ABSTRACT
Celery (Apium graveolens L.) is rich in nutrient substances and is cultivated worldwide. WRKY protein family is one of the largest transcription factor families in plants and is involved in growth and development, signal transduction, senescence and stress resistance. In this study, we identified 69 celery WRKY family transcription factors based on conserved WRKY domain sequence from the celery genomic and transcriptomic data. Phylogenetic analysis of celery and Arabidopsis WRKY proteins divided these celery WRKY proteins into three groups, seven subgroups. The numbers of WRKY proteins in celery and other 16 species were compared to illustrate the evolution of WRKY transcription factors in plants. Quantitative real-time polymerase chain reaction (qRT-PCR) was used to analyse the expression profiles of five selected AgWRKY genes under abiotic stress (abscisic acid, cold, drought and salt) treatments. The qRT-PCR results indicated that those selected AgWRKY family genes were upregulated under abiotic stress treatments. This study provides a basic research for further investigation of the structure and function of AgWRKY genes.
Introduction
Transcription factors (TFs) are proteins that can bind to sequence-specific DNA and interact with target genes [Citation1,Citation2]. TFs are found in large amounts in the plant genome, and have an important role in signalling pathways to regulate plant growth and development [Citation3–5]. In the Arabidopsis genome, more than 5% of the genes encode at least 1533 TFs [Citation6]; and 12.2% of the soybean genome genes code more than 5600 TFs [Citation7].
TFs can be classified as families on the basis of their DNA-binding domain [Citation8]. The WRKY family is one of the largest TF families and is mainly found in plants [Citation9]. WRKY proteins contain one or two WRKY domains that contain about 60 amino acids, which are composed of a peptide sequence WRKYGQK motif at the N-terminus followed by a C2H2 or C2HC zinc-binding motif [Citation9]. In some of the WRKY TFs, the conserved WRKYGQK domain also can be replaced by WRKYGKK, WRKYDQK or WRKYDHK [Citation10,Citation11]. WRKY TFs have been identified to interact with the W-box (TTGACT/C) and SURE (sugar responsive) cis element in the promoter region of large number of plant target genes [Citation12–14]. WRKY TFs can be divided into three groups in accordance with the number of WRKY domains and the structure of the zinc-finger motifs. Group I contains two WRKY domains with a C2H2 zinc-finger motif. Group II WRKYs have only one WRKY domain and a C2H2 zinc-finger motif, and can be further divided into five subgroups (IIa–IIe). Group III contains one domain and a C2HC zinc-finger motif [Citation9].
Since SPF1 was first reported in sweet potato in 1994 [Citation15], rapid progress has been made in the study of WRKY TFs. WRKY TFs have been characterized in Arabidopsis [Citation16], Cucumis sativus [Citation17], Oryza sativa [Citation18], Solanum lycopersicum [Citation19], Triticum aestivum [Citation20], Daucus carota [Citation21] and Camellia sinensis [Citation22]. WRKY proteins are involved in regulating seed germination, root formation, leaf senescence and other plant development processes [Citation23–27]. In recent years, more and more studies report that WRKY genes can also modulate the response of biotic and abiotic stress [Citation1,Citation25,Citation28]. For example, Arabidopsis mutant plants lacking in WRKY27 show delayed symptoms under bacterial wilt induced by Ralstonia solanacearum [Citation29]. Overexpression of WRKY25 or WRKY33 can improve NaCl tolerance in Arabidopsis [Citation30]. WRKY25, 26 and 33 in Arabidopsis have been found to concertedly mediate plant thermotolerance [Citation31]. In wheat, six TaWRKY genes were upregulated by salinity, abscisic acid (ABA) and polyethylene glycol (PEG) [Citation20]. Overexpression of three GmWRKY genes in Arabidopsis enhanced the tolerance to cold, salt and drought stresses [Citation32].
Celery (Apium graveolens L.), originated from the Mediterranean basin, is one of the most important vegetables in the Apiaceae family [Citation33]. This biennial herb is rich in dietary fibre, flavonoids, carotenoids and volatile oils, and is now cultivated worldwide [Citation34]. However, little reports on celery WRKY TFs have been found to date. In the present study, we identified 69 WRKY TFs from celery. The transcriptome data of celery have been built by our group [Citation35,Citation36]. Phylogenetic relationships and domain structure of AgWRKY family factors were analysed. The expression profiles of several AgWRKY genes under abiotic stress were detected. Our study could be used to investigate the structure and resistance to abiotic stress of WRKY genes in celery.
Materials and methods
Identification of putative WRKY genes in celery
The putative WRKY proteins were retrieved from A. graveolens transcriptome data [Citation35,Citation36]. The accession number of celery transcriptome data was SRA109935. The sequence of WRKY family factors from Arabidopsis were downloaded from the Arabidopsis Information Resource (http://www.arabidopsis.org/) [Citation9,Citation37]. WRKY factors from other species were retrieved from the Plant Transcription Factor Datbase (PlantTFDB) v3.0 (http://planttfdb.cbi.pku.edu.cn/index.php) [Citation38]. Putative celery AgWRKY TFs were predicted using HMMER 3.0 software (http://hmmer.janelia.org/) with default parameters. Then, the sequences obtained were submitted to NCBI database (http://ncbi.nih.gov/) for WRKY domain searching.
Analysis of celery AgWRKY family TFs
The multiple alignments of Arabidopsis and celery WRKY protein sequences was performed by ClustalW with default parameters [Citation39]. WRKYs in Arabidopsis are listed in Supplementary Table S1. A phylogenetic tree was constructed using MEGA 5.0 by the neighbour-joining method [Citation40]. The chemical and physical characteristics of AgWRKY amino acid sequences were analysed by the Expert Protein Analysis System (ExPASy) program (http://web.expasy.org/protparam/) [Citation41]. The conserved motifs of AgWRKYs were identified by MEME (Version 4.11.2) [Citation42]. The protein interaction networks were constructed by STRING software [Citation43].
Plant materials, growth conditions and stress treatments
Celery plants were grown in pots within a soil/vermiculite mixture (3:1) in phytotron. The phytotron programme was set as 25/18 °C (day/night) for 16/8 h; during the day the light intensity was 300 μmol m−2 s−1, the relative humidity was set at 75%. After two months, celery plants were transferred to 4 °C growth chamber or irrigated with 200 g·L−1 PEG6000, 200 mmol·L−1 ABA and 200 mmol·L−1 NaCl, respectively. Clipped leaf blades were sampled after 0, 1, 3, 6, 12, 24 and 48 h. All the samples were frozen in liquid nitrogen immediately and then stored at −70 °C for RNA extraction.
Total RNA extraction and cDNA reverse transcription
Total RNA of celery leaf blades was extracted using an RNA extraction Kit (Tiangen, Beijing, China) according to the manufacturer's instructions. cDNA was reverse transcribed from total RNA by a Prime Script RT reagent Kit (TaKaRa, Dalian, China) according to the manufacturer's instructions.
Quantitative real-time polymerase chain reaction (qRT-PCR) analysis
AgWRKYs from five subgroups (I, IIa, IIc, IId, III) were selected for qRT-PCR analysis. All the specific primers were designed by Primer Premier 6.0 and synthesized by Genscript Inc (Nanjing, China). Celery TUB-B gene was used to normalize the relative expressions of AgWRKYs [Citation44]. All the primers are listed in . The Bio-Rad IQ5 real-time PCR System (Bio-Rad, CA, USA) and the TaKaRa SYBR Premix Ex Taq (TaKaRa, Dalian, China) were used for qRT-PCR reaction. The operating instructions were as follows: 95 °C for 30 s, following 40 cycles of 95 °C for 5 s, 60 °C for 30 s and melting curve analysis (61 cycles) at 65 °C for 10 s. The 20-μL reaction system contained 10-μL SYBR Premix Ex Taq, 7.2 μL ddH2O, 2 μL diluted cDNA, forward primer 0.4 μL and reverse primer 0.4 μL. Each reaction was repeated three times. The relative gene expression level was calculated by Pfaffl's method [Citation45].
Table 1. The primer sequences used for qRT-PCR amplification in this study.
Results and discussion
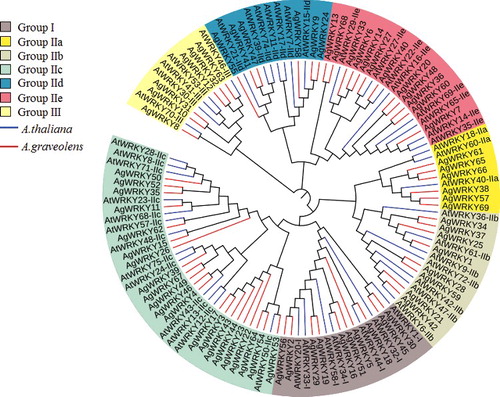
Identification and classification of AgWRKY TFs in celery
Using HMMER and BLAST, we searched the celery genome with the WRKY domain model (PF03106) to identify all the WRKY TFs in celery. A total of 69 AgWRKYs were identified and denoted from AgWRKY1 to AgWRKY69 (Supplementary Table S2). The WRKYGQK domain existed in 64 AgWRKY TFs, while the remaining 5 AgWRKYs contained WRKYGQF, WRKYRQK or WRKYGKK domains. The average amino acid length of the putative AgWRKY proteins is 365, ranging from 118 to 726. In accordance with the number of the WRKY domains and the structure of the zinc-finger motifs, the AgWRKYs were classified into three groups (). Group I contains 10 AgWRKYs with two WRKY domains with a C2H2 zinc-finger motif. Group II includes 53 AgWRKYs, which can be further divided into five subgroups. Group III members, six AgWRKYs, have one domain and a C2HC zinc-finger motif. A phylogenetic tree of WRKY proteins from celery and Arabidopsis was constructed (). Group I and group III aggregated into one clade, while group II was divided into five subgroups. Groups IIa and IIb composed of one branch, and groups IId and IIe detached from one clade. On the basis of further analysis of WRKYs in Arabidopsis and rice, the systematization of group II can be reorganized into IIa+IIb, IIc, IId+IIe [Citation46].
Table 2. Identified AgWRKY genes and their related information.
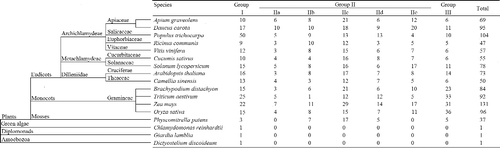
Evolution and distribution of WRKY transcription factors in different species
WRKY TFs have been identified in many species, including Giardia lamblia from Diplomonads and Dictyostelium discoideum from Amoebozoa [Citation46,Citation47]. We constructed a phylogenetic tree of 17 species in the evolutionary history and compared the number of WRKY TFs in each species () [Citation17,Citation19–22,Citation48–54]. The primitive eukaryote G. lamblia, slime mold D. discoideum and the green alga Chlamydomonas reinhardtii only have a single group I copy, which indicated that WRKY TFs are likely to have derived from group I. Brand et al. [Citation55] arranged the WRKY TF evolution relationship with group I as existing earliest, followed by IIa and IId, and group III as the youngest group in the WRKY TFs family. In moss Physcomitrella patens, groups IIa and IIe are absent, whereas group III exists, suggesting that group III WRKY TFs may have evolved earlier than groups IIa and IIb in moss [Citation49].
The higher plants contain a larger number of WRKYs and more subgroups than lower plants. The quantitative distribution of WRKY TFs in plants indicates that WRKY TFs family originated from group I. Within group II, group IIc has more WRKY TFs than other subgroups. In monocots, group III has more members than group I, which is opposite to eudicots. Group III may have a more important role in monocot plant physiological biochemical process [Citation21,Citation56–58].
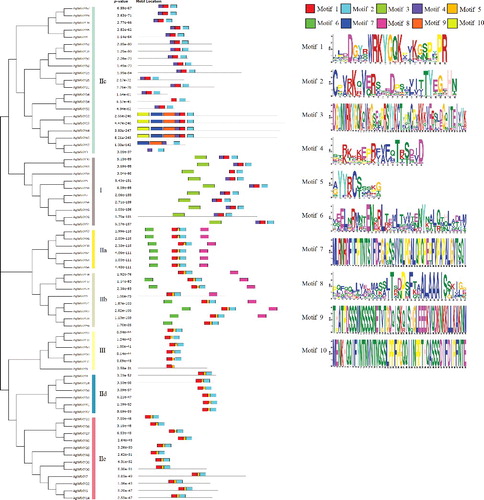
Motif discovery and physicochemical analysis of AgWRKY TFs
The conserved motifs of AgWRKY proteins were predicted by the MEME program. As shown in , motif 1 and motif 3 contain a WRKYGQK sequence. Most AgWRKYs contained motif 1. Group I members were shown to possess both motif 1 and motif 3. The motif composition in each subgroup was observed to be similar. For instance, most of the group IIc members contained motifs 1, 2 and 4. Group I possessed motifs 1, 2, 3 and 4, whereas group III contained motifs 1, 2 and 5. Both groups IIa and IIb contained motifs 1, 2, 5, 6 and 8, which indicated that these two subgroups were adjacent in evolutionary relationship.
The Protparam tool was used to predict AgWRKYs’ physical and chemical characteristics (Supplementary Table S3). The average molecular weight of the AgWRKYs was 40.35 kDa. The molecular weight variation of the members of group IIc was significant. However, the molecular weight variation in other groups did not change significantly. The theoretical isoelectric point (pI) ranged from 4.44 to 10.3, and the average pI for all proteins was 7.37. Several AgWRKY proteins were found to have pI values over 10, e.g. AgWRKY17, AgWRKY64, AgWRKY24, AgWRKY41 and AgWRKY47. In all AgWRKYs, the grand average of hydropathicity was less than zero, suggesting that AgWRKY proteins were hydrophilic. The percentage of aliphatic amino acids was twofold higher than that of aromatic amino acids in AgWRKY proteins, approximately. The positively charged amino acids in AgWRKYs were a bit more than the negatively charged amino acids at pH 6.5, except in group IIc, in which the proportion of negatively charged amino acids was a little higher.
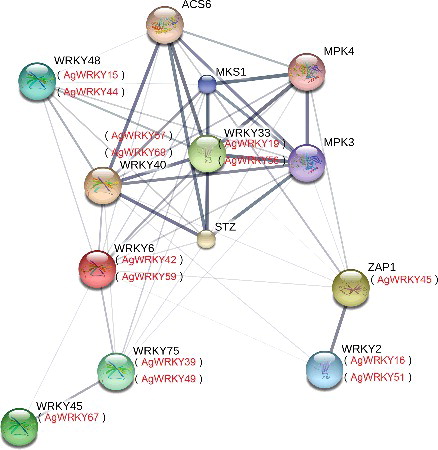
Interaction networks of AGWRKYs and related proteins
To investigate the relationship between AgWRKYs and related proteins, the protein interaction networks were integrated by STRING software using the Arabidopsis association model [Citation43]. In this study, we found 14 AgWRKY proteins associated with each other (Supplementary Table S4). Of the 14 AgWRKY proteins, AgWRKY16, AgWRKY19, AgWRKY45, AgWRKY51 and AgWRKY56 belonged to group I; AgWRKY57 and AgWRKY69 belonged to group IIa; AgWRKY42 and AgWRKY59 belonged to group IIb; AgWRKY15, AgWRKY39, AgWRKY44, AgWRKY49 and AgWRKY67 belonged to group IIc.
As shown in , the association between AgWRKY45 and AgWRKY16/AgWRKY51 was very strong, indicating these proteins may co-regulate some biological processes. Salt tolerance zinc (STZ) finger ZAT10 and MKS1 have strong associations with AgWRKY19, AgWRKY56, AgWRKY57 and AgWRKY69. In higher plants, ZAT10 and MKS1 play important roles in defence responses [Citation59,Citation60]. ZAT10 is a transcriptional repressor and is involved in plant tolerance to salinity, heat and osmotic stress [Citation59]. MKS1 can repress salicylic acid (SA)-dependent resistance and activate jasmonate (JA)-dependent gene expression [Citation60]. Those AgWRKYs may be also involved in the defence response of celery plants against abiotic stress.
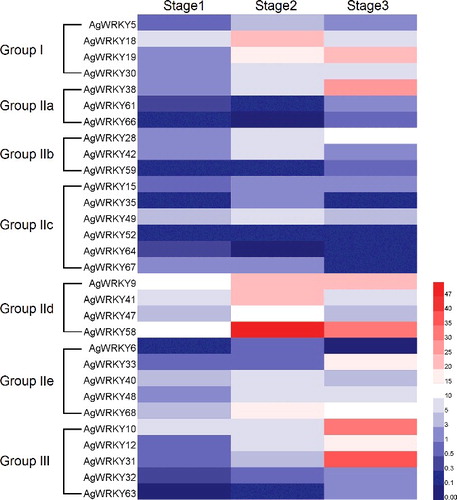
Expression level analysis of AgWRKY genes during different developmental stages in celery
According to the transcriptome data of celery [Citation35,Citation36], the transcript abundances of AgWRKY genes were analysed. The expression level analysis of 30 AgWRKY genes of celery leaves in three developmental stages (leaf length was 10, 20 and 30 cm) is shown in . The genes of groups I, IId and IIe of celery AgWRKY TFs show higher expression levels in stage 2 than in other stages. Groups IIa and III genes were more highly expressed in stage 3 than in stages 1 and 2. The expression levels of group IIc genes were lower than those of other groups. In three developmental stages, most of group III genes showed a low expression level, except for AgWRKY10 and AgWRKY31 genes in stage 3.
Expression profiles of the selected AgWRKY genes under abiotic stress treatments
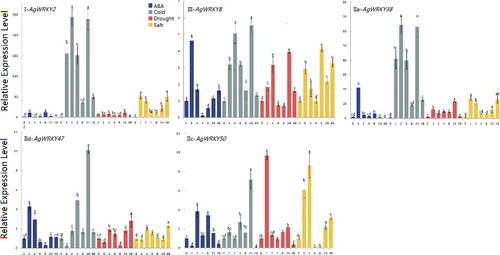
Evidence proved that WRKY TFs participate in a series of abiotic stresses. GmWRKY20 in wild soybean was upregulated under ABA treatment [Citation61]. Overexpression of OsWRKY8 in Arabidopsis improved the resistance to PEG and NaCl [Citation62]. Arabidopsis transgenic plants with GmWRKY21 can tolerate cold stress [Citation32]. Five AgWRKY TFs closely related to stress-related AtWRKYs [Citation31,Citation63–66] were selected to deduce their role under abiotic stress: AgWRKY2 from group I, AgWRKY8 from group III, AgWRKY38 from IIa, AgWRKY47 and AgWRKY50 from groups IId and IIc, respectively.
To reveal the expression patterns of AgWRKYs in different abiotic stresses, qRT-PCR was used to detect the expression levels of the five selected AgWRKYs (AgWRKY2, AgWRKY8, AgWRKY38, AgWRKY47 and AgWRKY50) in celery leaves (). Under ABA treatment, the expression levels of AgWRKY2, AgWRKY8, AgWRKY38 and AgWRKY47 showed an increased trend at first, followed by a reduction and then an increase. On the contrary, the expression levels of AgWRKY50 went down first and were then upregulated, with a final decline after 48 h. All the five selected AgWRKY genes were highly expressed under cold treatment, suggesting that these AgWRKYs may play important roles in the response to cold stress. Under drought treatment, AgWRKY2, AgWRKY8, AgWRKY38 and AgWRKY50 were upregulated during the first 24 h and then showed a decrease in expression. AgWRKY47 maintained a high expression level after 48 h drought treatment. The expression patterns of all the five AgWRKYs genes under salt treatment were similar; they increased at first and then declined, but were enhanced again at the end of the treatment. AtWRKY71 is reportedly induced by H2O2, mannitol and ABA [Citation66]. Its orthologous gene AgWRKY50 was also found to be upregulated under ABA, drought treatment and other abiotic treatments. High temperature can induce upregulation of AtWRKY39 [Citation31] and its orthologous gene in celery, AgWRKY47, was upregulated under different abiotic stresses in this study. These results demonstrated that the five AgWRKY TFs were involved in abiotic stress response. The results provided the foundation for investigating the roles of AgWRKYs in the resistance to abiotic stress in celery. This study expands the knowledge of WRKY TFs in celery and other higher plants. Further research should be performed to reveal the regulatory mechanism of WRKY TFs in the abiotic stress regulation network.
Conclusions
In this study, we identify 69 AgWRKY TFs from celery transcriptome data. AgWRKY proteins can be divided into three groups, of which group II contains five subgroups. Bioinformatics analysis predicted that AgWRKYs in the same subgroup share similar physicochemical properties. qRT-PCR results showed that the expression of AgWRKY genes can be upregulated or downregulated in response to various abiotic stresses. Our study will provide useful information for further research into the role of AgWRKY TFs in regulation mechanism in abiotic stress response. This study can provide potential useful information for the study of WRKY TFs in other plant species and molecular breeding of celery.
Supplementary_Data.zip
Download Zip (95.8 KB)Disclosure statement
The authors declare that there are no competing interests.
Additional information
Funding
References
- Pandey SP, Somssich IE. The role of WRKY transcription factors in plant immunity. Plant Physiol. 2009;150(4):1648–1655.
- Pan Y, Tsai CJ, Ma B, et al. Mechanisms of transcription factor selectivity. Trends Genet. 2010;26(2):75–83.
- Liu L, White MJ, MacRae TH. Transcription factors and their genes in higher plants functional domains, evolution and regulation. Eur J Biochem. 1999;262(2):247–257.
- Jin J, Zhang H, Kong L, et al. PlantTFDB 3.0: a portal for the functional and evolutionary study of plant transcription factors. Nucleic Acids Res. 2014;42:D1182–1187.
- Zhuang J, Zhang J, Hou XL, et al. Transcriptomic, proteomic, metabolomic and functional genomic approaches for the study of abiotic stress in vegetable crops. Crit Rev Plant Sci. 2014;33(2–3):225–237.
- Riechmann JL, Heard J, Martin G, et al. Arabidopsis transcription factors: genome-wide comparative analysis among eukaryotes. Science. 2000;290(5499):2105–2110.
- Schmutz J, Cannon SB, Schlueter J, et al. Genome sequence of the palaeopolyploid soybean. Nature. 2010;463(7278):178–183.
- Luscombe NM, Austin SE, Berman HM, et al. An overview of the structures of protein-DNA complexes. Genome Biol. 2000 [cited 2017 Aug 26];1(1):REVIEWS001. DOI: 10.1186/gb-2000-1-1-reviews001
- Eulgem T, Rushton PJ, Robatzek S, et al. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000;5(5):199–206.
- van Verk MC, Pappaioannou D, Neeleman L, et al. A novel WRKY transcription factor is required for induction of PR-1a gene expression by salicylic acid and bacterial elicitors. Plant Physiol. 2008;146(4):1983–1995.
- Guo C, Guo R, Xu X, et al. Evolution and expression analysis of the grape (Vitis vinifera L.) WRKY gene family. J Exp Bot. 2014;65(6):1513–1528.
- Sun C, Palmqvist S, Olsson H, et al. A novel WRKY transcription factor, SUSIBA2, participates in sugar signaling in barley by binding to the sugar-responsive elements of the iso1 promoter. Plant Cell. 2003;15(9):2076–2092.
- Ciolkowski I, Wanke D, Birkenbihl RP, et al. Studies on DNA-binding selectivity of WRKY transcription factors lend structural clues into WRKY-domain function. Plant Mol Biol. 2008;68(1-2):81–92.
- Brand LH, Kirchler T, Hummel S, et al. DPI-ELISA: a fast and versatile method to specify the binding of plant transcription factors to DNA in vitro. Plant Methods. 2010 [cited 2017 Aug 26];6:25. DOI: 10.1186/1746-4811-6-25
- Ishiguro S, Nakamura K. Characterization of a cDNA encoding a novel DNA-binding protein, SPF1, that recognizes SP8 sequences in the 5' upstream regions of genes coding for sporamin and beta-amylase from sweet potato. Mol Gen Genet. 1994;244(6):563–571.
- Dong J, Chen C, Chen Z. Expression profiles of the Arabidopsis WRKY gene superfamily during plant defense response. Plant Mol Biol. 2003;51(1):21–37.
- Ling J, Jiang W, Zhang Y, et al. Genome-wide analysis of WRKY gene family in Cucumis sativus. BMC Genomics. 2011 [cited 2017 Aug 26];12:471. DOI: 10.1186/1471-2164-12-471
- Qiu YP, Jing SJ, Fu J, et al. Cloning and analysis of expression profile of 13 WRKY genes in rice. Chinese Sci Bull. 2004;49(20):2159–2168.
- Huang S, Gao Y, Liu J, et al. Genome-wide analysis of WRKY transcription factors in Solanum lycopersicum. Mol Genet Genomics. 2012;287(6):495–513.
- Zhu XL, Liu SW, Meng C, et al. WRKY transcription factors in wheat and their induction by biotic and abiotic stress. Plant Mol Biol Rep. 2013;31(5):1053–1067.
- Li MY, Xu ZS, Tian C, et al. Genomic identification of WRKY transcription factors in carrot (Daucus carota) and analysis of evolution and homologous groups for plants. Sci Rep. 2016 [cited 2017 Aug 26];6:23101. DOI: 10.1038/srep23101
- Wu ZJ, Li XH, Liu ZW, et al. Transcriptome-wide identification of Camellia sinensis WRKY transcription factors in response to temperature stress. Mol Genet Genomics. 2016;291(1):255–269.
- Luo M, Dennis ES, Berger F, et al. MINISEED3 (MINI3), a WRKY family gene, and HAIKU2 (IKU2), a leucine-rich repeat (LRR) KINASE gene, are regulators of seed size in Arabidopsis. Proc Natl Acad Sci U S A. 2005;102(48):17531–17536.
- Jiang WB, Yu DQ. Arabidopsis WRKY2 transcription factor mediates seed germination and postgermination arrest of development by abscisic acid. BMC Plant Biol. 2009 [cited 2017 Aug 26];9:96. DOI: 10.1186/1471-2229-9-96
- Rushton PJ, Somssich IE, Ringler P, et al. WRKY transcription factors. Trends Plant Sci. 2010;15(5):247–258.
- Besseau S, Li J, Palva ET. WRKY54 and WRKY70 co-operate as negative regulators of leaf senescence in Arabidopsis thaliana. J Exp Bot. 2012;63(7):2667–2679.
- Schluttenhofer C, Yuan L. Regulation of specialized metabolism by WRKY transcription factors. Plant Physiol. 2015;167(2):295–306.
- Chen LG, Song Y, Li SJ, et al. The role of WRKY transcription factors in plant abiotic stresses. Biochim Biophys Acta. 2012;1819(2):120–128.
- Mukhtar MS, Deslandes L, Auriac MC, et al. The Arabidopsis transcription factor WRKY27 influences wilt disease symptom development caused by Ralstonia solanacearum. Plant J. 2008;56(6):935–947.
- Jiang YQ, Deyholos MK. Functional characterization of Arabidopsis NaCl-inducible WRKY25 and WRKY33 transcription factors in abiotic stresses. Plant Mol Biol. 2009;69(1-2):91–105.
- Li SJ, Fu QT, Chen LG, et al. Arabidopsis thaliana WRKY25, WRKY26, and WRKY33 coordinate induction of plant thermotolerance. Planta. 2011;233(6):1237–1252.
- Zhou QY, Tian AG, Zou HF, et al. Soybean WRKY-type transcription factor genes, GmWRKY13, GmWRKY21, and GmWRKY54, confer differential tolerance to abiotic stresses in transgenic Arabidopsis plants. Plant Biotechnol J. 2008;6(5):486–503.
- Li MY, Hou XL, Wang F, et al. Advances in the research of celery, an important Apiaceae vegetable crop. Crit Rev Biotechnol. Forthcoming 2017 [cited 2017 Aug 26]:1–12. DOI: 10.1080/07388551.2017.1312275
- Sowbhagya HB. Chemistry, technology, and nutraceutical functions of celery (Apium graveolens L.): an overview. Crit Rev Food Sci Nutr. 2014;54(3):389–398.
- Jia XL, Wang GL, Xiong F, et al. De novo assembly, transcriptome characterization, lignin accumulation, and anatomic characteristics: novel insights into lignin biosynthesis during celery leaf development. Sci Rep. 2015 [cited 2017 Aug 26];5:8259. DOI: 10.1038/srep08259
- Li MY, Wang F, Jiang Q, et al. Identification of SSRs and differentially expressed genes in two cultivars of celery (Apium graveolens L.) by deep transcriptome sequencing. Hortic Res. 2014 [cited 2017 Aug 26];1:10. DOI: 10.1038/hortres.2014.10
- Rhee SY, Beavis W, Berardini TZ, et al. The Arabidopsis Information Resource (TAIR): a model organism database providing a centralized, curated gateway to Arabidopsis biology, research materials and community. Nucleic Acids Res. 2003;31(1):224–228.
- Perez-Rodriguez P, Riano-Pachon DM, Correa LGG, et al. PlnTFDB: updated content and new features of the plant transcription factor database. Nucleic Acids Res. 2010;38(Database issue):D822–D827.
- Thompson JD, Higgins DG, Gibson TJ. Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22(22):4673–4680.
- Tamura K, Peterson D, Peterson N, et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28(10):2731–2739.
- Wilkins MR, Gasteiger E, Bairoch A, et al. Protein identification and analysis tools in the ExPASy server. Methods Mol Biol. 1999;112:531–552.
- Bailey TL, Boden M, Buske FA, et al. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res. 2009;37(Web Server issue):W202–W208.
- Szklarczyk D, Franceschini A, Wyder S, et al. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43(Database issue):D447–D452.
- Li MY, Wang F, Jiang Q, et al. Validation and comparison of reference genes for qpcr normalization of celery (Apium graveolens) at different development stages. Front Plant Sci. 2016 [cited 2017 Aug 26];7:313. DOI: 10.3389/fpls.2016.00313
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001 [cited 2017 Aug 26];29(9):e45. DOI: 10.1093/nar/29.9.e45
- Zhang Y, Wang L. The WRKY transcription factor superfamily: its origin in eukaryotes and expansion in plants. BMC Evol Biol. 2005 [cited 2017 Aug 26];5:1. DOI: 10.1186/1471-2148-5-1
- Rinerson CI, Rabara RC, Tripathi P, et al. The evolution of WRKY transcription factors. BMC Plant Biol. 2015 [cited 2017 Nov 30];15:66. DOI: 10.1186/s12870-015-0456-y
- Xie Z, Zhang ZL, Zou X, et al. Annotations and functional analyses of the rice WRKY gene superfamily reveal positive and negative regulators of abscisic acid signaling in aleurone cells. Plant Physiol. 2005;137(1):176–189.
- Rensing SA, Lang D, Zimmer AD, et al. The Physcomitrella genome reveals evolutionary insights into the conquest of land by plants. Science. 2008;319(5859):64–69.
- Wang YT, Pan YJ, Cho CC, et al. A novel pax-like protein involved in transcriptional activation of cyst wall protein genes in Giardia lamblia. J Biol Chem. 2010;285(42):32213–32226.
- He H, Dong Q, Shao Y, et al. Genome-wide survey and characterization of the WRKY gene family in Populus trichocarpa. Plant Cell Rep. 2012;31(7):1199–1217.
- Li HL, Zhang LB, Guo D, et al. Identification and expression profiles of the WRKY transcription factor family in Ricinus communis. Gene. 2012;503(2):248–253.
- Tripathi P, Rabara RC, Langum TJ, et al. The WRKY transcription factor family in Brachypodium distachyon. BMC Genomics. 2012;13:270. DOI: 10.1186/1471-2164-13-270
- Wei KF, Chen J, Chen YF, et al. Molecular phylogenetic and expression analysis of the complete WRKY transcription factor family in maize. DNA Res. 2012 [cited 2017 Aug 26];19(2):153–164.
- Brand LH, Fischer NM, Harter K, et al. Elucidating the evolutionary conserved DNA-binding specificities of WRKY transcription factors by molecular dynamics and in vitro binding assays. Nucleic Acids Res. 2013;41(21):9764–9778.
- Soltis P S, Soltis D E. The origin and diversification of angiosperms. Am J Bot. 2004;91(10):1614–1626.
- Jiang J, Ma S, Ye N, et al. WRKY transcription factors in plant responses to stresses. J Integr Plant Biol. 2017;59(2):86–101.
- Phukan U J, Jeena GS, Shukla RK. WRKY transcription factors: molecular regulation and stress responses in plants. Front Plant Sci. 2016 [cited 2017 Aug 26];7:760. DOI: 10.3389/fpls.2016.00760
- Mittler R, Kim Y, Song L, et al. Gain- and loss-of-function mutations in Zat10 enhance the tolerance of plants to abiotic stress. FEBS Lett. 2006;580(28-29):6537–6542.
- Andreasson E, Jenkins T, Brodersen P, et al. The MAP kinase substrate MKS1 is a regulator of plant defense responses. EMBO J. 2005;24(14):2579–2589.
- Luo X, Bai X, Sun X, et al. Expression of wild soybean WRKY20 in Arabidopsis enhances drought tolerance and regulates ABA signalling. J Exp Bot. 2013;64(8):2155–2169.
- Song Y, Jing SJ, Yu D Q. Overexpression of the stress-induced OsWRKY08 improves osmotic stress tolerance in Arabidopsis. Chinese Sci Bull. 2009;54(24):4671–4678.
- Shang Y, Yan L, Liu ZQ, et al. The Mg-chelatase H subunit of Arabidopsis antagonizes a group of WRKY transcription repressors to relieve ABA-responsive genes of inhibition. Plant Cell. 2010;22(6):1909–1935.
- Li J, Besseau S, Toronen P, et al. Defense-related transcription factors WRKY70 and WRKY54 modulate osmotic stress tolerance by regulating stomatal aperture in Arabidopsis. New Phytol. 2013;200(2):457–472.
- Schluttenhofer C, Pattanaik S, Patra B, et al. Analyses of Catharanthus roseus and Arabidopsis thaliana WRKY transcription factors reveal involvement in jasmonate signaling. BMC Genomics. 2014 [cited 2017 Aug 26];15:502. DOI: 10.1186/1471-2164-15-502
- Guo DS, Qin GJ. EXB1/WRKY71 transcription factor regulates both shoot branching and responses to abiotic stresses. Plant Signal Behav. 2016 [cited 2017 Aug 26];11(3):e1150404. DOI: 10.1080/15592324.2016.1150404