ABSTRACT
Medium-term storage for some medicinal plant species collected from Taif governorate was developed. First, the establishment of in vitro propagation system for Caper (Capparis spinosa L) and Lavender (Lavandula dentata L.) plants was studied using axillary buds as explants. Murashige and Skoag (MS) salts with different concentrations and combinations of auxins and cytokinens were used. Second, Shoot tips and nodal buds from in vitro culture plants (C. spinosa L, L. dentata L and Rhazya stricta Decne) were used as explants for in vitro conservation experiments. Eighteen different treatments were used as slow growth medium. After 12 months from conservation, in R. stricta Decne and C. spinosa L a highest percentage of survival rates (91.1% and 93.33%), respectively, were observed on MS + 10 g/L sucrose + 10 g/L sorbitol. However, in L. dentata L the highest percentage of survival rate (90%) was noted on MS + 15 g/L sucrose +10 g/L sorbitol. Random amplified polymorphic DNA (RAPD) analysis indicated high genetic stability of preserved plant species under investigation. These results suggested that, in vitro conservation using full strength MS salts with low concentrations of sucrose and sorbitol as a carbon source and osmotic agent, respectively, is a suitable slow growth medium for in vitro conservation of C. spinosa L, L. dentata and R. stricta Decne plants.
Introduction
In the flora of Saudi Arabia, there are 242 endemic and 600 rare and endangered species in the wild [Citation1]. Comprehensive studies on the desert flora in Saudi Arabia have been performed [Citation2].
Taif governorate is a high-altitude region, situated at about 2500 m above sea level in the western mountains of Saudi Arabia. In this region, about 261 species have been recorded, of these, 165 species have medicinal value [Citation3] that means it has a large number of medicinal plants that need to be surveyed.
Advances in biotechnology provide some important tools for conservation and management of plant genetic resources using tissue culture techniques and molecular biology. In vitro culture is an efficient method for ex situ conservation of medicinal and economically important plants [Citation4]. Several ex situ modern techniques for storage of vegetative propagated species have already been developed [Citation5].
Slow growth is one of the major tissue culture techniques used for storage of in vitro grown plants by restrict growth of in vitro materials [Citation6] and to increase the intervals between subcultures by various means, i.e. reduced temperature in the presence of growth retardants (i.e. abscisic acid), reduction in sucrose concentrations and/or addition of osmotically active additives (i.e. sorbitol and mannitol) under either low light intensity or complete darkness [Citation7] and reduction of the chemical compounds of the nutrient substance [Citation8]. The slow growth technique was applied to conserve germplasm of different plant species, i.e. Garcinia indica [Citation9], Glycyrrhiza glabra L. [Citation10], pear and apple [Citation11], and Tetrastigma hemsleyanum [Citation12].
The most important pre-requisite in micropropagation of plant species is a true-to-type clonal stability. Polymerase chain reaction (PCR)-based techniques such as random amplified polymorphic DNA (RAPD) and inter-simple sequence repeat are useful in analysis of genetic uniformity of micropropagated plantlets in many plant species because they are cost-effective, fast, simple, reliable. In addition, a small quantity of DNA sample is required that do not need any prior sequence information to design the primers and unlike restriction fragment length polymorphism they do not use radioactive probes [Citation13]. Some of wild medicinal plants were collected from Taif governorate and were studied in the present research.
Capparis spinosa L. is a perennial crop wild plant belongs to the Capparidaceae family native to the Mediterranean region [Citation14]. It has been known for centuries as condiment [Citation15]. Nowadays unopened flowers or young fruits are harvested from cultivated C. spinosa plants and are used in many traditional dishes and it has become a valuable and specialized crop of great economic importance in the Mediterranean region for local market and for export [Citation16].
Lavandula dentata L. is a small evergreen shrub that belongs to Lamiaceae family and is one of the important medicinal and aromatic plants [Citation17,Citation18]. It is also used as ornamental and melliferous plant. The essential oil of L. dentata L is widely used commercially in the fragrance industry [Citation19], food manufacturing [Citation20], as a relaxant in aromatherapy [Citation21] and as a therapeutic agent due to sedative, spasmolytic, antiviral and antibacterial activities [Citation22]. In vitro proliferations of several Lavandula species have been reported [Citation23].
Rhazya stricta Decne belongs to the family Apocynaceae. It is a glabrous, erect shrub [Citation24], distributed throughout western Asia to the north-west province of India and abundantly found in various regions of Pakistan. It is widely used in folk medicine [Citation25]. Rhazya stricta aqueous extract has been reported to have antimicrobial activity [Citation26,Citation27], central nervous system depressant properties and mutagenic activities of leaf aqueous extract on a wide range of cell types [Citation25]. There are few studies on application of in vitro propagation of R. stricta [Citation28], evaluation of genetic stability of in vitro propagated R. stricta plants [Citation29], establishment of transgenic R. stricta hairy roots modulate terpenoid indole alkaloid production [Citation30].
The objectives of the study were to establish a micropropagation system; preserve some economically and medicinally important wild plant species using a slow growth technique and evaluation of genetic stability of preserved plant germplasm. To our knowledge, the present study is the first to report for medium-term preservation of Harmal (R. stricta Decne).
Materials and methods
Plant materials and explants preparation
Juvenile nodal buds of some collected plant germplasm (C. spinosa L, L. dentata L and R. stricta Decne), from Al Hada and El Shafa regions in Taif, Saudi Arabia, were excised from wild plants and used as explants. The explant materials subsequently were surface sterilized in 20% of sodium hypochlorite (NaOCl) solution containing drops of (Tween-20) for 20 min, then it was rinsed three to four times with sterile distilled water (s.d. H2O). The sterilized explant materials were used for in vitro culture experiments.
Establishment of in vitro propagation
Culture media preparation and culture conditions
Murashige and Skoag [Citation31] (MS) medium with 3% (w/v) sucrose, 0.7% (w/v) phytoagar and different concentrations and combinations of cytokinins and auxins were used for in vitro culture stages. Before adding phytoagar, the pH was adjusted to 5.7 using 1.0 N potassium hydroxide and 1.0 N hydrochloric acid. Culture media were autoclaved for 20 min at 121 °C and 1.5 k/cm2 pressure. MS medium without growth regulators was used as a control for all in vitro culture treatments. Incubation conditions 25 ± 2 °C, 16/8 h light/dark and 3000 Lux light intensity (fluorescent light) were used for in vitro culture stages.
In vitro culture
Surface sterilized nodal bud explants of (C. spinosa L and L. dentata L) were cultured for three to four weeks in shoot initiation medium containing full strength MS medium supplemented with different concentrations and combinations of BA, IBA and NAA + 3% sucrose + 0.7% (w/v) phytoagar (). The explants which formed Shoots were subcultured on the same fresh media for three to four weeks. For shoot multiplication and elongation, induced shoots were subcultured on the same media for another three to four weeks. This step was repeated 3–4 times on the same medium for mass production. For rooting stage, elongated shoots (3–4 cm length) were transferred in half and full strength of MS media supplemented with different concentrations of NAA for a further three to four weeks (). For acclimatization stage, good rooted plantlets were carefully washed with warm H2O to remove adhered agar and traces of the medium; then they were transplanted to plastic pots diameter (15 cm) containing sterile peat moss under greenhouse conditions. The top of the pots was covered with transparent plastic.
Table 1. Effect of different concentrations and combinations of BA, IBA and NAA on shoot initiation, multiplication and elongation stages of C. spinosa and L. dentata plants.
Table 2. Effect of medium strength and different concentrations of NAA on root formation stage of C. spinosa and L. dentata plants.
In vitro preservation ‘slow growth technique’
Shoot tips and nodal buds from in vitro culture plants of C. spinosa L, L. dentata L and R. stricta Decne, shoot tips and nodal buds of R. stricta Decne were excised from in vitro propagated plants according to El-Tarras et al. [Citation28], and were used as explants for in vitro conservation experiments. Eighteen different treatments of full and half strength of MS salts with different concentrations and combinations of sucrose and sorbitol as a carbon source and osmotic agent, respectively, were used as slow growth media to detect the effect of media strength and kind of carbon source on the responses of growth and survival rates of different plant species (). All in vitro cultures were incubated at the same conditions except the temperature was decreased to 22 ± 2 °C.
Table 3. Effect of MS medium strength, carbon source and osmotic agent on induction of slow growth in C. spinosa L, L. dentata and R. stricta Decne plants after 12 months from preservation.
Statistical analysis
All experiments were carried out in three replicates. For in vitro culture, data were collected from different experiments four weeks after culture. For in vitro preservation, data for two parameters, height of regenerated shoots and percentage of survival rates were collected every two months of preservation. A statistical analysis of data, analysis of variance and the comparison between the mean value of treatments were carried out using Duncan Test at a level of 5% of probability (.01 = < p <.05), using Statistical Assistance Software (ASSISAT) Version 7.6 beta (2014).
Genetic stability of preserved germplasm
DNA isolation
After 12 months of preservation on slow growth medium, total genomic DNA was isolated from leaf tissues from a high percentage survival rate treatment and none preserved plant as control using the cetyl trimethyl ammonium bromide method [Citation32]. The quality of DNA was determined through electrophoresis on 1% agarose gel.
Random amplified polymorphic DNA (RAPD)
Fifteen RAPD primers were screened. Amplification reaction was performed with 25 μL volume containing Tris-HCl 10 mmol/L, KCl 50 mmmol/L, MgCl2 1.5 mmmol/L, Taq DNA polymerase 0.6 U, dNTP 0.2 mmmol/L of each, RAPD primer 10 pmoles and DNA template 20 ng. PCR reaction, an initial denaturation cycle for 3 min at 94 °C followed by 39 cycles comprising 1 min at 94 °C, 1 min at 35 °C and 2 min at 72 °C. An additional cycle of 5 min at 72 °C was used for final extension. Amplification products were separated by electrophoresis in 1.5% agarose gels and stained with ethidium bromide. A photographic record was taken under UV gel doc system.
Results and discussion
Establishment of in vitro propagation
Axillary bud explants of C. spinosa L and L. dentata L plants started to germinate after one week from culture on MS medium supplemented with different concentrations of BAP, IBA and NAA.
In C. spinosa L, no significant variations were noticed between shoot initiation medium free growth regulators and that supplemented with BA alone and with IBA, the percentages of the average number of explants produced shoots ranged between (83.3% and 100%). On the other hand, the average number of initiating shoots decreased with the increase of BA concentration 0.5–2 mg/L in the presence of 0.5 mg/L NAA (). The highest and lowest average number of shoots/explant (5.17 and 1.47) were produced on medium fortified with 0.5 mg/L BA + 0.5 mg/L IBA and 1.5 mg/L BA + 0.5 mg/L IBA, respectively, and the highest average number (1.54) of elongated shoots was noticed on 0.5 mg/L BA ( and A,B)). As shown in , the elongated shoots were rooted on all treatments and the highest percentage of the average number of rooted shoots (56.7%) was observed on half-strength MS medium without NAA and full MS with 1.5 mg/L NAA ((C)).
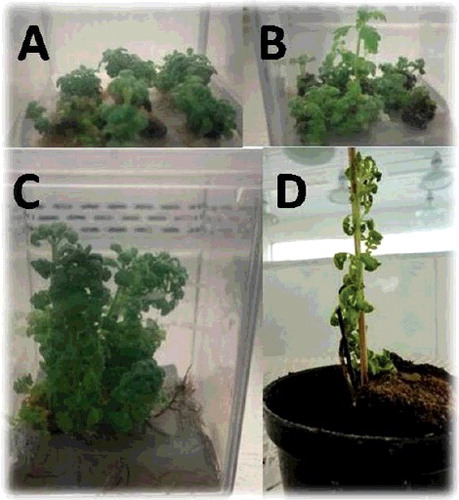
Figure 1. In vitro propagation stages of Caper (C. spinosa L). (A) Shoot multiplication after four weeks on MS fortified with 0.5 mg/l BA + 0.5 mg/l IBA. (B) Elongated shoots were noticed after four weeks from sub-culture on MS supplemented with 0.5 mg/l BA. (C) Rooted plantlets on half-strength MS without NAA. (D) Acclimatized plantlet under greenhouse conditions.

In vitro propagation of C. spinosa has been reported in different studies, Rodriguez et al. [Citation33] demonstrated that a reduced concentration of BAP (2 μmmol/L/) induced plantlet regeneration on single shoots. Culturing nodal shoot segments of C. spinosa in the presence of 4 μmmol/L BAP, 0.3 μM/l IAA and 0.3 μmmol/LGA3 achieved 20-fold multiplication per 20 days [Citation33]. Musallam et al. [Citation34] reported that multiple shoot production of C. spinosa was obtained at woody plant medium supplemented with 0.8 mg/L Kin, 0.05 mg/L IBA and 1.0 mg/L GA3. According to Al-Mahmood et al. [Citation35], maximum root formation (75%) was produced at 0.6 mg/L IBA, when charcoal was added to the medium at 0.5 g/L. Higher rooting responses (70%) were obtained after a 20-day incubation period in darkness on solid half-strength MS fortified with 30 μmmol/LIAA. High rooting response of shoots (95%) were observed after a 4 h pulse treatment period in darkness with 100 mg/L IAA solution, followed by culture on solid half-strength MS basal medium [Citation36]. As shown in , in both half and full strength MS medium, the increase of the average number of shoots formed callus was noticed according to the increase of NAA concentrations. This result is in agreement with the previous studies by Rodríguez et al. [Citation33] and Musallam et al. [Citation34]. They found that NAA was the most inducing to callus, microshoot formed callus failed to develop functional roots, and roots differentiated in callus do not have a vascular connection with the stem.
In L. dentata L, a high percentage of average number of explants produced shoots (90%) were noticed in shoot initiation medium with 0.5 mg/L BA + 0.5 mg/L IBA and 1.5 mg/L BA + 0.5 mg/L IBA (). For shoot multiplication, as shown in , average number of shoots/explants were increased from (2–8 shoots/explant), the highest average number of shoots/explant (8) was produced on MS medium with 2 mg/L BA + 0.5 mg/L IBA ((A)), the lowest average number of shoots/explants (2) was observed on medium containing 0.5 and 1 mg/L BA. The highest average number of elongated shoots (1.63) was obtained on medium supplemented with 1.5 mg/L BA ((B)). The results showed that rooting medium containing half-strength MS with 0.5 and 1.5 mg/L NAA produced a highest average number of rooted shoots (27, 26), respectively ( and (C)).
Figure 2. In vitro propagation stages of Lavender (L. dentata L). (A) Shoot multiplication after four weeks on MS with 2 mg/l BA + 0.5 mg/l IBA. (B) Elongated shoots were noticed after four weeks from sub-culture on MS supplemented with 1.5 mg/l BA. (C) Rooted plantlets on half-strength MS 0.5 mg/L. (D) Acclimatized plantlet under greenhouse conditions.
Previous studies on in vitro propagation of some Lavandula species demonstrated that MS medium was superior for L. dentata L [Citation37]. The efficiency of BA on shoot multiplication was also shown for different Lavandula species [Citation23,Citation37,Citation38]. Echeverrigaray et al. [Citation38] reported that the highest multiplication rate for in vitro propagated L. dentata plants was obtained using MS medium supplemented with a combination of 2.2 μmol/L BAP and 2.5 μmol/LIBA. As shown in , number of shoots/explant increased with the addition of BA to the culture medium, this result is in contrast with the result obtained by Zuzarte et al. [Citation23]. They demonstrated that the highest number of shoots was noticed when nodal segment explants of L. pedunculata were cultured on MS medium supplemented with 0.25 mg/L BA and a higher percentage of rooting (73.16%) occurred when elongated shoots were cultured on MS medium without auxins. In vitro rooting of L. stoechas shoots resulted in 100% rooting on MS medium contained 1.0 mg/L NAA [Citation39].
The authors observed that, during the in vitro culture experiments, vitrified shoot numbers were increased, according to the increase of BAP concentrations. In C. spinosa L, as shown in the highest number of vitrified shoots (7) was observed on medium supplemented with 1.5 mg/L and 2 mg/L of BA in the presence of 0.5 mg/L NAA. However, in L. dentata L the highest number of vitrified shoots (13) was noticed on medium containing 1.5 mg/L BAP + 0.5 mg/L IBA (). Effect of high concentration of BA on shoot vitrification was studied earlier in different reports [Citation40]. High percentage of vitrified shoots was observed on medium containing 1.0 mg/L BA [Citation41]. Vitrification could be represented by poorly developed vascular bundles, abnormal functioning and stomata abnormal wax quality [Citation42] and it is the consequence of culture conditions [Citation43]. Good rooted plantlets of C. spinosa and L. dentata L were successfully acclimatized in greenhouse with survival rates 65% and 80%, respectively, as shown in (D) and (D).
Chalak et al. [Citation44] successfully rooted shoots of C. spinosa but the survival rate of acclimatized plantlets was only 40%. According to Al-Mahmood et al. [Citation35], survival rate of 85% was achieved, when plantlets of C. spinosa were transferred for acclimatization under greenhouse conditions. Survival rates of acclimatized plants ranged (50%–94%) for different Lavandula species [Citation37,Citation38].
In vitro preservation ‘slow growth technique’
In C. spinosa L, as shown in , the highest percentage of survival rate (93.33%) was noticed on MS medium + 20 g/L sucrose and MS medium + 10 g/L sucrose + 10 g/L sorbitol, with a plant height average (13.03 and 11.1 cm) in the case of axillary buds and shoot tips, respectively.
In L. dentata L, the result shown that MS medium with 15 gm/L sucrose + 15 gm/L sorbitol showed a highest percentage of survival rate (90%) with average of plant height (2.0 cm) and (88.73%) with average of plant height (1.85 cm) in the case of shoot tips and axillary bud, respectively ().
In R. stricta Decne, as shown in , in the case of shoot tips, the highest percentage of survival rates (91.1% and 71.1%) and mean average of plant height (3.30 and 3.26 cm) were observed after 12 months from preservation on slow growth medium fortified with 10 g/L sucrose + 10 g/L sorbitol and 20 g/L sucrose + 10 g/L sorbitol, respectively. However, in the case of axillary bud explants, the highest percentage of survival rate (61.1%) and mean average of plant height (3.51 cm) were noticed on MS medium with 10 gm/L sucrose + 10 gm/L sorbitol.
Slow growth is a very simple method for germplasm conservation and has been reported in several studies [Citation35,Citation44–46]. Divakaran et al. [Citation45] demonstrated that addition of mannitol (10–15 g/L) and reduction of sucrose to lower level (15–10 g/L) induced slow growth of Vanilla planifolia cultures and subsequently 80%–90% of the cultures could be maintained for a period of 360 days. Survival of C. spinosa plantlets was 100% after four months with 3% sorbitol, if stored in the light conditions, however survival and re-growth rates of plantlet decreased according to the increase of sorbitol concentration up to 12% [Citation35]. Decreased shoot height of African violet (Saintpaulia ionantha wendl.) was noticed when sorbitol was used as an osmotic agent [Citation44]. In full MS treatment as shown in , the increase of sucrose and sorbitol resulted in a decrease in the percentage of survival rates and plant height for both axillary buds and shoot tips. Addition of sucrose (0–30 g/L) in full MS storage medium resulted in overgrowth of Vanilla planifolia cultures [Citation45]. According to Tahtamoni et al. [Citation47], high concentrations of osmotic agents in the medium cause a negative water potential and reduce the optimal turgor pressure needed for cell division and inhibit growth.
Genetic stability of preserved germplasm
RAPD analysis
The PCR amplification products of control plant and three in vitro preserved treatments (C. spinosa L. and L. dentata L) and two in vitro preserved treatments of R. stricta Decne were plotted together for comparison. A total of 15 random RAPD primers were tested for initial screening, among them only 5 primers gave clear and reproducible loci.
RAPD analysis of C. spinosa L
The number of scorable loci for each RAPD primer varied from 4 (OPG-09) to 7 (OPC-07, OPE-03 and OPG-03). The 5 RAPD primers produced 121 total scorable loci, with an average of 4–7 loci per primer. Each primer generated a set of amplification products ranging in size from 490 to 2250 bp. The maximum number of 7 loci was present within the size range of 580–2250 bp at the primer (OPC-07), followed by 4 loci of less than 475 bp in size to highest 1495 bp at the primer (OPG-09).
RAPD analysis of L. dentata L
The number of scorable loci for each RAPD primer varied from 4 (OPF-05) to 13 (OPB-03). The 5 RAPD primers produced 179 total scorable loci, with an average of 4–13 loci per primer. Each primer generated a set of amplification products ranging in size from 162 to 3000 bp. The maximum number of 13 loci was present within the size range of 270–2820 bp, followed by 4 loci of less than 600 bp in size to highest 1720 bp at the primer (OPG-10). The banding reveals that the plantlets after preservation were identical. However, some bands were absent in some other treatments.
RAPD analysis of R. stricta Decne
The number of scorable loci for each RAPD primer varied from 5 (OPC-03) to 8 (OPB-05). The 5 RAPD primers produced 138 total scorable loci, with an average of 5–8 loci per primer. Each primer generated a set of amplification products ranging in size from 100 bp (OPB-05) to 2300 bp (OPB3). The maximum number of 8 loci was present within the size range of 100–2000 bp, followed by 5 loci of less than 300 bp in size to highest 1000 bp at the same primer (OPC-03). The banding reveals that the plantlets after preservation were identical. However, some bands were absent in some other treatments. A pair wise Jaccard's similarity values between preserved and non-preserved plants ranged from 84% to 96% (average 90%), 88% to 96% (average 92%) and 91% to 97% (average 94%) in C. spinosa L, L. dentata L and R. stricta Decne (–), respectively.
Figure 3. Polymerase chain reaction (PCR) amplification products obtained with a random amplified polymorphic DNA (RAPD) of Caper (Capparis spinosa). (a) Primer (OPC-07) and (b) Primer (OPG-03). Lane M – 1 kb DNA Marker (500 bp to 10 kbp), Lane Nb represents control (none preserved) plant and lanes 3, 6 and 7 represent preserved plantlets.
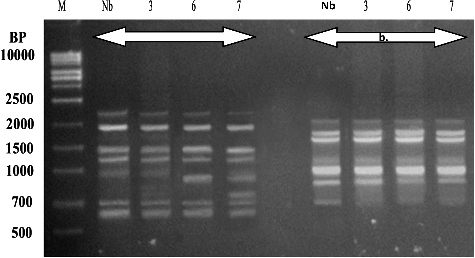
Figure 4. Polymerase chain reaction (PCR) amplification products obtained with a random amplified polymorphic DNA (RAPD) of Lavender (L. dentata L.). (a) Primer (OPG-10) and (b) Primer (OPF-05). Lane M – 1 kb DNA Marker (500 bp to 10 kbp), Lane Lb represents control (none preserved) plant and lanes 6 and 12 represent preserved plantlets
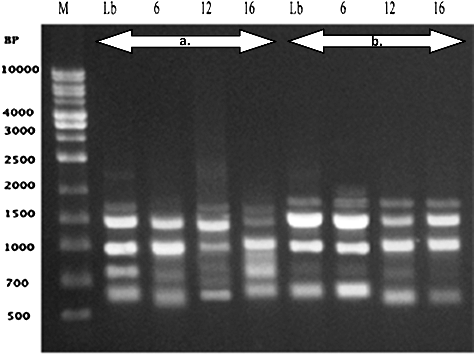
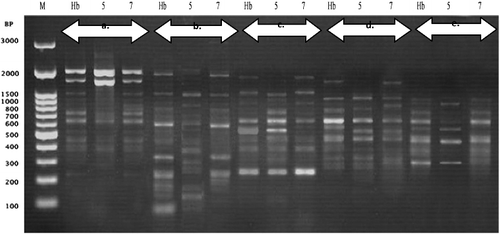
Figure 5. Polymerase chain reaction (PCR) amplification products obtained with a random amplified polymorphic DNA (RAPD) of Harmal (R. stricta Decne). (a) Primer (OPB-03), (b) Primer (OPB-05), (c) Primer (OPB-10), (d) Primer (OPC-02) and (e) Primer (OPC-03). Lane M molecular marker (100 bp to 3 kbp), Lane Hb represents control (none preserved) plant and lanes 5 and 7 represent preserved plantlets.
Our results corroborate the reports of genetic stability of preserved dormant buds of Morus germplasm [Citation48]. These results are in agreement with those by Saker et al. [Citation49] who mentioned that no significant variations were observed in tissue cultures derived date palm plantlets, RAPD analysis showed genetic variation in only 4% of analysed plants (70 regenerants), which were incubated for 6–12 months under 25 ˚C.
Conclusion
In this study, in vitro propagation systems for C. spinosa L and L. dentata L plants were established using axillary buds as explants. MS medium with different concentrations and combinations of auxin and cytokinen were used in different in vitro culture stages. Full strength MS with low concentrations of sucrose and sorbitol as a carbon source and osmotic agent, respectively, is a suitable slow growth medium for in vitro conservation and RAPD analysis indicated high genetic stability of preserved plant species under investigation. Further work will be carried out to survey and collect economically and medicinally important wild plants, from different regions in Taif province, for both in vitro propagation and preservation of plant germplasm
.
Acknowledgement
Authors would like to thank Vice – Presidency of Graduate Studies and Academic Research – Taif University for financial support of the project (ex situ preservation for endemic and rare medicinal plants in Taif governorate) [project number 1-435-3586].
Disclosure statement
All authors named in the manuscript have no conflict of interest; they are entitled to the authorship and have approved the final version of the submitted manuscript.
Additional information
Funding
References
- Rahman MA, Mosa JS, Al-Said MS, et al. Medicinal plant diversity in the flora of Saudi Arabia 1: a report on seven plant families. Fetoterapia. 2004;75: 149-161
- Abd El-Ghani M. Phenology of ten common plant species in western Saudi Arabia. J Arid Environ. 1997;35:673–683.
- Al-Sodany YM, Bazaid SA, Mossalam H A. Medicinal plants in Saudi Arabia: I. Sarrwat mountains at Taif, KSA. Acad J Plant Sci. 2013;6:134–145.
- Fay MF. In what situation is in vitro culture appropriate to plant conservation? Biodiv Conserv. 1994;3:176–183.
- Benson E, Danaher JE, Pimbley IM, et al. In vitro micropropagation of Primula scotia: a rare Scottish plant. Biodivers Conserv. 2000;9:711–726.
- Engelmann F. In vitro conservation methods. In: Callow JA, Ford-Lloyd BY, Newbury HJ, editors. Biotechnology and plant genetic resources. CABI Wallingford. U.K.; 1997. p. 119–161.
- Zandvoort EA, Hulshof MJH, Staritsky G. In vitro storage of Xanthosoma spp. under minimal growth conditions. Plant Cell Tiss Org Cult. 1994;36:309–316.
- Engelmann F. In vitro conservation of tropical plant germplasm – a review. Euphytica. 1991;57:227–243.
- Malik SK, Chaudhary R, Kalia RJ. Rapid in vitro multiplication and conservation of Garcinia indica: a tropical medicinal tree species. Sci Hortic. 2005;106:539–553.
- Uzundzhalieva K, Ruseva R, Kachakova S. Ex situ and in vitro conservation of Glicyrrhiza glabra L. Crop wild relative from Fabaceae. Ecologia Balkanica.2014;5:9–13.
- Sedlak J, Paprstein F, Bilavcik A, et al. Adaptation of apple and pear plants to in vitro conditions and to low temperature. Acta Hortic. 2001;560:457–460.
- Peng X, Zhang TT, Zhang J. Effect of subculture times on genetic fidelity, endogenous hormone level and pharmaceutical potential of Tetrastigma hemsleyanum callus. Plant Cell Tiss Org Cult. 2015;122:67–77. DOI:10.1007/s11240-015-0750-2
- Lakshmanan V, Venkataramareddy SR, Neelwarne B. Molecular analysis of genetic stabilityin long-term micropropagated shoots of banana using RAPD and ISSR markers. Electron J Biotechnol. 2007;10:1–8.
- Vidaeus L. Jordan – conservation of medicinal and herbal plants project. The World Bank; 2002. Available from:http:/www.gefweb.org.
- Sozzi C. Capper and caperberies. In: Peter V, editor. Handbooks of herb and spices. 1st ed. New York (NY): CRC Press; 2006. p. 235–237.
- Carra A, Sajeva M, Abbate L, et al. In vitro plant regeneration of caper (Capparis spinosa L.) from floral explants and genetic stability of regenerants. Plant Cell Tiss Organ Cult. 2012;109:373–381.
- Nobre J. In vitro cloning and micropropagation of Lavandula stoechas from field-grown plants. Plant Cell Tiss Org Cult. 1996;46:151–155.
- Sudriá C, Piñol MT, Palazon J, et al. Influence of plant growth regulators on the growth and essential oil content of cultured Lavandula dentata plants. Plant Cell Tiss Org Cult. 1999;58:177–184.
- Paul JP, Brophy JJ, Goldsack RJ, et al. Analysis of the volatile components of Lavandula canariensis L. Mill., a Canary Islands endemic species, growing in Australia. Biochem Syst Ecol. 2004;32:55–62.
- Kim NS, Lee DS. Comparison of different extraction methods for the analysis of fragrances from Lavandula species by gas chromatography–mass spectrometry. J Chromatogr A. 2002;982:31–47.
- Ghelardini C, Galeotti N, Salvatore G, et al. Local anaesthetic activity of the essential oil of Lavandula angustifolia. Planta Med. 1999;65:700–703.
- Gamez MJ, Jimenez J, Navarro C, et al. Study of the essential oil of Lavandula dentata L. Pharmazie. 1990;45:69–70.
- Zuzarte MR, Dinis AM, Cavaleiro C, et al. Trichomes, essential oils and in vitro propagation of Lavandula pedunculata (Lamiaceae). Ind Crop Prod. 2010;32:580–587.
- Western AR. The flora of the United Arab Emirates: an introduction. Dubai: Al Bayan Commercial Printing Press, United Arab Emirates University; 1989.
- Baeshen NA, Lari SA, Aldoghaither HA, et al. Biochemical evaluation of the effect of Rhazya stricta aqueous leaves extract in liver and kidney functions in Rats. Nature Sci. 2010;8(4):136–142.
- Bashir AK, Abdalla AA, Hassan ES, et al. Alkaloids with antimicrobial activity from the roots of Rhazya stricta Dence growing in United Arab Emirates. Arab Gulf J Sci Res. 1994;12:119–131.
- Bashir AK, Abdalla AA, Wasfi IA, et al. Phytochemical and antimicrobial studies on the leaves of Rhazya stricta growing in United Arab Emirates. Fitotrepia. 1994;65(1):84–85.
- El-Tarras A, El-Awady AM, Attia OA, et al. In vitro multiplication of the important medicinal plant, harmal (Rhazya stricta Decne). J Med Plants Res. 2012;6(19):3586–3590.
- El-Awady MAM, El-Dessoky SD, Attia OA, et al. Evaluation of genetic fidelity of in vitro raised plants of the important medicinal plant harmal (Rhazya stricta Decne) using RAPD and ISSR markers. Int J Agri Sci Res. 2014;4(3):115–124.
- Akhgari A, Yrjönen T, Laakso I, et al. Establishment of transgenic Rhazya stricta hairy roots to modulate terpenoid indole alkaloid production. Plant Cell Rep. 2015;34(11):1939–1952.
- Murashige T, Skoog F. A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant. 1962;15:473–479.
- Doyle JJ, Doyle JL. A rapid total DNA preparation procedure for fresh plant tissue. Focus. 1990;12:13–15.
- Rodríguez R, Rey M, Cuozzo L, et al. In vitro propagation of caper (Capparis spinosa L.). In Vitro Cell Dev Biol. 1990;26(5):531–536.
- Musallam I, Duwayri M, Shibli R. Micropropagation of caper (Capparis spinosa) from wild plants. Fun Plant Sci Biotechnol. 2011;5:17–21.
- Al-Mahmood HJ, Shatnawi MA, Rida AS, et al. Clonal propagation and medium-term conservation of Capparis spinosa: a medicinal plant. J Med Plants Res. 2012;6(22):3826–3836.
- Chalak L, Elbitar A. Micropropagation of Capparis spinosa L. subsp rupestris Sibth and Sm. by nodal cuttings. Indian J Biotechnol. 2006;5:555–558.
- Jordan AM, Calvo MC, Segur J. Micropropagation of adult Lavandula dentata plants. J Hortic Sci Biotechnol. 1998;73:93–96.
- Echeverrigaray S, Basso R, Andrade LB. Micropropagation of Lavandula dentata from axillary buds of field-grown adult plants. Biol Plant. 2005;49(3):439–442.
- Al-Bakhit AAM, Sawwan JS, Al-Mahmoud MS. In vitro propagation of two Lavandula species: Lavandula angustifolia and Lavandula latifolia L, Medica. J Agric Sci. 2007;3(1):16–25.
- Constantine DR. Micropropagation in the commercial environment. In Withers L, Alderson P G, editors. Plant tissue culture and its agricultural applications. London: Butterworth; 1986. p. 175–186.
- Li Q, Deng M, Zhang J, et al. Shoot organogenesis and plant regeneration from leaf explants of Lysionotus serratus D. Don. Sci World J. 2013; 2013:Article ID 280384. Available from:https://doi.org/10.1155/2013/280384.
- Asghari F, Hossieni B, Hassani A, et al. Effect of explants source and different hormonal combinations on direct regeneration of basil plants (Ocimum basilicum L.). AJAE. 2012;3(1):12–17.
- Genkov T, Ivanova I. Effect of cytokinin-active phenylurea derivatives on shoot multiplication, peroxidase and superoxide dismutase activities of in vitro cultured of carnation. Plant Physiol. 1995;21(1):73–83.
- Moges AD, Karam NS, Shibli RA. Slow growth in vitro preservation of African Violet (Saintpaulia ionantha wendl.) shoot tips. Adv Hortic Sci. 2003;17(4):223–230.
- Divakaran M, Babu KN, Peter KV. Conservation of Vanilla species, in vitro. Sci Hortic. 2006;110(2):175–180.
- Gianní S, Sottile F. In vitro storage of plum germplasm by slow growth. Hortic Sci. 2015;42(2):61–69.
- Tahtamouni RW, Shibli RA, Ajlouni MM. Growth responses and physiological disorders in wild pear (Pyrus syriaca Bioss.) during slow-growth in vitro preservation on osmo stressing media. Plant Tiss Cult. 2001;11(1):15–23.
- Choudhary R, Chaudhury R, Malik SK, et al. Genetic stability of mulberry germplasm after cryopreservation by two-step freezing technique. Afr J Biotechnol. 2013;12:5983–5993.
- Saker MM, Bekheet SA, Taha HS, et al. Detection of somaclonal variations in tissue culture-derived date palm plants using isoenzyme analysis and RAPD fingerprints. Biol Plant. 2000;43:347–351.
