation characterize the summers, with an annual mean temperature of 14.5 °C for the last two decades. Between May and September, the temperature is usually over 30 °C, while it is rarely below 0 °C between November and April. In the vegetation period (between 15th March and 20th December), the daily mean temperature is approximately 8 °C, which lasts for about 280 days [Citation27,Citation28]. Istanbul receives an average of 640 mm total precipitation per year, with 40% falling in the winter. The minimum rainfalls are in July and August, while December and January are the wettest months [Citation13,Citation27,Citation29].
According to Davis (1965–1985), the scientific description of C. australis is as follows: ‘Tree to 20 (–25) m. Young twigs velutinous. Leaves 4–12(–15) × (2–)3–4(–5) cm, ovate-lanceolate to lanceolate, oblique at base, usually long-acuminate, sharply serrate, scabrid above, velutinous and brownish-green or greyish-green beneath. Petiole to 1.5(–2) cm. Drupe 9–12 mm diam., brownish to blackish. Pedicel to 3.5 cm, longer than next internode. Stone reticulate-rugose. Open rocky slopes and in thickets, rarely in forest, usually solitary, often planted, 50–700 (–1000) m. Described from South Europe and North Africa, North-West Turkey, South Anatolia, Africa, South Europe, West Transcaucasia.’ It is usually grown as an ornamental plant because of its long life and resistance to air pollution [Citation21,Citation30–32].
Sample collection and preparation
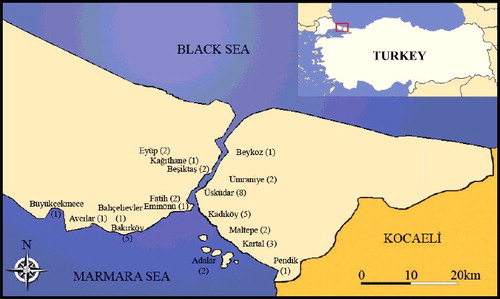
Plant (washed/unwashed leaf, branch and bark) and soil samples were collected from 40 different localities in Istanbul during the summer period, especially in July and August. The number of samples per site was distributed as follows: urban (14), roadside (14), suburban (7) and rural (control) areas (5) (). About 200 g of fresh leaf samples were taken from the leafy part of the plants and then samples were divided into two groups. One group was completely washed with distilled water to remove the particulate matter, while the other group was left untreated. Bark samples (10 × 2 cm) were taken from the main stem (about 1 m above the soil) and wounded sites were sealed with grafting wax to prevent microbial contamination. Soil samples (about 1000 g) were taken from a depth of about a 10 cm. Then, the soil samples were dried (at 80 °C) and fed through a 2-mm sieve. The plant samples were oven-dried at 80 °C for 24 h, ground in a micro hammer cutter and fed through a 1.5-mm sieve. Plant samples were then stored in plastic bags.
Figure 1. Map showing the study area (Istanbul) and districts. In these districts, the roadside (RS), urban (U), suburban (SU) and control (C) localities are as follows: Büyükçekmece (1 RS), Avcılar (1 U), Bahçelievler (1 U), Bakırköy (1 RS, 1 SU and 3 U), Eminönü (1 U), Fatih (1 RS and 1 U), Beşiktaş (1 U and 1 C), Kağıthane (1 SU), Eyüp (2 RS) on the European side and Pendik (1 SU), Kartal (2 RS, 1 SU), Maltepe (2 RS), Kadıköy (2 RS, 3 U), Üsküdar (3 RS, 1 U, 2 SU, 2 C), Beykoz (1 SU), Ümraniye (2 U), Adalar (2 C) on the Asian side.
Analytical techniques
About 0.5 g of dried plant samples were transferred into polytetrafluoroethylene (PTFE) vessels (XP-1500) and then 10 mL of 65% HNO3 (Merck, Darmstadt, Germany) were added. The samples were mineralized in a microwave oven (CEM MARS5). The soil samples were weighed as 0.3 g and transferred into Teflon vessels and then 5 mL of 65% HNO3, 3 mL of 37% HCl (Merck, Darmstadt, Germany) and 2 mL of 48% HF (Merck, Darmstadt, Germany) were added. The soil samples were also mineralized in a microwave oven. After cooling, all samples were filtered using Whattman filters, and made up to 25 mL with water in volumetric flasks and stored in plastic bags. Standard calibration techniques were used for Cd, Cu, Pb and Zn in samples. Measurements were done using an atomic absorption spectrophotometer (ASS; Perkin Elmer model 1100).
Statistical analysis
Statistical calculations such as multivariate analysis of variance (MANOVA) with Tukey's post-hoc HSD and Pearson correlation were performed using IBM SPSS Statistics 20 based on the dry weight of samples. The statistical significance was indicated as **P < 0.01 (2-tailed).
Results and discussion
The mean concentrations of heavy metals such as Cd, Cu, Pb and Zn in plant samples (washed/unwashed leaves, branches and bark) and soil samples are presented in . In this study, the average highest and lowest Cd values were measured to be 0.65 ± 0.13 mg/kg in unwashed leaves from the roadside and 0.30 ± 0.06 mg/kg in washed leaves from the control (rural) area, respectively (-A). The average Cd content in the soil ranged within 0.53 ± 0.01–1.02 ± 0.04 mg/kg (-B). For Cu, the average highest (19.94 ± 1.17 μg/g) value was in unwashed leaves from the roadside, whereas the lowest one (5.99 ± 0.21 μg/g) was detected in washed leaf samples from the control area (-C). Moreover, the average Cu content in the soil was between 25.26 ± 1.01 and 71.03 ± 3.55 mg/kg (-D). In this study, the Pb content largely varied, and the average highest value was measured in the bark samples (14.90 ± 2.96 mg/kg) collected near the roadside, whereas the lowest value (1.19 ± 0.12 mg/kg) was measured in branch samples in the control area (-E). The average Pb content in the soil ranged between 27.4 ± 2.41 and 51.55 ± 4.74 mg/kg (-F). For Zn, the average highest value was 42.53 ± 3.08 mg/kg in unwashed leaves from the roadside and the lowest value was 14.34 ± 0.71 mg/kg in branch samples from the control area (-G).
Figure 2. Mean Cd (A), Cu (C), Pb (E) and Zn (G) contents (μg/g) in plant [unwashed leaves (![]()
![Figure 2. Mean Cd (A), Cu (C), Pb (E) and Zn (G) contents (μg/g) in plant [unwashed leaves (Display full size), washed leaves Display full size), branches (Display full size), bark ( Display full size)] and soil (B, D, F and H) samples of C. australis from the roadside, urban, suburban and rural (control) areas.](/cms/asset/f98e8c31-933e-4081-ad01-2357c6cf21db/tbeq_a_1353922_f0002_b.gif)
It has been reported that the normal limits of Cd in plants are between 0.2 and 0.8 mg/kg and values between 5 and 30 mg/kg are accepted as toxic [Citation33,Citation34]. In our study, the average highest Cd value was found in unwashed leaf samples from the roadside (0.65 ± 0.13 μg/g). In literature, the normal limits of Cu in plant tissues are in the range of 4–15 μg/g and between 20 and 100 μg/g are accepted as toxic levels [Citation34–36]. In this study, the average highest Cu value was measured in unwashed leaf samples from the roadside: 19.94 ± 1.17 μg/g, which is very close to the toxic value. In addition, the Cu values in unwashed leaf samples from the roadside (19.94 ± 1.17 μg/g) and urban areas (17.97 ± 0.86 μg/g) and from bark samples from the roadside (18.96 ± 0.78 μg/g) and urban areas (17.86 ± 0.76 μg/g) were also higher than the normal range.
According to literature, the normal Pb concentration range in plant tissues varies between 0.1 and 10 μg/g, and levels between 30 and 300 μg/g are considered toxic [Citation28]. In our study, the average highest Pb value was measured in unwashed leaf samples from the roadside (14.90 ± 2.96 μg/g). In addition, the Pb content (11.29 ± 1.18 μg/g) in unwashed leaf samples collected from urban areas was higher than the normal limits.
The normal limits of Zn in plants are reported to be 20–100 μg/g [Citation37]. Between 100 and 400 μg/g are considered toxic [Citation38]. Herein, the highest value was measured in unwashed leaf samples (42.53 ± 3.08 μg/g) from the roadside. Thus, in this study, the Zn content was within the normal range. However, the highest and lowest Zn levels in the soil samples were determined to be 81.61 ± 5.20 mg/kg and 47.46 ± 2.28, respectively (-H).
The washing procedure caused a reduction (4–34%) in the levels of all the four studied heavy metals in the leaf samples (), the highest reduction being observed for Pb.
Table 1. Total percentage of Cd, Cu, Pb and Zn removed by washing procedure from leaf samples of C. australis collected at different stations.
In this study, the highest Cd content (1.02 ± 0.04 μg/g), Cu (71.03 ± 3.55 μg/g), Pb (51.55 ± 4.74 μg/g) and Zn content (81.61 ± 5.20 μg/g) were measured in the soil samples from the roadside. According to the literature, the normal concentration range of these heavy metals in soils is as follows: Cd 0.05–1 μg/g, Cu 10–40 μg/g, Pb 10–30 μg/g and Zn 10–300 μg/g [Citation28,Citation31]. Thus, the soil Cu and Pb values exceeded the normal range.
The correlation coefficients between the concentration of heavy metals in soil and washed leaf samples are shown in . The results indicated a positive correlation, with correlation matrix (R) values of >0.51 and >0.76, between the Cd content in the soil and the Cd content in leaves; between the Cu content in the soil and the Cu and Pb content in leaf samples and between the Zn content in the soil and the Pb and Zn content in leaf samples.
Table 2. Correlation matrix (R) between heavy metal concentrations in soil and washed leaf samples.
In this study, the mean heavy metal concentrations in the samples from the roadside were slightly higher than the ones in the urban and suburban samples and significantly higher than the ones in the rural samples. This indicated that the source of pollution is most likely traffic related in the investigated area/s. This suggestion is in agreement with many previous studies [Citation3,Citation8,Citation39,Citation40]. Moreover, the soil samples were observed to include the highest average content of the studied metals. In plant parts, from highest to lowest, the measured heavy metal concentration ranked as follows: unwashed leaves, washed leaves, bark and branches for Pb; unwashed leaves, bark, branches and washed leaves for Cd and Cu; and unwashed leaves, bark, washed leaves and branches for Zn. Besides, the relative abundance of the tested elements in the plant samples was Zn > Cu > Pb > Cd.
It has also been reported that Cd, Cu, Pb and Zn are the major roadside polluters, mainly released from burning fuels, worn tires, oil leakages and abrasion of metallic parts and batteries [Citation41]. Rapid industrialization, high traffic intensity and vast fertilizer applications have been also widely reported in high quantity release of heavy metals into the biosphere [Citation42,Citation43]. In addition, the industrial activities and sewage sludge are the major important factors affecting the metal concentration in soil [Citation44]. Especially, automobile exhaust gas is one of the potential Pb contributors in urban areas with serious effects on humans and the environment [Citation45,Citation46].
Moreover, the high Cu levels in plant samples from the roadside could be related to the use of car brakes. It has been reported that each car brake applied contributes to the Cu pollution in bays, beaches and creeks [Citation47]. When a car is braked, Cu dust is deposited on the roadside since the brake pads are made of copper; subsequently, this dust is washed out into the water sources with rainfalls or irrigation water and could be toxic to aquatic life, particularly at the base of the food chain. In our study, there was also significant atmospheric deposition on plant leaves, which was removed in the washing procedure in order to distinguish the air and soil related contamination (). In the washed leaf samples, maximum reduction was observed in the Cu and Pb levels, correlating with the atmospheric deposition of these elements. However, the Pb and Cd concentrations were below the toxic levels in the plant and soil samples. The findings also reflected some variations in the source and heavy metal uptake mode by plants, which is in compliance with many earlier reports [Citation3,Citation8,Citation13,Citation17,Citation18,Citation48–52].
In the roadside samples, the total percentage of Cd, Cu, Pb and Zn removal from plant leaves through the washing procedure was higher than that in the samples from rural sites. All these results indicated that Pb and Cu could have been accumulated in the environment via atmospheric deposition related to road distance and traffic volume. In other studies, Pb uptake has been also demonstrated to occur in the root system as well as through the plant leaves [Citation43,Citation50]. In this study, the removal of a high percentage of Pb and Cu from the plant leaves by washing suggests the areal deposition of these two elements [Citation3,Citation8,Citation13].
The mean metal concentrations in both the plant and the soil samples were observed to decrease as follows: roadside > urban > suburban > rural site. In addition, the heavy metal concentrations were high in the areas with heavy traffic jams. High values were also measured in areas with reduced air circulation due to the high-rise buildings. The Pb, Cd and Zn contents in plant and soil samples were lower than the permitted concentrations (noxious level), with the exception of some localities. Only the Cu concentrations exceeded the limited risk values. Moreover, the high metal concentrations in the bark may result from its long-lived age and thorny structures. Thus, barks can be effectively used for biomonitoring purposes. However, the branches are not as old as the bark, and they do not have thorny structures. In addition, they do not possess flat and wide structures. These could be the reasons why the heavy metal accumulation was minimum in the branch samples. In contrast, the leaves could be regarded as suitable biomonitor organs due to their shape and horny structures.
Overall, C. australis was a good accumulator of all the four studied heavy metals. Hence, these woody plants could be recommended for further biomonitoring programmes. Furthermore, C. australis is reported to have lower trace element content today than 15–20 years ago due to improvements such as the use of unleaded fuels, the development and control of new technologies in the automobile sector and other branches of industry and the efforts to increase the green lands [Citation40]. On the other hand, the main sources of pollution are being shifted to rural areas, which calls for strict, reliable and efficient biomonitoring measures in terms of environment sustainability [Citation9–12].
Conclusions
In this study, C. australis was demonstrated to have a capability to accumulate some heavy metals proportionally to their concentration in the soil/environment. Thus, the results suggested that C. australis could be used as a biomonitor plant for Cd, Cu, Pb and Zn. In addition, the barks of this plant could also be effectively used for long-term biomonitoring of heavy metal depositions.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Avino RB, Lopez–Moya JR, Navarro-Avino JP. Health implications: trace elements in cancer. In: Prasad MNV, editor. Trace elements as contaminants and nutrients: consequences in ecosystems and human health. Hoboken: (NJ): Wiley; 2008. p. 495–521.
- Ugulu I, Dogan Y, Baslar S, et al. Biomonitoring of trace element accumulation in plants growing at Murat Mountain. Int J Environ Sci Technol. 2012;9:527–534.
- Severoglu Z, Ozyigit II, Dogan I, et al. The usability of Juniperus virginiana L. as a biomonitor of heavy metal pollution in Bishkek City, Kyrgyzstan. Biotechnol Biotec Eq. 2015;29(6):1104–1112.
- Yilmaz R, Sakcali S, Yarci C, et al. Use of Aesculus hippocastanum L. as a biomonitor of heavy metal pollution. Pak J Bot. 2006;38(5):1519–1527.
- Banerjee T. Srivastava RK. Assessment of the ambient air quality at the Integrated Industrial Estate Pantnagar through the air quality index (AQI) and exceedance factor (EF). Asia-Pacific Jrnl of Chem Eng. 2011;6(1):64–70.
- Yasar U, Ozyigit II, Yalcin IE. et. al. Determination of some heavy metals and mineral nutrients of bay tree (Laurus nobilis L.) in Bartin City, Turkey. Pak J Bot. 2012;44:81–89.
- Jadia CD, Fulekar MH. Phytoremediation of heavy metals: Recent techniques. Afr J Biotechnol. 2012;8(6):921–928.
- Osma E, Ozyigit II, Leblebici Z, et al. Determination of heavy metal concentrations in tomato (Lycopersicon esculentum Miller) grown in different station types. Rom Biotechnol Lett. 2012;17(1):6962–6974.
- WHO. Toxicological evolution of certain food additives. Joint FAO/WHO expert committee of food additives. WHO Food Additives Series; No 17. Geneva: World Health Organization; 1982.
- WHO. Evaluation of certain food additives and contaminants. WHO Technical Report Series; No 683. Geneva: World Health Organization; 1982.
- WHO. Evaluation of certain food additives and contaminants. Thirty-third report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series; No 776. Geneva: World Health Organization; 1989.
- WHO. Evaluation of certain food additives and contaminants. WHO Technical Report Series; No 837. Geneva: World Health Organization; 1993.
- Yasar U, Ozyigit II, Serin M. Judas tree (Cercis siliquastrum L. subsp. siliquastrum) as a possible biomonitor for Cr, Fe and Ni in Istanbul (Turkey). Rom Biotech Lett. 2010;15(1):4983–4992.
- Yasar U, Ozyigit II. Use of human hair as a potential biomonitor for zinc in the Pendik District Istanbul Turkey. Rom Biotech Lett. 2009;14(3):4477–4484.
- Kulshreshtha S, Mathur N, Bhatnagar P, et al. Bioremediation of industrial waste through mushroom cultivation. J Environ Biol. 2010;31(4):441–444.
- Prasad NMV, Freitas HMO. Metal hyperaccumulation in plants-biodiversity prospecting for phytoremediation technology. Electron J Biotechnol. 2003;6(3):285–321.
- Akguc N, Ozyigit II, Yarci C. Pyracantha coccinea Roem. (Rosaceae) as a biomonitor for Cd, Pb and Zn in Mugla province (Turkey). Pak J Bot. 2008;40(4):1767–1776.
- Aksoy A, Osma E, Leblebici Z. Spreading pellitory (Parietaria judaica L.): a possible biomonitor of heavy metal pollution. Pak J Bot. 2012;44:123–127.
- Malakootian M, Nouri J, Hossaini H. Removal of heavy metals from paint industries wastewater using Leca as an available adsorbent. Int J Environ Sci Technol. 2009;6(2):183–190.
- Wittig R. General aspects of biomonitoring heavy metals by plants. In: Markert B, editor. Plants as biomonitors indicators for heavy metals in the terrestrial environment. Weinheim: VCH Publisher; 1993. p. 3–28.
- Davis PH. Flora of Turkey and the East Aegean Islands. Vol. 1-9. Edinburg: Edinburg University Press; p. 1965–1985.
- El-Alfy TSM, El-Gohary HMA, Sokkar NM. et.al. Botanical and genetic characteristics of Celtis australis L. and Celtis occidentalis L. grown in Egypt. Bull Fac Pharm, Cairo University. 2011;49(1):37–57.
- Altay V, Ozyigit II, Yarci C. Urban flora and ecological characteristics of the Kartal District (Istanbul): A contribution to urban ecology in Turkey. Sci Res Essays. 2010;5(2):183–200.
- Tuik. The Official Web Site of Turkish Statistic Institution [Internet]. 2016. [cited 2016 Aug]. Available from: http://www.turkstat.gov.tr/
- Özdemir H, Borucu G, Demir G. et. al. İstanbul'daki Çocuk Oyun Parklarında Partikül Madde (PM2,5 ve PM10) Kirliliğinin incelenmesi [Investigation of particulate Matter (PM2.5 and PM10) pollution in children's playgrounds in Istanbul]. Ekoloji. 2010;20(77):72–79. Turkish.
- Kottek M, Grieser J, Beck C, et al. World map of the Köppen-Geiger climate classification updated. Meteorologische Zeitschrift. 2006;15(3):259–263.
- Akman Y. İklim ve Biyoiklim [Climate and Bioclimate]. Ankara: Palme Yayın-Dağıtım; 1990. Turkish.
- Altay V, Ozyigit II, Yarci C. Urban ecological characteristics and vascular wall flora on the Anatolian side of Istanbul, Turkey. Maejo Int J Sci Technol. 2010;4(3):483–495.
- DM I. The Official Website of Turkish State Meteorological Service [Internet]. 2016. [cited 2016 Aug]. Available from: https://www.mgm.gov.tr
- Maikhuri RK, Semwal RL, Rao KS, et al. Growth and ecological impacts of traditional agroforestry tree species in Central Himalaya, India. Agroforest Syst. 2000;48:257–272.
- Ota A, Višnjevec AM, Vidrih R, et al. et.al. Nutritional, antioxidative, and antimicrobial analysis of the Mediterranean hackberry (Celtis australis L.). Food Sci Nutr. 2017;5(1):160–170.
- Baytop T. Türkçe Bitki Adlari Sözlügü [A Dictionary of vernacular names of wild plants of Turkey], No. 578. Ankara: The Turkish Language Society; 1994.
- Ross SM. Sources and forms of potentially toxic metals in soil-plant systems. In: Ross SM, editor. Toxic metals in soil-plant systems. Chicester: John Wiley; 1994. p. 3–25.
- Kabata-Pendias A, Pendias H. Trace elements in soils and plants. Boca Raton: CRC Press; 2001. p. 73–98.
- Allaway WH. Agronomic controls over the environmental cycling of trace elements. Adv Agronom. 1968;20:235–274.
- Bowen HJM. Environmental chemistry of the elements. London: Academic Press; 1979.
- Blum WEH, Horak O, Mentler A, et al. Trace elements. In: Sabljic A. editor. Environmental and ecological chemistry. Vol. 2. Oxford: UNESCO – EOLSS; 2012. p. 156–164.
- Kabata-Pendias A, Wiacek K. Effects of sulphur deposition on trace metal solubility in soils. Environ Geochem Health. 1986;8(4):95–98.
- Baycu G, Tolunay D, Ozden H. et.al. Ecophysiological and seasonal variations in Cd, Pb, Zn, and Ni concentrations in the leaves of urban deciduous trees in Istanbul. Environmental Pollution. 2006;143(3):545–554.
- Yetimoglu EK, Ercan O, Tosyali K. Heavy metal contamination in street dusts of Istanbul (Pendik to Levent) E-5 highway. Annali di Chimica. 2007;97(3–4):227–235.
- Garba ST, Ahmed I, Akan JC, et al. Distribution pattern of the heavy metals: Pb, Zn, Cd and Cu in roadside soils of Maiduguri Metropolis, Borno State Nigeria. Int Res J Pure Appl Chem. 2014;4(5):486–493.
- Bozdogan E. Heavy metal concentration in leaves of Melia azedarach as a biomonitor of traffic-related air pollution. Oxid Commun. 2016;39(1):756–764.
- Štrbac S, Gavrilović M, Budakov L. Bioaccumulation of metals in the trees of Novi Sad, Serbia. J Toxicol Env Heal A. 2016;79(18):804–807.
- Motto HL, Danies RP, Chilko DM. et.al. Lead in soils and plants: Its relationship to traffic volume and proximity to highways. Environ Sci Technol. 1970;4:231–237.
- Fergusson JE, Kim ND. Trace elements in street and house dusts: sources and speciation. Sci Total Environ. 1991;100:125–150.
- Baker DE, Senft JP, Copper AB. Heavy metals in soils. London: Blackie Academic & Professional; 1995. p. 179–205.
- Dogan Y, Baslar S, Ugulu IA. Study on detecting heavy metal accumulation through biomonitoring: Content of trace elements in plants at Mount Kazdagi in Turkey. Appl Ecol Environ Res. 2014;12(3):627–636.
- Grigoratos T, Martini G. Brake wear particle emissions: a review. Environ Sci Pollut R. 2015;22(4):2491–2504.
- Albasel N, Cottenir A. Heavy metal contamination near major highways, industrial and urban areas in Belgian grassland. Water Air Soil Poll. 1985;24(1):103–110.
- Aksoy A, Ozturk M. Phoenix dactylifera L. as a biomonitor of heavy metal pollution in Turkey. J Trace Microprobe T. 1996;14(3):605–614.
- Aksoy A, Sahin U. Elaeagnus angustifolia L. as a biomonitor of heavy metal pollution. Turk J Bot. 1999;23:83–87.
- Aksoy A, Sahin U, Duman F. Robinia pseudo-acacia L. as a possible biomonitor of heavy metal pollution in Kayseri. Turk J Bot. 2000;24:279–284.
