?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Fair skins are traditionally viewed as beautiful in Eastern regions of the world, and whitening effect is therefore eternally pursued. The aim of this study was to evaluate the whitening effectiveness of a traditional Chinese medicine (TCM) formula using laser Doppler technology. In addition, a melanin and erythema meter (Mexameter MX18/MPA-9, Courage&Kahazaka) and VISIA facial imaging booth (VISIA-CR, Canfield) were used to establish parallel comparisons. Pre- and post-treatment results showed that the TCM formula exhibits the best skin whitening effect. This was followed in effectiveness by the vitamin C group. The negative control and blank groups showed no whitening effect. The obtained results indicated that skin micro-circulation was enhanced in the TCM formula group. Therefore,this method could provide sufficient nutritive substances to fibroblasts and collagen, which in turn remove cell metabolic waste and other harmful substances such as superoxide anion in a timely manner. This improves the overall health of skin cells.
Introduction
The main chemical components of licorice (Glycyrrhiza uralensis Fisch) are flavonoids and triterpenoids, with a small amount of coumarin, lignin, alkaloid and multiple amino acids [Citation1]. Substantial research has been conducted on the components of licorice. It has been found that licorice extracts, particularly flavonoid, have a whitening effect and can remove oxygen radicals safely and effectively to protect healthy skin. Therefore, licorice is widely used in cosmetics [Citation2].
The active constituents of licorice have the capacity to restrain melanin formation by inhibiting the activity of tyrosinase and hindering polymerization of 5, 6-dihydroxyindole. The ultimate result is whitening of the skin. Flavonoids, as an active ingredient of licorice, generally have sun-blocking effects. Due to the conjugated aromatic rings in the molecular structure of flavonoids, they show strong absorbance of ultra violet (UV) and visible light. Firstly, the molecules absorb high-energy UV light. Then, the molecules make a transition from a ground state to an activated state, return to the ground state and finally release low-energy, harmless rays [Citation2,Citation3].
Vitamin C, known as ascorbic acid (ASA), is a water-soluble vitamin. It is also a co-substrate for oxygenize and hydroxylase enzymes related to the biosynthesis of carnitine, pro-collagen and neurotransmitters, which are all necessary for the formation, growth and reproduction of bone, cartilage and skin. Additionally, ASA acts as a powerful antioxidant for protecting low-density lipoproteins from oxidation, thereby whitening the skin by reducing harmful oxidants and promoting iron absorption [Citation4].
Currently, many techniques are used to measure the micro-circulation of skin. Some of these techniques include the Doppler laser, plethysmography and angioscopy. Among these, plethysmography is very sensitive to blood flow variations, and the variation of optical transmission exerts great influence on measurement results. At the same time, this equipment's data accuracy is not considered reliable enough for research studies [Citation5], and so it is not commonly used to measure micro-circulation. Angioscopes use a microscope to observe the condition of blood capillaries, and software to perform graph analysis. This is not applicable for real-time monitoring of subjects in a laboratory setting [Citation5].
The Mexameter® MX18 spectrophotometer is commonly used to evaluate skin colour and it is the cosmetic field's gold standard in skin colour testing [Citation6–10]. However, the results tend to vary occasionally, due to variations when the probe contacts the skin. This has a significant impact on the final testing results.However, non-contact instruments, such as a laser Doppler imager, can successfully avoid this drawback and offer more reliable skin colour test results.
When using a traditional laser Doppler flowmeter (LDF), a probe is placed on the subject's skin. However, studies show that even very low pressure (<2 kPa) applied to the skin's surface limits blood flow. Therefore, any probe that directly contacts the skin must have an impact on blood flow; generating inconsistencies in the variation of blood flow and actual skin conditions. Additionally, the subject's blood flow can be greatly affected by their physical condition, mood and other factors such as temperature, humidity and environmental noise during testing. For these reasons, LDF could not be used to measure subjects’ micro-circulation data in common conditions [Citation2].
A non-contact laser Doppler imager (LDI), on the other hand, has the capacity to generate an image within a defined region. This makes blood flow independent of space variation, and keeps the subject relaxed during the measurement process [Citation11]. If a subject performs any minor movement during testing, the traditional LDI-generated image will be blurred, and the data accuracy will decrease. However, a new generation of non-contact LDIs can measure the blood perfusionin a defined tissue region continuously, producing colour images of blood perfusion conditions while processing data simply and accurately [Citation12].
For the present study, we considered various aspects, including monitor accuracy, user-friendliness and environmental conditions during testing. We then adopted a non-contact LDI to test our subjects’ facial skin and to evaluate the whitening effect of two formulas. We randomly formed four groups out of 120 participants. We collected melanin and blood perfusion values, as well as facial images, in real-time. We then applied our intervention and followed up with participants later on to perform the same measurements. We compared the initial and final values to determine the effect caused by our different formulas. We also analyzed inadequacies, such as ineffective sampling mechanisms, to ensure that each enrolled subject applied the formulas per the study's specifications. Our goal was to avoid operation variations caused by different data collection staffs as much as possible.
Subjects and methods
Study cohort
One hundred and twenty healthy female volunteers (aged 36–49 years, Beijing) were enrolled in the study. Our testing area was defined as the left cheek. Volunteers were divided randomly into four groups, who were required to meet the following requirements:
| (1) | Absence of hormone drugs and immunosuppressive agents for a period of at least 1 month before enrollment; | ||||
| (2) | Full participation and completion of the cross-sectional study, from beginning to end; | ||||
| (3) | Absence of recent history of gastrointestinal disorders, pregnancy, severe disease, surgery, severe allergic reactions to food, or current use of any medication; | ||||
| (4) | Absence of any dermatological or systemic disorders; and | ||||
| (5) | Good overall health (absence of cold, flu, headache, fever or any other adverse symptoms on the testing day). | ||||
Subject confidentiality
Subject confidentiality was maintained throughout the study. Once we obtained informed consent from each subject, we assigned subject numbers. All information and data relating to subjects and their participation in this study were considered confidential. All data used in the analysis and reporting of this evaluation omitted identifiable references to any subject enrolled in the study.
Informed consent
Signed, written, informed consent was mandatory for all study subjects. We obtained such consent prior to the initiation of any study-specific procedures. The study was approved by the ethics committee at the Chinese Academy of Traditional Chinese Medicine.
Study instruments
For this study, we used: a melanin and erythema meter (Mexameter MX18/MPA-9, Courage&Kahazaka); a laser Doppler blood perfusion imager (PIM3, PeriMed) and a VISIA facial imaging booth (VISIA-CR, Canfield).
Data collection staff training
We developed an instrument operation training program for operation staff, and each staff member was evaluated to meet our requirements.
Study methods
The 120 female subjects enrolled in this study were randomly divided into four groups (n = 30). Prior to this clinical trial, we collected real-time, skin base data by using melanin and skin blood perfusion values, as well as facial images. We then analyzed the baseline data for each study group. Prior to our intervention, we ensured there were no significant differences between the four groups in terms of baseline data. After clinical interventions, we used the same methods to collect skin data, and then analyzed the collected data via paired t-test to evaluate our samples’ whitening effects.
Study procedures
Prior to the beginning of the study, 120 subjects were randomly assigned into four groups. A different formula was offered to each group, as shown in .
Table 1. Group information.
Day 1: baseline skin data collection
Prior to measurement, the subjects were instructed to clean their faces and use napkins without fluorescent reagent to wipe them dry (the napkins were provided by the laboratory). The subjects signed informed consent forms and completed a questionnaire in an environment of 50 ± 10% humidity at 26 ± 1 °C, which took approximately 20 min. Left cheek real-time melanin and blood perfusion values were measured, and real-time facial images were taken.
The subjects were given 10 g of formulation and were instructed to evenly apply it on their faces after their regular skin care routine, once in the morning and once in the evening. The subjects were instructed to do this for 28 continuous days.
Day 28: Post-treatment skin data collection
Prior to measurement, the subjects were instructed to clean their faces and use napkins without fluorescent reagent to wipe them dry (these were again provided by the laboratory). The subjects rested for 20 min. We then measured the subjects’ left cheek real-time melanin and blood perfusion values, and took real-time facial images.
Statistical analysis
Using a mean value for each group, we assessed the significance of the differences between the four groups. Additionally, we used SPSS (Statistical Package for Social Sciences) v. 19.0 to determine the effectiveness based on the level of significance of the differences between pre- and post-treatment values. We used paired t-test to process data collected within each group.
Results and discussion
Left cheek real-time melanin value
The melanin content values are shown in . Prior to sample application, there were no significant differences between the four study groups in terms of melanin values. The blank group's melanin value did not decrease after using the test sample, but actually tended to increase (). In this group, there was no significant variation between the baseline and post-treatment melanin values (P > 0.05). We applied the same comparison to our negative control group. In the TCM and positive control groups, the melanin values decreased dramatically, but the TCM group showed a significant difference (P < 0.05), whereas the positive control group did not (P > 0.05).
Table 2. Melanin values* measured pre- and post-treatment.
Left cheek real-time blood perfusion value
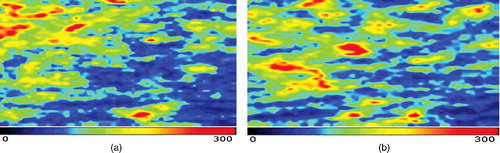
The results from the blood perfusion measurements are shown in and –. Prior to using the samples, there was no significant difference between the four groups in regards to the blood perfusion values. The blood perfusion values in the blank group decreased at the post-treatment stage, but did not show a significant difference (P > 0.05). We applied the same observation to the negative control group. In the TCM and positive control groups, the blood perfusion values increased dramatically. However, the TCM group showed a significant difference (P < 0.05), but the positive control group did not (P > 0.05).
Table 3. Real-time blood perfusion values* measured pre- and post-treatment.
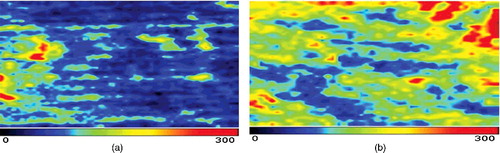
Figure 1. Comparison between pre- (a) and post-treatment blood perfusion (b) images in the blank group.
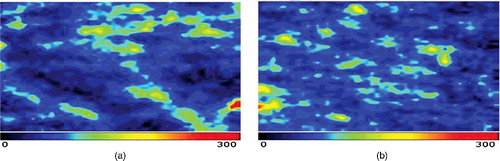
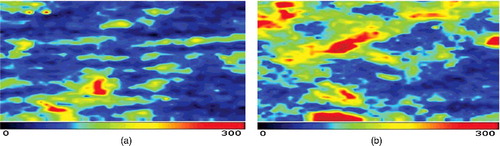
Figure 2. Comparison between pre- (a) and post-treatment blood perfusion (b) images in the negative control group.
Real-time facial image
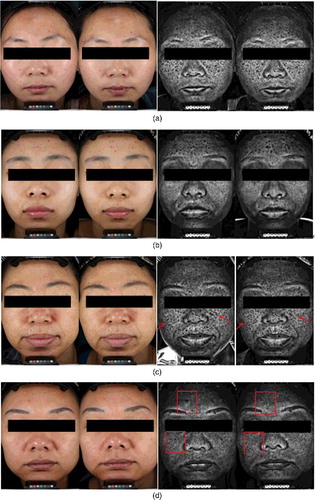
Reference facial images were taken under UV and visible light (). The blank and negative control groups showed no visible difference, whereas the TCM formula and positive control groups showed no obvious difference under visible light, but showed a decreased melanin value under UV-light.
Figure 5. Comparison between pre- and post-operation facial images: blank group (a), negative control group (b), TCM formula group (c) and positive control group (d).
Skin micro-circulation is a complex, dynamic process that plays a key role in determining skin colour, temperature adjustments and skin metabolism [Citation13]. Skin micro-circulation transports oxygen and nutrients into cells/tissues. It also removes metabolic waste through substances exchanged in capillaries, thereby improving the overall health of skin [Citation14,Citation15]. In terms of whitening, enhanced skin micro-circulation could provide sufficient nutritive substances to fibroblasts and collagen, facilitating the removal of cell metabolic waste and various harmful substances such as free radicals in a timely manner. This potentially maintains the health of skin cells while strengthening the whitening effects [Citation16].
Traditional medical theory conceptualizes the whitening mechanism as mainly promoting blood circulation, and reducing melanin and anti-oxidation. The most important element for whitening is improving the blood micro-circulation [Citation17]. Ancient prescriptions of traditional medicine for whitening skin primarily seek to enhance blood circulation. When promoting micro-circulation, the amount of red blood cells passing through blood vessels in unit intervals are increased, resulting in increased red oxyhemoglobin and pigment simultaneously. This leads to a flushing complexion [Citation18]. Skin micro-circulation transports oxygen and nutrients into tissues and also removes metabolic waste through substances exchanged in capillaries, thereby improving the overall health of the skin. When the blood circulation in the body is insufficient or poor, it leads to a dim complexion and pigmentation [Citation19]. For example, an increase in blood viscosity leads to the generation of cholesterol, which is associated with blood stasis and obstacles in skin microcirculation [Citation20–22].
Overall, our results demonstrated significant skin whitening effectiveness of the TCM formula. In addition, according to our previous safety experiment (such as patch test), the negative rate is above 98%, indicating that the formula safety is quite outstanding. It could be speculated that the whitening effect could most likely result from providing sufficient nutrition to fibroblasts and collagen and enhancing the timely removal of cell metabolic waste and other harmful substances. Overall, this leads to an improvement in the health of skin cells [Citation23]. Further research needs to focus on the correlations of other skin evaluation indices (e.g. water de-absorption, skin moisture content, etc.) and skin micro-circulation.
Conclusions
In this study, we employed different methodologies to evaluate traditional Chinese medicine. Our study was performed under stringent laboratory conditions to ensure the accuracy and stability of the obtained data. Moreover, we relied on a laser Doppler imager testing method that is noninvasive and does not contact the skin directly. We used this as our evaluation methodology to avoid the drawbacks of probe sensitivity and to obtain accurate results. The obtained results demonstrated that the TCM formula showed significant skin whitening effectiveness after 28 days of application.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Parvez S, Kang M, Chung HS, et al. Survey and mechanism of skin depigmenting and lightening agents. Phytother Res. 2006;20(11):921–934.
- Jones K, Hughes J, Hong M, et al. Modulation of melanogenesis by aloesin: a competitive inhibitor of tyrosinase. Pigment Cell Res. 2002;15(5):335–340.
- Ephrem E, Elaissari H, Greige-Gerges H. Improvement of skin whitening agents efficiency through encapsulation: current state of knowledge. Int J Pharma. 2017;526:50–68.
- Combs GF, McClung JP. The vitamins. 5th ed. Amsterdam: Academic Press; 2017. Chapter 10, Vitamin C; p. 267–295.
- Ndhlla AR, Amoo SO, Stafford GI, et al. Antimicrobila, anti-inlfammatory and mutagenic investigation of the South African tree aloe (Aloe barberae). J Ethnopharmacol. 2009;124(3):404–408.
- Shin JW, Yoon SW, Jeong JB, et al. Different responses of the melanin index to ultraviolet irradiation in relation to skin color and body site. Photodermatol Photoimmunol Photomed. 2015;30(6):308–315.
- Treesirichod A, Chansakulporn S, Wattanapan P. Correlation between skin color evaluation by skin color scale chart and narrowband reflectance spectrophotometer. Ind J Dermatol. 2014;59(4):339–342.
- Park SB, Huh CH, Choe YB, et al. Time course of ultraviolet-induced skin reactions evaluated by two different reflectance spectrophotometers: DermaSpectrophotometer®; and Minolta spectrophotometer CM-2002®. Photodermatol Photoimmunol Photomed. 2002;18(1):23–28.
- Del BS, Bernerd F. Variations in skin colour and the biological consequences of ultraviolet radiation exposure. Br J Dermatol. 2013;169(s3):33–40.
- Stamatas G, Kollias N. Visual versus spectroscopic analysis of skin color reactions: separation of contributing chromophores. Int Symp Biomed Optics. 2002;4613:208–211.
- Forrester KR, Stewart C, Tulip J, et al. Comparison of laser speckle and laser Doppler perfusion imaging: measurement in human skin and rabbit articular tissue. Med Biol Engin Comput. 2002;40(6):687–697.
- Bonté F. Skin miniaturization mechanisms: new data. Retour Au Numéro. 2011;69(3):135–141.
- Thornton MJ. Oestrogen functions in skin and skin appendages. Expert Opin Ther Targets. 2005;9(3):617–629.
- Li L, Mac-Mary S, Marsaut D, et al. Age-related changes in skin topography and microcirculation. Arch Dermatol Res. 2006;297(9):412–416.
- Lorencini M, Brohem CA, Dieamant GC, et al. Active ingredients against human epidermal aging. Ageing Res Rev. 2014;15(4):100–115.
- Yu SY, Chiu JH, Yang SD, et al. Biological effect of far-infrared therapy on increasing skin microcirculation in rats. Photodermatol Photoimmunol Photomed. 2006;22(2):78–86.
- Downie MM, Sanders DA, Maier LM, et al. Peroxisome proliferator-activated receptor and farnesoid X receptor ligands differentially regulate sebaceous differentiation in human sebaceous gland organ cultures invitro. Br J Dermatol. 2004;151(4):766–775.
- Schoonjans K, Staels B, Auwerx J. The peroxisome proliferator activatedreceptors (PPARS) and their effects on lipid metabolism and adipocyte differentiation. Biochim Biophys Acta. 1996;1302(2):93–109.
- Alexiev U, Volz P, Boreham A, et al. Time-resolved fluorescence microscopy (FLIM) as an analytical tool in skin nanomedicine. Eur J Pharma Biopharm. 2017;116:111–124.
- Tousoulis D, Simopoulou C, Papageorgiou N, et al. Endothelial dysfunction in conduit arteries and in microcirculation. Novel therapeutic approaches. Pharmacol Therap. 2014;144(3):253–267.
- Alsheikh-Ali AA, Kuvin JT, Karas RH. Risk of adverse events with fibrates. Am J Cardiol. 2004;94(7):935–938.
- Henderson AJ, Lasselin J, Lekander M, et al. Skin colour changes during experimentally-induced sickness. Brain Behavior Immun. 2017;60:312–318.
- He YF, Meng H, Dong YM. Assessment of various traditional Chinese medicine formulas on skin micro-circulatory perfusion. Bio-Med Mater Engin. 2015;26:S951–S958.