?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
As a class of important cell growth regulators, expansins have been studied for over 20 years. Since Neolamarckia cadamba (Roxb.) Bosser was praised as a ‘miraculous tree’ at the World Forestry Congress in 1972 due to its rapid growth. A lot of research has been carried out to uncover the underlying mechanisms of this rapid growth. Based on previous findings and our research, we hypothesized that expansins may play an important role in such growth. Quantitative real-time polymerase chain reaction (qRT-PCR) analysis revealed that the N. cadamba expansin family member NcEXPA8 is highly expressed in all four young tissues, particularly in the cambium region, suggesting that NcEXPA8 acts as a key regulator of secondary growth in this gene family. Overexpression of NcEXPA8 in Arabidopsis thaliana increased the diameter and height of the main stem. It also promoted interfascicular fiber cell elongation and cell-wall thickness but did not alter the cellulose content in the cell wall. These results suggested that expansins act as activators during secondary fiber cell elongation during tip growth to promote plant growth.
Introduction
Expansin (EXP) is a cell-wall protein that induces pH-dependent cell-wall extension and stress relaxation in a characteristic and unique manner [Citation1,Citation2]. Although the exact mechanism is still unclear, it is hypothesized that EXP disrupts non-covalent bonds (hydrogen bonds) between the cellulose microfibrils and matrix glucans sticking to the microfibrils, thereby allowing turgor-driven polymer creep and softening of plant cell walls [Citation3,Citation4]. Expansins are composed of α-expansin (EXPA), β-expansin (EXPB), expansin-like A (EXPLA) and expansin-like B (EXPLB) subfamilies [Citation5,Citation6]. Since two expansins that induce cell-wall extension were first isolated from the elongation zone of cucumber seedling hypocotyls [Citation7], expansins have been extensively investigated in Arabidopsis thaliana, rice (Oryza sativa), poplar (Populus trichocarpa), maize (Zea mays) and other plant species [Citation7–10]. Several reports have demonstrated that expansins play important roles in different developmental processes. In rice, the expansin gene OsEXPA8 is critical for root system architecture, influencing primary root length and the lateral root number [Citation11,Citation12]. On the other hand, OsEXPA4 is expressed preferentially in the internode elongation zone [Citation13,Citation14], and both overexpression and downregulation of OsEXPA4 alter the coleoptile and mesocotyl length, respectively [Citation14,Citation15]. Similarly, in poplar, PtEXPA1 is abundantly expressed in the cambium [Citation16], and the overexpression of PttEXPA1 promotes internode elongation [Citation17]. In potato (Solanum tuberosum), expression of nine EXPA genes is associated with cell expansion in developing tubers and rapidly growing etiolated stems [Citation18]. In A. thaliana, AtEXPA2, which is exclusively expressed in germinating seeds [Citation19], controls seed germination [Citation20]. In Coffea arabica fruits, CaEXPA1 and CaEXP3 regulate grain size during growth and maturation [Citation21,Citation22]. Expansins are also involved in the stress response [Citation23–25]; however, little is known about the mechanism by which expansins operate in plants during the adaptive responses to adverse stresses.
Neolamarckia cadamba (syn. Anthocephalus chinensis), a member of the Rubiaceae family, is widely distributed throughout South Asia and South China [Citation26]. It has received a praise as ‘a gem of a tree’ in the Philippines [Citation27] and was credited as ‘a miraculous tree’ at the World Forestry Congress in 1972 because of its rapid growth. We previously cloned 16 growth-related genes: NcEXPA1-16 (GenBank accession numbers FJ417847, JF922686–JF922700) [Citation26]. Due to their important roles in cell-wall extension, it is necessary to determine their biological function(s). Here, we investigated the expression patterns of 16 NcEXPAs in different growth tissues and found that NcEXPA8 was preferentially expressed at a high level in the cambium region. Additionally, NcEXPA8 belongs to the secondary xylem-specific expansin subgroup [Citation26], therefore we speculated that NcEXPA8 may play an important role in xylem formation in N. cadamba. To investigate the biological functions of NcEXPA8 during stem growth in plants, we ectopically expressed this gene in A. thaliana and examined the macrostructure variations, such as leaf shape, stem diameter and plant height, as well as the microstructure variations, such as cell-wall thickness, cell area and cell length, and stem component variations (e.g. cellulose content).
Materials and methods
Generation of NcEXPA8-overexpressing Arabidopsis
The NcEXPA8 open reading frame (ORF) was amplified with NcEXPA8-specific primers (forward: 5’-CGggatccATGGCTATTGTGGTTTTGACCT-3’; reverse: 5’-CgagctcCGGTTAGACTCTGAAGTTCTTT CC-3’) using the existing PMD19-NcEXPA8 as the template. BamHI and SacI restriction sites were introduced at the ends of the forward and reverse primers, respectively. The polymerase chain reaction (PCR) products were then subcloned into the PMD19 vector (Takara, Dalian, China), digested with BamHI and SacI restriction enzymes and ligated to the CaMV 35S promoter in sense orientation by insertion of the NcEXPA8 fragments at the BamHI and SacI sites of the pBI121 binary vector. The resulting construct was used to transform A. thaliana ecotype Columbia by the floral dip method [Citation28]. After screening with kanamycin sulphate, transgenic plants were transferred to soil, cultivated at a light intensity of 150 µmol m2 L−1 s−1 under a 16-h light/8-h dark photoperiod and 22 ± 1 °C light/20 ± 1 °C dark in an illumination incubator, and confirmed by PCR analysis using the above-mentioned primers.
Plant materials and growth conditions
Wild-type (Col-0) and NcEXPA8 transgenic homozygous plants of A. thaliana (T2 generation, the second generation of transgenic plants) were cultivated as described above. Ten-day-old stems were collected from wild-type (WT) and T2 homozygous plants. N. cadamba trees were grown in the open filed under natural conditions. Four growth tissues, buds, young leaves, cambium region and young roots were collected from a 1-year-old N. cadamba plant in July in Guangzhou China as previously described [Citation26]. Each tissue was collected from three individual plants for three biological replicates. All samples were immediately cut into pieces and frozen in liquid nitrogen for RNA extraction.
Quantitative real-time polymerase chain reaction (qRT-PCR) analysis
Total RNA from each sample was extracted using the CTAB (cetyl trimethylammonium bromide) method plus the OMEGA Plant RNA Isolation Kit (E.Z.N.A.™ Plant RNA Kit (R6827-01)) [Citation29]. Total RNA (500 ng) was reverse-transcribed into first-strand cDNA using the PrimeScript™ RT Master Mix Kit (Takara, Dalian, China) according to the manufacturer's instructions. The cDNA was diluted 15-fold and used as the template for qRT-PCR. qRT-PCR was performed using the SYBR Premix Ex Taq™ Kit (Takara, Japan) in a final reaction volume of 20 µL, which included 2 µL reverse-transcribed cDNA, 1 µL of 10 µmol/L forward primer, 1 µL of 10 mol/L reverse primer, 10 µL SYBR Premix Ex Taq and 6 µL ddH2O. The thermocycling conditions (CFX96, BIO-RAD, Hercules, USA) were as follows: initial denaturation at 95 °C for 30 s, amplification and quantification at 95 °C for 5 s, 58 °C for 30 s, and 72 °C for 15 s for 40 cycles (with a single fluorescence measurement) and an infinite hold at 10 °C. The specificity of the PCR amplification products was determined using a melting curve program (from 65 to 95 °C with a heating rate of 0.1 °C per second and continuous fluorescence measurement) after the final PCR cycle. The primers used for qRT-PCR are shown in . Cyclophilin (JX902587) and AtPP2AA3 (NM_101 203) [Citation30,Citation31] were used as internal references for N. cadamba and A. thaliana, respectively.
Table 1. Primers used for qRT-PCR analysis.
Stem survey of T2 generation transgenic homozygous plants
On the 10th day after bolting, the stem was harvested for total RNA extraction, according to the OMEGA Plant RNA Isolation Kit. The extracted total RNA was reverse-transcribed into first-strand cDNA as described above. On the 65th day after planting, the stem (5 mm above ground) was cut using a LeicaVT1000S vibratome equipped with a razor blade. The stem section was observed under an Olympus BX43F light microscope and measured using soft Image-Pro Express 6.3. The height of each line plant was measured and analyzed by Student's t-test. All tests on WT plants were performed during the same growth stage as they were performed on the control.
Semi-thin sections
On the 10th day after bolting, the stem (1 cm above the rosette) was cut into 1–2-mm segments and immediately fixed in FAA [5% (v/v) formalin, 5% (v/v) glacial acetic acid and 65% (v/v) ethyl alcohol] for 24 h at 4 °C. Segments were dehydrated in a series of gradient ethanol and embedded in Epon812 (SPI Supplies Division of Structure Probe Inc., West Chester, PA, USA). Polymerization was carried out for 24 h at 40 °C, followed by 24 h at 60 °C. Segments were cut into 1-μm thick sections on a Leica RM2155 microtome (Leica, Germany), and semi-thin sections were loaded on slides and stained with 0.5% toluidine blue. The semi-thin sections were then cover-slipped with a thin coat of Neutral Balsam and dried at 38 °C for 48 h. After staining, stem sections were observed under an Olympus BX43F light microscope and cell length was measured and analyzed as described above.
Determination of cellulose content
On the 10th day after bolting, the stem segment (1 cm in length) was collected from the bottom and each sample contained five segments from the same line with three biological replicates. The cellulose content was measured according to the description by Shatalov and Mellerowicz [Citation32,Citation33]. In brief, samples were ground into a fine powder, incubated in 95% ethanol for 30 min at 65 °C, cooled to room temperature and centrifuged at 4325 g. The sediment was washed twice in 95% ethanol and extracted overnight in methanol/chloroform (2/3 v/v). Extracted material was centrifuged and washed five times in 95% ethanol. The cell-wall sediment was dried overnight at 65 °C and then freeze-dried. Cell wall material (approximately 1 mg) was hydrolyzed in 1 mL of 2 mol/L trifluoroacetic acid at 120 °C for 90 min. The undigested material (mostly crystalline cellulose) was centrifuged at 3460 g for 5 min. Cellulosic material was digested to glucose monomers by Saeman hydrolysis [Citation32]. The sediment was dispersed in 0.2 mL of 72% H2SO4 at 20 °C for 30 min, diluted to 1 mol/L H2SO4 by the addition of 2.2 mL water and hydrolyzed for 2.5 h at 100 °C. The mixture was then centrifuged at 1600 g for 5 min, and 2 mL of the supernatant was transferred to a 15-mL tube, mixed with 50 µL of 80% phenol and 5 mL of concentrated sulphuric acid, and placed in a water bath at 25–30 °C for 15 min using water instead of the supernatant as a control. The glucose content of the supernatant was assayed using the colourimetric anthrone assay. First, a series of diluted pure glucose solutions were reacted instead of the above-described supernatant, and the absorption was read at 490 nm on a spectrophotometer (UV2800, Unique, Shanghai, China) so that a standard curve could be generated. Thus, the glucose content of the supernatant was calculated based on absorbance by comparison to the standard curve. Cellulose content was calculated according to the following formula:
Where C is the concentration of glucose measured, mg/L; W is the weight of the cell wall, mg.
Data analysis
The results are mean values with standard deviation (±SD). Statistical analysis was performed using Student's t-test.
Results and discussion
Expression patterns of NcEXPA1-16
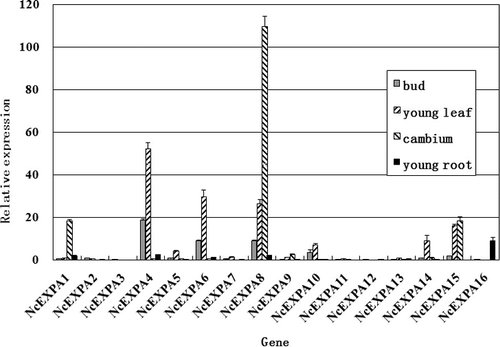
To determine the NcEXPA1-16 expression patterns in different tissues, the transcriptional expression levels of NcEXPA1-16 were investigated in four growth tissues in N. cadamba (bud, young leaf, cambium region and young root) by qRT-PCR. As shown in , the transcript levels of NcEXPA8 were the highest in the cambium region, followed by NcEXPA1 and NcEXPA15. However, the transcript levels of the rest members were low or barely detectable in the cambium region. In the young leaf, the expression levels of NcEXPA4, NcEXPA6 and NcEXPA8 were more than 20-fold higher than that of the reference gene Cyclophilin. In the four tissues, the expression levels of NcEXPA3, NcEXPA11 and NcEXPA12 were extremely low, while NcEXPA16 was expressed only in the young root. Therefore, these results strongly suggest that the NcEXPA8 gene plays an important role in xylem formation in N. cadamba.
Overexpression of NcEXPA8 in A. thaliana plants promotes stem growth
NcEXPA8 belongs to the secondary xylem-specific expansin subgroup [Citation26] and was preferentially expressed in the cambium region (). To investigate the biological functions of NcEXPA8 during stem growth in plants, we expressed the gene in A. thaliana by introducing the construct 35Spro:NcEXPA8.
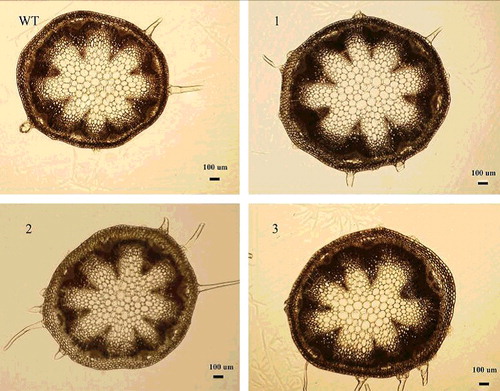
Three independent transgenic homozygous lines in the T2 generation were obtained. The expression levels of the three lines were all high in the 10-day-old stem (). On the 65th day after planting, the main stem of A. thaliana was completely mature. Compared to WT, the stem transverse sectional area 5 mm above ground increased, to some extent, in three transgenic lines (), with statistically significant differences between line 1, line 3 and WT (). Overexpression of NcEXPA8 also increased the plant height (Supplementary Figure 1S), with statistically significant differences between lines 2, 3 and WT (), indicating that there are additional effects of NcEXPA8 on Arabidopsis aerial tissue growth. A number of studies have analyzed EXPA gene expression patterns to predict the function of the EXPA gene in aerial growth. Our study also provides strong evidence that the EXPA protein promotes apical growth.
Figure 2. Relative expression of NcEXPA8 in the stem of transgenic Arabidopsis lines and WT. Note: Left to right: line 1, line 2, line 3 and WT.
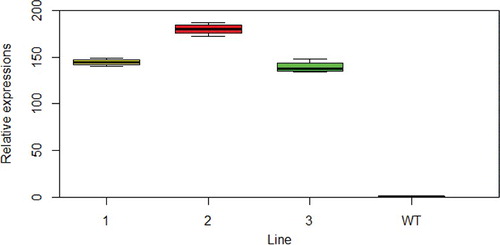
Figure 4. Effects of NcEXPA8 overexpression on Arabidopsis stem transverse sectional area. Note: *P < 0.05 according to Student's t-test (n = 10).
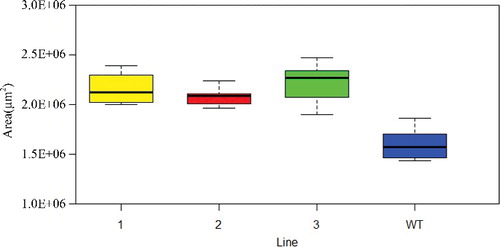
Table 2. Effects of NcEXPA8 overexpression on Arabidopsis height of WT and different transgenic lines.
Overexpression of NcEXPA8 promotes interfascicular fiber cell elongation and cell wall thickening
Since NcEXPA8 was highly expressed in the cambium region () and belongs to the secondary xylem-specific expansin subgroup [Citation26], we speculated that it may play an important role in the secondary growth of xylem. Overexpression of NcEXPA8 not only promoted interfascicular fiber cell elongation, but also increased the thickness of interfascicular fiber cells ( and ). The mean lengths of the fiber cells of the three transgenic lines were 538.74, 445.87 and 467.76 µm, respectively, while that of the WT was only 355.89 µm. The differences between all transgenic lines and WT were statistically significant according to Student's t-test (). As fiber tip intrusive growth occurs, fiber cell growth and developmental processes are initiated and elongation, ultimately resulting in the fully differentiated fibers to be much longer than the original fiber. This process requires softening of the primary cell walls in the tip of the fiber. Therefore, the regulatory factors involved in cell-wall expansion are needed during this process. Expansins are a main class of proteins that soften the cell wall without the involvement of additional active proteins [Citation3,Citation34,Citation35]. To date, there have been two in situ hybridization experiments demonstrating that EXPA mRNA is located mostly in the tip of the original fiber cells and is proposed to play an important role in fiber intrusive elongation [Citation36,Citation37]. In the present study, we demonstrated that overexpression of the EXPA gene significantly promoted interfascicular fiber cell elongation, indicating that it is of crucial importance in softening the tip of Arabidopsis interfascicular fiber cells by NcEXPA8.
Figure 5. Longitudinal section of an Arabidopsis fiber cell. Note: The arrow indicates the fiber cell. Left to right: WT, line 1, line 2 and line 3. Scale bar = 100 µm.
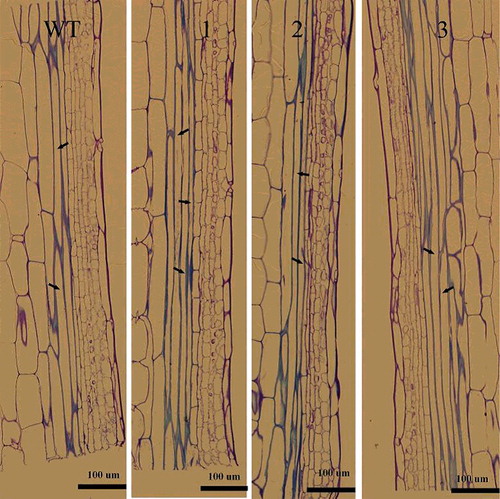
Figure 6. Fiber cell wall thickness (transverse section) in Arabidopsis WT and transgenic lines. Scale bar = 5 µm.
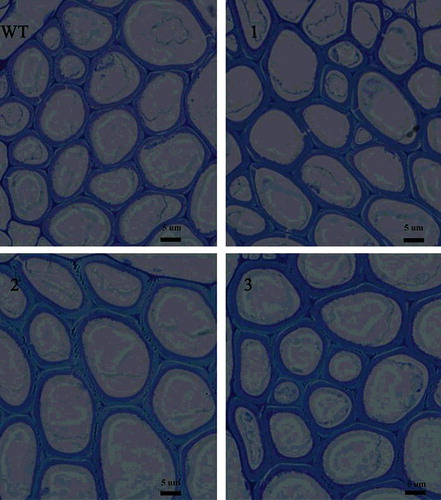
Table 3. Effects of NcEXPA8 overexpression on Arabidopsis fiber cell length of WT and different transgenic lines.
Table 4. Effects of NcEXPA8 overexpression on Arabidopsis fiber cell-wall thickness of WT and different transgenic lines.
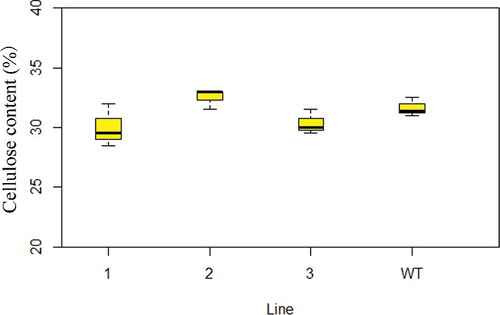
The thickness of the transverse section of the fiber cells is shown in . The mean thickness in the three transgenic lines was 1.199, 1.216 and 1.376 µm, respectively; the differences between both transgenic lines (2 and 3) and WT was statistically significant according to Student's t-test (). However, overexpression of NcEXPA8 did not alter the cellulose content in the cell wall (). This is contrary to the only two studies in which the EXPA gene was reported to be involved in regulating the cellulose content in the cell wall. Dal Santo et al. [Citation38] found that PhEXPA1 anti-sense transgenic plants exhibited smaller petals, a thinner cell wall and decreased cellulose content. Overexpression of two EXPAs from Chinese fir in tobacco resulted in fiber cell wall thickening and an increase in cellulose content in the stem section [Citation39], which may be related to the mechanism of action of expansins and to different growth environments in different species. In this present study, overexpression of NcEXPA8 disrupted non-covalent bonds (hydrogen bonds) between fiber cell wall polysaccharides in Arabidopsis, resulting in cell-wall softening; it also assisted other cell-wall–modifying enzymes to assemble the cell wall in a more efficient way so as to promote the precipitation of the secondary wall, but it did not alter the cellulose content of cell walls.
Overexpression of NcEXPA8 does not alter endogenous gene expression
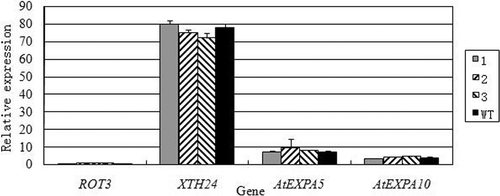
Plant cell growth, specifically cell-wall growth, is the result of the synergistic effects of many regulatory factors and proteins as well as environmental or hormonal stimulation. To determine whether the expression of other endogenous genes involved in stem growth are altered following NcEXPA8 overexpression, the transcriptional levels of AtROT3 (NM_119801), AtXTH24 (NM_119173), AtEXPA5 and AtEXPA10 genes (http://www.arabidopsis.org/browse/genefamily/expansin.jsp) were analyzed. However, no significant difference was found between WT and transgenic Arabidopsis lines, as shown in . In the stems collected on the 10th day after bolting in WT and transgenic lines, the expression levels of endogenous genes involved in stem growth were similar and/or unaffected by the transgene, indicating that endogenous gene expression is not affected by the transgene. Therefore, we conclude that the phenotypic changes observed in transgenic Arabidopsis plants resulted from overexpression of the NcEXPA8 gene. The specific function of NcEXPA8 may explain the rapid growth of N. cadamba, and could allow for improvement of the growth of other woody plants through manipulating this single gene without inducing other deleterious effects. Further studies should be performed to examine how the expression of this gene in N. cadamba and other relevant tree species affects their biomass production.
Conclusions
The obtained results demonstrated that overexpression of NcEXPA8 can accelerate the secondary growth and can change the leaf morphogenesis of A. thaliana. NcEXPA8 was shown to have a high expression value not only in the cambium, but also in bud and young leaves of N. cadamba. Thus, we could conclude that NcEXPA8 plays an important role in xylem and leaf development of N. cadamba.
Supplementary_Data.pdf
Download PDF (378 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Perini MA, Sin IN, Martinez GA, et al. Measurement of expansin activity and plant cell wall creep by using a commercial texture analyzer. Electron J Biotechnol. 2017;26:12–19.
- Cosgrove DJ, Li LC, Cho HT, et al. The growing world of expansins. Plant Cell Physiol. 2002;43(12):1436–1444.
- Cosgrove DJ. Loosening of plant cell walls by expansins. Nature. 2000;407(6802):321–326.
- Cosgrove DJ. Catalysts of plant cell wall loosening. F1000Res. 2016;5(F1000 Faculty Rev):119.
- Marowa P, Ding A, Kong Y. Expansins: roles in plant growth and potential applications in crop improvement. Plant Cell Rep. 2016;35(5):949–965.
- Guimaraes LA, Mota APZ, Araujo ACG, et al. Genome-wide analysis of expansin superfamily in wild Arachis discloses a stress-responsive expansin-like B gene. Plant Mol Biol. 2017;94(1–2):79–96.
- McQueen-Mason S, Durachko DM, Cosgrove DJ. Two endogenous proteins that induce cell wall extension in plants. Plant Cell. 1992;4(11):1425–1433.
- Rose JK, Bennett AB. Cooperative disassembly of the cellulose–xyloglucan network of plant cell walls: parallels between cell expansion and fruit ripening. Trends Plant Sci. 1999;4(5):176–183.
- Li Z-C, Durachko DM, Cosgrove DJ. An oat coleoptile wall protein that induces wall extension in vitro and that is antigenically related to a similar protein from cucumber hypocotyls. Planta. 1993;191(3):349–356.
- Cosgrove DJ, Zhang T, Vavylonis D, et al. Nanoscale movements of cellulose microfibrils in primary cell walls. Nat Plants. 2017;3:17056.
- Wang Y, Ma N, Qiu S, et al. Regulation of the α-expansin gene OsEXPA8 expression affects root system architecture in transgenic rice plants. Mol Breed. 2014;34(1):47–57.
- Ma N, Wang Y, Qiu S, et al. Overexpression of OsEXPA8, a root-specific gene, improves rice growth and root system architecture by facilitating cell extension. PLoS One. 2013;8(10):e75997.
- Cho HT, Kende H. Expression of expansin genes is correlated with growth in deepwater rice. Plant Cell. 1997;9(9):1661–1671.
- Xu, Q, Krishnan S, Merewitz E, et al. Gibberellin-regulation and genetic variations in leaf elongation for tall fescue in association with differential gene expression controlling cell expansion. Sci Rep. 2016;6:30258.
- Choi D. Regulation of expansin gene expression affects growth and development in transgenic rice plants. Plant Cell. 2003;15(6):1386–1398.
- Gray-Mitsumune M. Expansins abundant in secondary xylem belong to subgroup A of the alpha-expansin gene family. Plant Physiol. 2004;135(3):1552–1564.
- Gray-Mitsumune M, Blomquist K, McQueen-Mason S, et al. Ectopic expression of a wood-abundant expansin PttEXPA1 promotes cell expansion in primary and secondary tissues in aspen. Plant Biotechnol J. 2008;6(1):62–72.
- Jung J, O Donoghue EM, Dijkwel PP, et al. Expression of multiple expansin genes is associated with cell expansion in potato organs. Plant Sci. 2010;179(1–2):77–85.
- Yan A, Wu M, Yan L, et al. AtEXP2 is involved in seed germination and abiotic stress response in Arabidopsis. Plos One. 2014;9:e85208.
- Li D, Huang X, Liu Z, et al. Effect of AtR8 lncRNA partial deletion on Arabidopsis seed germination. Mol Soil Biol. 2016;7(7):1–7.
- Budzinski IGF, Santos TB, Sera T, et al. Expression patterns of three α-expansin isoforms in Coffea arabica during fruit development. Plant Biol. 2011;13(3):462–471.
- Zhang T, Li Y, Zhou Y, et al. Cloning and expression analysis of a homologous expansin gene EXP2 in Picea wilsonii. J For Res. 2016;27(2):247–255.
- Geilfus C, Ober D, Eichacker LA, et al. Down-regulation of ZmEXPB6 (Zea mays β-Expansin 6) protein is correlated with salt-mediated growth reduction in the leaves of Z. mays L. J Biol Chem. 2015;290(18):11235–11245.
- He X, Zeng J, Cao F, et al. HvEXPB7, a novel β-expansin gene revealed by the root hair transcriptome of Tibetan wild barley, improves root hair growth under drought stress. J Exp Bot. 2015;66(22):7405–7419.
- Zhou S, Han Y, Chen Y, et al. The involvement of expansins in response to water stress during leaf development in wheat. J Plant Physiol. 2015;183:64–74.
- Ouyang KX, Liu MQ, Pian RQ, et al. Isolation and analysis of α-expansin genes in the tree Anthocephalus chinensis (Rubiaceae). Genet Mol Res. 2013;12(2):1061–1073.
- Fox JED. Anthocephalus chinensis, the Laran tree of Sabah. Econ Bot. 1971;25(3):221–233.
- Zhang X, Henriques R, Lin S, et al. Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat Protoc. 2006;1(2):641–646.
- Ouyang K, Li J, Huang H, et al. A simple method for RNA isolation from various tissues of the tree Neolamarckia cadamba. Biotechnol Biotechnol Equip. 2014;28(6):1008–1013.
- Czechowski T, Stitt M, Altmann T, et al. Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol. 2005;139(1):5–17.
- Oh E, Yamaguchi S, Hu J, et al. PIL5, a phytochrome-interacting bHLH protein, regulates gibberellin responsiveness by binding directly to the GAI and RGA promoters in Arabidopsis seeds. Plant Cell. 2007;19(4):1192–1208.
- Shatalov A, Evtuguin DV, Pascoal Neto C. (2-O-alpha-D-galactopyranosyl-4-O-methyl-alpha-D-glucurono)-D-xylan from Eucalyptus globulus Labill. Carbohydr Res. 1999;320(1–2):93–99.
- Mellerowicz EJ, Baucher M, Sundberg B, et al. Unravelling cell wall formation in the woody dicot stem. Plant Mol Biol. 2001;47(1–2):239–274.
- Brummell DA, Harpster MH, Civello PM, et al. Modification of expansin protein abundance in tomato fruit alters softening and cell wall polymer metabolism during ripening. Plant Cell. 1999;11(11):2203–2216.
- Sampedro J, Cosgrove DJ. The expansin superfamily. Genome Biol. 2005;6(12):242.
- Fleming AJ, McQueen-Mason S, Mandel T, et al. Induction of leaf primordia by the cell wall protein expansin. Science. 1997;276(5317):1415–1418.
- Liquori CL, Ricker K, Moseley ML, et al. Myotonic dystrophy type 2 caused by a CCTG expansion in intron 1 of ZNF9. Science. 2001;293(5531):864–867.
- Dal Santo S, Fasoli M, Cavallini E, et al. PhEXPA1, a Petunia hybrida expansin, is involved in cell wall metabolism and in plant architecture specification. Plant Signal Behav. 2011;6(12):2031–2034.
- Wang G, Gao Y, Wang J, et al. Overexpression of two cambium‐abundant Chinese fir (Cunninghamia lanceolata) α‐expansin genes ClEXPA1 and ClEXPA2 affect growth and development in transgenic tobacco and increase the amount of cellulose in stem cell walls. Plant Biotechnol J. 2011;9(4):486–502.