ABSTRACT
α-Terpineol is one of the main monoterpenes found in the grape berry (Vitis vinifera L.) with its concentrations varying in different varieties. The present study was conducted to identify associations between the α-terpineol synthase (α-TPS) gene, a regulator of α-terpineol metabolism, and α-terpineol content in different grapevine varieties. We used a core collection of 61 grape accessions to identify causal single nucleotide polymorphisms in α-TPS, and evaluated the α-terpineol content in two consecutive years (2014 and 2015). Twenty SNPs were detected in the α-TPS coding region and were used in an association analysis. We found that the α-terpineol levels were higher in the varieties with a T/G genotype at S133 than in the varieties with other genotypes at this site. Additionally, we found S1556 (C/T) with a functional effect on α-terpineol content related to the regulation of gene transcription. This study suggests a relationship between α-TPS and α-terpineol content, with the identified polymorphism sites potentially assisting in the molecular breeding of grapevines.
Introduction
Aroma plays an essential role in high-quality winemaking and table grapes (Vitis vinifera L.), contributing to the economic success of a cultivar. Flavour is considered a biochemically and genetically complex trait [Citation1]. Linalool, geraniol, nerol, citronellol and α-terpineol are isoprenoid molecules responsible for the specific aromas found in grapes and wines [Citation2]. However, α-terpineol has different aroma characters from those of the other isoprenoid molecules. The levels of linalool and geraniol increase significantly in most cultivars, while α-terpineol levels vary only slightly with time during ripening [Citation3,Citation4]. Grape aroma is caused by free forms, such as terpenes, norisoprenoids and thiol, which are volatile and have low boiling points [Citation5]. Monoterpenoid levels can range from zero to 1 mg·kg−1 in grapes, depending on the grapevine variety [Citation6]. α-Terpineol is a common monoterpene with the strongest fragrance in grapes [Citation7], and it plays an important role in grapevine aroma [Citation8]. The presence of α-terpineol in berries or wine might contribute peach, anise or fruity characters to the bouquet [Citation9].
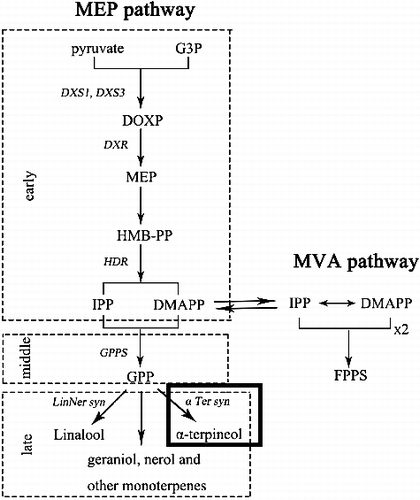
In grapevine, the cytoplasmic mevalonic acid and plastidial 2-methyl-D-erythritol-4-phosphate (MEP) pathways synthesize the precursors of all terpenoids [Citation10,Citation11]. The MEP pathway is a dominant route for the biosynthesis of monoterpenes in grapes [Citation12]. α-Terpineol synthase (α-TPS) catalyses the last reaction in the MEP pathway. Previous studies have shown that α-TPS plays a role in regulating the biosynthesis of α-terpineol in the MEP pathway. The functional identification of α-terpineol synthase provided evidence that V. vinifera has the genetic and biochemical capacity to form this monoterpenol compound directly from geranyl diphosphate (GPP) by employing the reaction mechanism of a typical monoterpene synthase [Citation13]. Studies have shown a sharp increase in the abundance of α-TPS transcripts at 82 days post half-anthesis, but the monoterpenol α-terpineol was released at relatively constant levels from the pre-export verification of conformity (PVOC) pool of developing berries [Citation14]. The accumulation of terpenes is related to the α-TPS transcript profiles in developing grapes [Citation15]. It is well known that mutations in a functional gene might trigger a series of biochemical consequences. For example, a major quantitative trait locus (QTL) on linkage group 5 collocates with a VvDXS gene encoding the first enzyme of the terpenoid biosynthesis pathway [Citation2]. The single nucleotide polymorphism (SNP) mutations in VvDXS (the first gene in the MEP pathway) are the main cause of the Muscat flavour in grapes [Citation16]. The substitution of a lysine (K) with an asparagine (N) at position 284 of the VvDXS amino acid sequence affects the monoterpene content of Muscat-flavoured and neutral grapevine varieties.
As the last gene in the pathway, α-TPS directly affects the biosynthesis of α-terpineol in grapevines. It is, therefore, necessary to elucidate the regulatory mechanism of α-TPS. However, previous studies conducted only limited analyses on the other flavour characters in grapevine. In this study, we supposed that one or more SNP(s) in the α-TPS sequence could affect the biosynthesis of α-terpineol in grapevine. Therefore, we performed a series of experiments aimed at finding an SNP site that affects the α-terpineol content by conducting an association study between α-terpineol content and α-TPS in different varieties of grapevine.
Materials and methods
Plant material
The plant material consisted of 61 grapevine varieties (Table 1S in the Online Supplementary Appendix) obtained from Shenyang Agricultural University, China (E123°24′ N41°50′), with the accessions selected to maximize flavour diversity. Grapes were sampled when they were fully ripe in September 2014 and 2015. Grape clusters were randomly picked from three plants and 300 g of each sample was stored at −80 °C for monoterpenoid analysis and RNA extraction.
Phenotypic evaluation
The phenotypic evaluation was repeated over two consecutive years (2014 and 2015). The α-terpineol content of all grapevine varieties was evaluated using the solid phase microextraction method described by Wu et al. [Citation17]. The gas chromatography mass spectrometry (GC-MS) (7890A-5795C; Agilent, Palo Alto, CA, USA) oven programme was as follows: 60 °C, 2 °C·min−1 to 100 °C, 4 °C·min–1 to 210 and 210 °C for 3 min. It was equipped with a VF-max column (30 m × 0.25 mm × 0.25 μm; Agilent) and helium (flow rate of 1.0 mL·min−1) was used as the carrier gas. The MS detector scanned within a mass range of 30–500 m·z−1 and the mass spectra were retrieved using the NIST11 spectral library. A calibration curve was obtained using the juice of the neutral grapes supplemented with α-terpineol (25.1 μg·kg−1) (Tokyo Chemical Industry, Tokyo, Japan) dissolved in MilliQ water and ethanol (1:1, v/v; six scores). A calibration curve was calculated every time a new column was used in the chromatograph.
Genotyping
Total RNA was extracted from the pericarp tissue of each sample using the cetyltrimethylammonium bromide method. First-strand cDNA was synthesized from the total RNA, using a Takara (Tokyo, Japan) PrimeScript™ II 1st Strand cDNA Synthesis Kit according to the manufacturer's instructions. The full open reading frame (ORF) of α-TPS cDNA was amplified by polymerase chain reaction (PCR) with the forward (5′-ATGGCTCTTTCCATGCTTTCTTCAA-3′) and reverse (5′- TTATTCAGAACTCAAACTGGGAATGG-3′) primers using the high-fidelity Phusion polymerase (Takara, Tokyo, Japan). The PCR cycling conditions were as follows: initial denaturation at 95 °C for 5 min, followed by 35 cycles of denaturation at 95 °C for 30 s, annealing at 60 °C for 1.5 min and extension at 72 °C for 30 s, and a final elongation step at 72 °C for 10 min. The PCR products were purified from the agarose gel, using the PCR Purification Kit (Takara, Tokyo, Japan) according to the manufacturer's instructions. The purified fragments were subcloned and 20 clones were randomly selected. Subsequently, a pair of alleles was obtained from the α-TPS sequence of each grapevine variety.
Association tests
To avoid false-positive associations, all of the 61 accessions were genotyped at 78 genome-wide microsatellite [simple sequence repeat (SSR)] loci that were obtained from our previous studies. The SSR data were used to infer the population structure using the software STRUCTURE 2.3 [Citation18], which was also used to calculate a Q-matrix [Citation18,Citation19]. A general linear model was combined with the Q-matrix to obtain association results using TASSEL version 5.0 [Citation20].
Data analysis
Data are presented as mean values with standard deviation (±SD). Gene sequences were aligned using the MegAlign software (DNAStar) [Citation21]. Tajima's D and Fu and Li's D* tests were performed using the DnaSP program [Citation18,Citation22]. The neutral mutation parameter θ was calculated for all mutations [Citation23]. Nucleotide diversity was analysed using the parameter π, that is, the average number of nucleotide differences per site between the two sequences [Citation24]. Gene diversity, often referred to as expected heterozygosity, was calculated by means of PowerMarker V3.25 software, where Pij is the frequency of the jth allele for the ith locus [Citation25]. Decay of linkage disequilibrium (LD) with distance in base pairs (bp) between sites within each locus was evaluated by nonlinear regression [Citation26]. The linkage disequilibrium (r2) between two loci in the genome and the exact test for multi-locus association were calculated as described by Zaykin et al. [Citation27] using PowerMarker V3.25 software [Citation25].
Results and discussion
Distribution of the main α-terpineol content in grapes
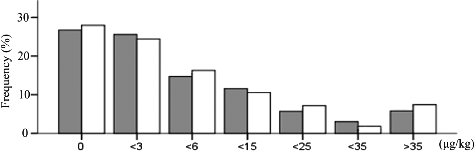
We found minor differences in the α-terpineol content of 61 grapevine varieties between the two years (). The distribution of α-terpineol content was unimodal and continuous for two years. A wide range of α-terpineol concentrations were observed. The concentrations of α-terpineol were mainly concentrated in a narrow range between 0 and 6 μg·kg−1 (nearly 70%).
α-TPS nucleotide diversity
The α-TPS ORF was 1773 bp long; the ratio of synonymous mutations to non-synonymous mutations was 1:1 and the SNP frequency in the nucleotide sequence was 1/93. The values of π and θ were 0.0034 and 0.325, respectively, and the values of Tajima's D and Fu and Li's D* were negative. The P-value was less than 0.05; therefore, it could be suggested that the evolution of this population has been affected by positive selection (). Twenty SNPs were found in the α-TPS ORF sequences, and the protein sequences consisting of 591 amino acids were predicted from the sequenced cDNA. Among the 20 detected SNPs, 10 non-synonymous changes (I38S, A144S, M185L, K230N, E245G, E306Q, F317S, S441A, E492K and T519I) were predicted in the corresponding amino acid sequences. The distribution of varieties with different genotypes is shown in . A relatively low rate of nucleotide variation in α-TPS was observed in the grapevine varieties considered. The frequency of SNPs in the α-TPS coding region found in study was slightly lower than that reported in other grapevine studies, wherein a frequency of one SNP per 47–64 bp was detected on average [Citation28–32]. This might be due to differences in the sample size and uneven distribution of polymorphisms in the grapevine genome. No indels were detected in the coding region, consistent with the results of Emanuelli et al. [Citation33]. Tajima's D and Fu and Li's D* values were significantly negative, suggesting that α-TPS might have undergone positive selection during evolution.
Table 1. Nucleotide diversity of α-TPS in grapevine.
Table 2. Characteristics of SNPs in α-TPS coding regions.
Association analysis
Candidate gene association study has been widely used in plants for associating plant genetic variations with the phenotypes they produce [Citation34,Citation35]. In this study, four non-synonymous mutation sites (S113, S734, S950 and S1556) were significantly associated with the α-terpineol content of different grapevine varieties in 2014 and 2015 (). In detail, S113 accounted for up to 12.64% and 11.37% (2014 and 2015, P < 0.01), S734 accounted for 9.53% and 9.16% (2014, P < 0.01; 2015, P < 0.05), S950 accounted for 5.25% and 4.93% (2014 and 2015, P < 0.05), and S1556 accounted for 11.38% and 10.13% (2014 and 2015, P < 0.01) of α-terpineol variation in 2014 and 2015, respectively.
Table 3. Associations found between α-terpineol and SNP markers in 2014 and 2015.
α-Terpineol has been described as a major aromatic determinant because of its high concentrations in grapevine [Citation33,Citation36,Citation37]. Therefore, candidate–gene association studies between α-TPS and α-terpineol are an effective strategy to further investigate the relationship between aroma and genetic variation. SNPs might cause relevant changes in gene expression or in protein function, followed by alterations in the relevant phenotype [Citation38]. In this study, S734 and S950 were found to have low contribution to α-terpineol variation in two years; therefore, these sites are very unlikely to be the functional sites in the regulation of monoterpene metabolism (). The obtained results suggest that S113 and S1556 could have high contribution to α-terpineol variation, making these sites associated with a high probability to the regulation of α-terpineol content in 2014 and 2015 ().
At site S113, the individuals with genotype TG (32% of all accessions) showed higher α-terpineol levels than the individuals with genotype GG or TT. At site S1556, the individuals with genotype CT (27% of all accessions) had higher α-terpineol levels than those with genotype AG or GG (). These SNPs were located in the coding region and caused non-synonymous changes in the corresponding amino acids (I38S and T519I). The mean content of α-terpineol in S113 (TG) and S1556 (CT) was higher than that in the other genotypes (). It is reasonable to assume that S113 (TG) and S1556 (CT) are potentially functional sites involved in the regulation of α-terpineol metabolism, with the results of this study verifying that S113 (TG) and S1556 (CT) were associated with high α-terpineol varieties. This analysis approach has been used to obtain reasonable results in some other studies [Citation2,Citation33]. Some reports have attributed the Muscat flavour in grapevine mainly to SNP (K284N) mutations in VvDXS (the first gene in the MEP pathway) () [Citation2,Citation15,Citation30]. However, there is still little insight into the flavour characters in grapevine. Expression of the late-pathway α-TPS gene showed an increase in the relative transcript abundance, suggesting a correlation between α-TPS gene and α-terpineol content [Citation14]. No correlation was observed between the early-pathway VvDXS gene and α-terpineol content [Citation14,Citation15]. However, α-TPS expression can have an immediate impact on the α-terpineol content, providing more accurate assessment of the substance content. Moreover, our results have validated the possibility of the existence of SNPs in α-TPS.
Table 4. Means α-terpineol concentrations according to the genotype at S113 and S1556.
Owing to their high concentration in grapevine cultivars, linalool, geraniol, nerol, citronellol and α-terpineol are often described as the major aromatic determinants [Citation33,Citation37]. Therefore, we were unable to identify the aromatic type by α-terpineol content only. Further insight into the combination of the biosynthesis pathways of the other aroma compounds (linalool, geraniol, nerol and citronellol) could throw light on the regulatory mechanisms that determine the aroma in grape berries. The VvTPS gene family is one of the largest gene families of specialized metabolism in grapevine where TPS enzymes contribute to berry and wine flavour [Citation39]. α-TPS is a member of the TPS-d subfamily. Our results of cloning and association study of α-TPS provide evidence for the functional characterization of α-TPS in the MEP pathway and VvTPS gene family. As an example of how a single genetic mutation can cause a functional alteration of the gene, previous studies have indicated that VvGuaS, an enzyme responsible for the production of the rotundone precursor, α-guaiene, is encoded by a novel allele of the previously characterized grapevine gene VvTPS24, and that two specific polymorphisms are responsible for the functional differences between VvTPS24 alleles [Citation40]. Since random mutations can cause a variety of functional variations, further analyses, including a larger number of grapevine varieties, are required to elucidate the functional effect of the putative causal mutations identified in this study.
Conclusions
In this study, we found high-level synthesis of α-terpineol in grapes bearing mutations in α-TPS. The SNPs S113 and S1556 in α-TPS were significantly associated with the α-terpineol content. These novel SNPs could potentially be used for identifying grapevine varieties with high α-terpineol content and could be used as a functional marker for marker-assisted breeding to accelerate the breeding process in grapes. However, it is necessary to study the functional effects of these polymorphisms to gain a further accurate judgment of their effects on aroma. This is an important step in understanding the genetic characteristics of aromatic substances in grape berries.
Supplementary_Data.pdf
Download PDF (113.7 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Dunemann F, Ulrich D, Malysheva-Otto L, et al. Functional allelic diversity of the apple alcohol acyl-transferase gene MdAAT1 associated with fruit ester volatile contents in apple cultivars. Mol Breeding. 2012;29:609–625.
- Duchêne E, Butterlin G, Claudel P, et al. A grapevine (Vitis vinifera L.) deoxy-D-xylulose synthase gene colocates with a major quantitative trait loci for terpenol content. Theor Appl Genet. 2009;118:541–552.
- Nitsch M, Hey M, Rühl EH, et al. Monoterpene levels in different grapevine (Vitis vinifera L.) cultivars and clones during fruit ripening. Acta Hortic. 2014;1046:471–476.
- Sasaki K, Takase H, Matsuyama S, et al. Effect of light exposure on linalool biosynthesis and accumulation in grape berries. Biosci Biotechnol Biochem. 2016;80(12):2376–2382.
- Lund ST, Bohlmann J. The molecular basis for wine grape quality – a volatile subject. Science. 2006;311(5762):804–805.
- Ebeler SE. Analytical chemistry: unlocking the secrets of wine flavor. Food Rev Intl. 2001;17:45–64.
- Vladimira I, Košmerl T, Pejić I, et al. Clone candidates differentiation of grapevine Vitis vinifera ‘Škrlet bijeli’ using aroma compounds detected by gas chromatography–mass spectrometry. Acta Agric Slovenica. 2016;107:483–496.
- Hernandez-Orte P, Concejero B, Astrain J, et al. Influence of viticulture practices on grape aroma precursors and their relation with wine aroma. J Sci Food Agric. 2015;95:688–701.
- Skinkis PA, Bordelon BP, Wood KV. Comparison of monoterpene constituents in Traminette, Gewurztraminer, and Riesling winegrapes. Am J Enol Vitic. 2008;59:440–445.
- Nagegowda DA. Plant volatile terpenoid metabolism: Biosynthetic genes, transcriptional regulation and subcellular compartmentation. FEBS Lett. 2010;584:2965–2973.
- Pokhilko A, Bou-Torrent J, Pulido P, et al. Mathematical modelling of the diurnal regulation of the MEP pathway in Arabidopsis. New Phytol. 2015;206:1075–1085.
- Mateo JJ, Jiménez M. Monoterpenes in grape juice and wines. J Chromatography A. 2000;881:557–567.
- Martin DM, Bohlmann J. Identification of Vitis vinifera (-)-α-terpineol synthase by in silico screening of full-length cDNA ESTs and functional characterization of recombinant terpene synthase. Phytochemistry. 2004;65(9):1223–1229.
- Martin DM, Chiang A, Lund ST, et al. Biosynthesis of wine aroma: transcript profiles of hydroxymethylbutenyl diphosphate reductase, geranyl diphosphate synthase, and linalool/nerolidol synthase parallel monoterpenol glycoside accumulation in Gewürztraminer grapes. Planta. 2012;236:919–929.
- Sun L, Zhu BQ, Sun XR. Terpenes biosynthesis related gene transcript profiles and terpenes accumulation of ‘Alexandria’ grape. Sci Agric Sinica. 2014;47(7):1379–1386.
- Battilana J, Emanuelli F, Gambino G, et al. Functional effect of grapevine 1-deoxy-D-xylulose-5-phosphate synthase substitution K284N on Muscat flavor formation. J Exp Bot. 2011;62(15):5497–5508.
- Wu YW, Pan QH, Qu WJ, et al. Comparison of volatile profiles of nine litchi (Litchi chinensis Sonn.) cultivars from Southern China. J Agric Food Chem. 2009;57(20):9676–9681.
- Pritchard, JK, Stephanes M, Rosenberg NA. Association mapping in structured populations. Am J Hum Genet. 2000;67:170–181.
- Falush D, Stephens M, Pritchard JK. Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genet. 2003;164:1567–1587.
- Bradbury PJ, Zhang Z, Kroon DE, et al. TASSEL: software for association mapping of complex traits in diverse samples. Bioinformatics. 2007;23(19):2633–2635.
- Burland TG. DNASTAR's Lasergene sequence analysis software. Methods Mol Biol. 2000;132:71–91.
- Rozas J, Sánchez dBJC, Messeguer Peypoch X, et al. DnaSP, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics. 2003;19(18):2496–2507.
- Nei M, Li WH. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci USA. 1979;76:5269–5273.
- Watterson GA. On the number of segregating sites in genetical models without recombination. Theor Popul Biol. 1975;7:256–276.
- Liu K, Muse SV. PowerMarker: an integrated analysis environment for genetic marker analysis. Bioinformatics. 2005;21(9):2128–2129.
- Jorde LB. Linkage disequilibrium and the search for complex disease genes. Genom Res. 2000;10(10):1435–1444.
- Zaykin D, Zhivotovsky L, Weir BS. Exact tests for association between alleles at arbitrary numbers of loci. Genetica. 1995;96(1–2):169–178.
- Salmaso M, Faes G, Segala C, et al. Genome diversity and gene haplotypes in the grapevine (Vitis vinifera L.), as revealed by single nucleotide polymorphisms. Mol Breeding. 2004;14:385–395.
- Lijavetzky D, Cabezas JA, Ibáñez A, et al. High throughput SNP discovery and genotyping in grapevine (Vitis vinifera L.) by combining a re-sequencing approach and SNPlex technology. BMC Genom. [cited 2016 Jun 11]. 2007;8(1):424. DOI:10.1186/1471-2164-8-424.
- This P, Lacombe T, Cadle-Davidson M, et al. Wine grape (Vitis vinifera L.) color associates with allelic variation in the domestication gene VvmybA1. Theor Appl Genet. 2007;114:723–730.
- Le Cunff L, Fournier-Level A, Laucou V, et al. Construction of nested genetic core collections to optimize the exploitation of natural diversity in Vitis vinifera L. subsp sativa. BMC Plant Biol. 2008;8(1):31–31.
- Eyduran SP, Ercisli S, Akin M, et al. Genetic characterization of autochthonous grapevine cultivars from Eastern Turkey by simple sequence repeats (SSRs). Biotechnol Biotechnol Equip. 2016;30(1):26–31.
- Emanuelli F, Battilana J, Costantini L, et al. A candidate gene association study on Muscat flavor in grapevine (Vitis vinifera L.). BMC Plant Biol. 2010; [cited 2013 Oct 12];10:241. DOI:10.1186/1471-2229-10-241.
- Castillo AM, Sánchez-Díaz RA, Vallés MP. Effect of ovary induction on bread wheat anther culture: ovary genotype and developmental stage, and candidate gene association. Front Plant Sci. 2015; [cited 2016 Mar 10];6:402. DOI:10.3389/fpls.2015.00402
- Zhang L, Li QP, Dong HJ, et al. Three CCT domain-containing genes were identified to regulate heading date by candidate gene-based association mapping and transformation in rice. Sci Rep. 2015; [cited 2016 Aug 24];5:7663. DOI:10.1038/srep07663.
- Ribereau-Gayon P, Boidron JN, Terrier A. Aroma of Muscat grape varieties. J Agric Food Chem. 1975;23:1042–1047.
- Gunata YZ, Bayonove CL, Baumes RL, et al. The aroma of grapes. Extraction and determination of free and glycosidically bound fractions of some grape aroma components. J Chromatogr A. 1985;331:83–90.
- Mercati F, Lorenzis GD, Brancadoro L, et al. High-throughput 18 K SNP array to assess genetic variability of the main grapevine cultivars from Sicily tree. Genet Genom. 2016;12(3):59–59.
- Martin DM, Aubourg S, Schouwey MB, et al. Functional annotation, genome organization and phylogeny of the grapevine (Vitis vinifera) terpene synthase gene family based on genome assembly, FLcDNA cloning, and enzyme assays. BMC Plant Biol. 2010; [cited 2013 Dec 11];10(1):226. DOI:10.1186/1471-2229-10-226.
- Drew DP, Andersen, TB, Sweetmann C, et al. Two key polymorphisms in a newly discovered allele of the Vitis vinifera TPS24 gene are responsible for the production of the rotundone precursor – guaiene. J Exp Bot. 2016;67(3):799–808.