ABSTRACT
The aim of this work was to demonstrate the feasibility of nisin and lactic acid production from corn stover through simultaneous saccharification and fermentation using Lactococcus lactis subsp. lactis ATCC 11454. The optimum conditions for nisin and lactic acid production were cellulose concentration of 30 g/L, a cellulase loading of 25 FPU/g cellulose and an initial pH of 6.0 in a 10 g/L yeast extract and 10 g/L peptone broth. The optimized bioprocess achieved nisin titer of 1635 IU/mL and lactic acid concentration of 15.2 g/L. The results indicated that simultaneous saccharification and fermentation with corn stover is a promising bioprocess for the economic production of nisin and lactic acid simultaneously.
Introduction
Nisin is a natural antimicrobial peptide produced by Lactococcus lactis subsp. lactis, which inhibits Gram-positive bacteria effectively. It is used as preservative in the food industry worldwide. Nisin is produced by culturing L. lactis subsp. lactis in culture media, such as Man Rogosa and Sharpe medium (MRS), complete medium (CM), M17 [Citation1–3]. However, these culture media are unsuitable for large-scale nisin production because of their high costs. In industrial processes, pasteurized milk and yeast extract treated with protease have been used as a substrate. Moreover, alternative substrates including barley extract [Citation4], corn soluble and sago starch [Citation5] have been used to reduce the costs.
Lactic acid, which can also be used as a preservative and antimicrobial agent, accumulates in the fermentation broth during nisin production [Citation6]. Nisin production has been studied by controlling the concentration of lactic acid, which affects cell growth and yield [Citation7]. Optimizing the fermentation process to produce nisin and lactic acid simultaneously is attractive. Extracting lactic acid in stillage-based media to increase yields of both nisin and lactic acid has been investigated [Citation8]. Cheese whey or potato hydrolysate can be used as medium components to produce nisin and lactic acid [Citation9,Citation10].
Lignocellulose receives attention for the production of biochemicals such as lactic acid and ethanol because it is cheap, abundant and renewable. Simultaneous saccharification and fermentation (SSF) is a good strategy for lactic acid production from lignocellulose by lactic acid bacteria [Citation11]. As an abundant crop residue, corn stover was used to produce lactic acid with SSF and good performances of lactic acid yields were obtained [Citation12–15].
In this study, nisin and lactic acid were produced simultaneously using corn stover by SSF with Lactococcus lactis subsp. lactis ATCC 11 454. The objective was to demonstrate the feasibility of nisin and lactic acid production by SSF using corn stover as the carbon source and to optimize the substrate concentration, cellulase concentration, pH and nitrogen source. Nisin and lactic acid are both preservatives. Moreover, nisin can modify ruminal fermentation [Citation16] and exogenous cellulase could increase the fibre and total tract digestibility in ruminants [Citation17,Citation18]. The product mixture of the process including nisin, lactic acid, cellulase and polysaccharides can be used as a preservative of ruminal animal feed directly without separation and purification. Moreover, it could potentially promote nutrition absorption and manipulate ruminal fermentation.
Materials and methods
Raw materials and enzyme
The corn stover was pretreated with steam explosion and then, washed six times with water to remove soluble components. The average composition of the samples was 32.6% cellulose, 26.4% hemicelluloses and 31% lignin, which was determined using the laboratory analytical protocol developed by the National Renewable Energy Laboratory (Golden, USA) [Citation19]. The corn stover was stored at −20 °C. A commercially available cellulase Cellic CTec2 (Denmark, Novozymes) was used.
Culture and fermentation medium
Lactococcus lactis subsp. lactis ATCC 11 454, a nisin producing strain, was grown on MRS medium at 37 °C with agitation at 160 rpm for 20 h and the initial pH was adjusted to 6.5. One liter of MRS medium contained 10 g peptone, 10 g yeast extract (YE), 10 g glucose, 0.05 g MnSO4, 2 g K2HPO4, 5 g CH3COONa, 0.2 g MgSO4.7H2O, 2 g triammonium citrate and 1 g Tween 80.
Micrococcus flarus NCIB 8166, obtained from China General Microbiological Culture Collection Center (Beijing, China), was the test organism used in the agar diffusion bioassay for the quantification of nisin in a 0.5% YE, 1% peptone and 1% NaCl medium and incubated at 37 °C at 160 rpm for 12 h.
SSF experiment
SSF was conducted in 250 mL flasks containing 100 g medium, which contained corn stover, nitrogen source, 0.05 g/L MnSO4, 2 g/L K2HPO4, 5 g/L CH3COONa, 0.2 g/L MgSO4, 2 g/L triammonium citrate and 1 g/L Tween 80. Doses of 10 g/L CaCO3 were added to the medium to maintain a stable pH during fermentation. The flasks were sealed with rubber stoppers and equipped with syringe needles to vent the generated carbon dioxide.
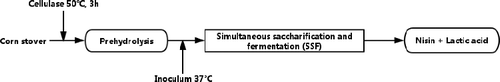
The fermentation process for nisin and lactic acid production was a prehydrolysis phase and an SSF phase (). After the cellulase was added to the medium, the flasks were incubated in a rotary shaker at 50 °C. After a 3 h prehydrolysis, each flask was inoculated with 5% (v/v) 12-h preculture seed and cultured at 37 °C. Samples were taken at 12 h intervals during the course of the 120 h fermentation. All experiments were performed in duplicate.
Figure 1. Flow chart of the process used to assess SSF of pretreated corn stover to produce nisin and lactic acid in an integrated process.
To determine the optimum conditions for nisin and lactic acid production from corn stover, carbon source concentration, nitrogen source, enzyme concentration and initial pH on SSF were investigated. The corn stover was diluted to the cellulose concentration of 10, 20, 30, 40 and 50 g/L and used as the carbon source. Cellulase loadings used in SSF experiments were 10, 15, 20, 25 or 50 FPU/g cellulose. The initial pH was adjusted to 5.0, 5.5, 6.0, 6.5 or 7.0 with 10% NaOH or 10% HCl. The effect of the nitrogen source on nisin and lactic acid production from corn stover was assayed at concentrations of yeast extract 0, 10 or 30 g/L supplemented with 0, 10 or 30 g/L peptone.
Analytical methods
Samples were treated with 5 mol/L H2SO4 to pH 2 and centrifuged at 14,000 g for 10 min to release lactic acid as calcium lactate, resulting from the reaction with the buffering agent CaCO3. Sugars and lactic acid were quantified by high performance liquid chromatography (HPLC) with a BioRad HXP87H column using a refractive index detector. The mobile phase was 0.005 mol/L H2SO4 and operated at 0.6 mL/min and 65 °C. For the assay of nisin titer, the samples were adjusted to pH 2 with a few drops of 5 mol/L HCl, heated in a boiling water bath for 10 min, cooled to room temperature and centrifuged (14,000 g) for 10 min. The supernatant was diluted 10-fold with 0.02 mol/L HCl and the nisin concentration was analysed by an agar well diffusion assay [Citation20].
Statistical analysis
The statistical analyses were performed using the SPSS version 21.0 software (IBM/SPSS, Chicago, IL, USA). The results are expressed as the mean values with standard error of the means (±SEM). For descriptive purposes, the mean values are presented as median values with standard deviation (±SD). A two-tailed P < 0.05 was considered statistically significant. Independent t-tests were used to compare the parameters between the two groups.
Results and discussion
Effect of corn stover concentration on nisin and lactic acid production
For the enzymatic hydrolysis of lignocellulose in SSF, increasing the concentration of lignocellulose leads to higher glucose concentrations, which in turn yields more products [Citation21]. As a drawback, higher concentrations of lignocellulose may increase the end-product enzyme inhibition. To investigate the effect of corn stover on nisin and lactic acid production, pretreated corn stover at different cellulose concentrations (10, 20, 30, 40 and 50 g/L) were evaluated. As shown in , the production of nisin and lactic acid were dependent on the cellulose concentration.
Figure 2. Effect of cellulose on simultaneous saccharification and fermentation for nisin and lactic acid production.
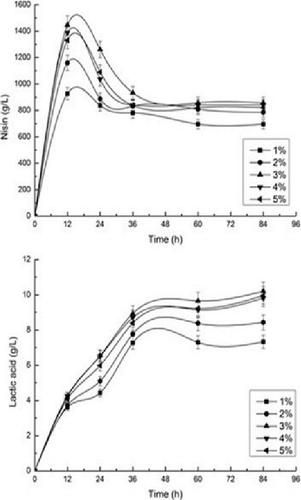
The nisin titer reached the highest value at 12 h, then decreased at different cellulose concentration. It is reported nisin titer decreased dramatically after reaching the peak value, which was suspected to be a result of proteolytic degradation and for adsorption of nisin by producer cells [Citation22]. The decrease of nisin by proteolysic degradation could be inhibited by protease inhibitor. The concentration of lactic acid increased sharply from zero hour to 36 h, and then increased slowly after 36 h. With increasing the substrate concentrations from 10 to 30 g/L, the nisin and lactic acid concentration increased because of the increasing total amount of sugars released from the substrate. The concentration of nisin titer increased from 927 to 1,446 IU/mL, while the concentration of lactic acid increased from 7.33 to 9.97 g/L as the initial substrate concentration increased from 10 to 30 g/L. An excess of dry weight would adversely affect the fermentation process, the nisin titer decreased slightly when the concentration of cellulose exceeded 30 g/L. The concentration of cellulose at 30 g/L was optimum for nisin and lactic acid production.
Higher concentrations of cellulose produced more fermentable sugars, which were liberated by the cellulase. However, high substrate concentration in the form of fibrous solid materials poses the following problems: (1) The solid portion of pretreated lignocellulose is gradually hydrolyzed into the liquid slurry containing monosaccharide sugars, oligomers sugars and insoluble lignin/ashes under the catalysis of cellulase enzymes during the SSF process; (2) The increased concentrations of inhibitors such as acetic acid, furfural and ethanol hamper the performance of cells and enzymes; (3) High viscosity results in more power consumption in the fermentor and lowered mixing and heat transfer efficiency [Citation23]. The hydrolysis step is inefficient when the substrate load exceeds a threshold value of the dry weight because of an increase in the viscosity [Citation24]. For the production of lactic acid, the finding that a higher initial cellulose concentration inhibits its utilization and product yield may be because of the inhibition of free lactic acid and calcium lactate on the activity of cellulase and an increase in substrate viscosity [Citation25]. Therefore, the nisin titer and lactic acid increased from 10 to 30 g/L cellulose, and then decreased from 30 to 50 g/L cellulose. The optimum cellulose concentration was 30 g/L.
Effect of cellulase concentration on nisin and lactic acid production
To determine the optimum cellulase loading in SSF, different cellulase loadings were investigated (). The nisin titer increased from 860 to 1,378 IU/mL as the cellulase loading increased from 10 to 25 FPU/g cellulose. By increasing the initial enzyme loading from 10 to 25 FPU/g, the nisin titer was increased by 60%. The concentration of lactic acid increased from 7.79 to 10.46 g/L with the increased cellulase concentration from 10 to 25 FPU/g. These results indicate that an increase in cellulase loading improves the corn stover conversion efficiency and product yields.
Figure 3. Effect of enzyme (cellulase) addition on simultaneous saccharification and fermentation for nisin and lactic acid production from pretreated corn stover.
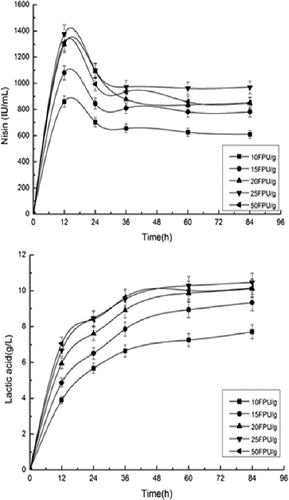
Enzymatic hydrolysis is the most promising means to yield fermentable sugars from pretreated lignocellulosic biomass, and is necessary to utilize cellulose as a carbon source [Citation11]. The higher cellulase concentration increases the hydrolysis rates to release more glucose, which was immediately consumed by L. lactis subsp. lactis to produce nisin and lactic acid in this work. However, the cost of enhancing the cellulase concentration to improve the production should be taken into account. The high cost of cellulases is one of the major bottlenecks of lignocellulosic materials production [Citation26]. Moreover, there is a probability of saturating cellulase with the corn stover [Citation27]. Therefore, taking into account both substrate utilization and economics, the cellulase loading of 25 FPU/g (cellulose) could be considered ideal for nisin and lactic acid production using the SSF process.
Effect of initial pH on nisin and lactic acid production
Initial pH is an important factor that influences both the strain growth and enzyme activities. An appropriate pH value is important for nisin and lactic acid production. To determine the effect of initial pH on the production of nisin and lactic acid in SSF, the initial pH was set at 5.0, 5.5, 6.0, 6.5 or 7.0 (). The highest nisin titer was 1,419 IU/mL when the initial pH was 6.0, the nisin production at initial pH 5.5 followed behind it, since the activity of cellulases was higher and cells grow better at pH 6.0 than at other pH values. At pH 6.0, the maximum lactic acid concentration was 9.02 g/L.
Figure 4. Effect of initial pH value on simultaneous saccharification and fermentation for nisin and lactic acid production from pretreated corn stover.
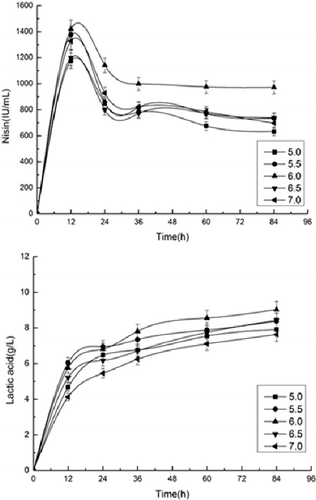
The pH values affect SSF, which includes the saccharification of cellulose, production of nisin and lactic acid and cell growth. There are different optimum pH values for each part. The process for the hydrolysis of cellulose is preferred to be carried out at pH 5.0, which is appropriate for the activity of cellulase [Citation28]. The optimum pH for the production of lactic acid is pH 6.0 [Citation29]. However, the appropriate pH for the growth of L. lactis subsp. lactis is pH 6.8, which is higher than the optimum pH for the hydrolysis of cellulose or production of lactic acid [Citation30].
The pH for nisin production (pH 5.5–6.0) is lower than that for the cell growth of L. lactis [Citation10]. pH is an important factor for the production of nisin. The pH values may affect nisin production by different mechanisms. Environmental pH during fermentation may alter microbial growth, which subsequently modifies the production, adsorption and desorption of nisin to the cell membrane, the post-translational modification or the secretion of nisin from the cells, and the gene regulation for the biosynthesis of nisin [Citation10]. When the fermentation was conducted at pH values 6.5 and 7.0, there are adverse effects on nisin fermentation, which are caused by the adsorption of nisin on the producer cells [Citation31]. At pH 6.4, reportedly about 1% and 23% of activity were lost after the nisin preparation was stored at 4 and 20 °C for 3 h, respectively, and the higher the pH value is, the more the nisin activity decreases [Citation32]. However, pH values such as 6.0 and 5.5 decrease cell growth [Citation33]. Therefore, a moderate initial pH value of 6.0 is suitable for cell growth in the whole process of nisin and lactic acid production, and the highest nisin titer and lactic acid production were obtained at pH 6.0 in this work.
Effect of nitrogen source concentration on nisin and lactic acid production
As popular nitrogen sources, yeast extract and peptone, which are used as a rich nutrient source of vitamin B and amino acids, were employed to produce nisin or lactic acid [Citation17]. YE and peptone have shown to aid the highest nisin or lactic acid production among alternative nitrogen sources as reported by Liu et al. [Citation3]. To determine the optimum nitrogen source concentration for the production of nisin and lactic acid in SSF, fermentations were carried ot at concentrations of 0, 10 or 30 g/L yeast extract supplemented with peptone at concentrations of 0, 10 or 30 g/L ().
Table 1. Effect of peptone and yeast extract (YE) on the production of lactic acid and nisin.
Nisin and lactic acid increased with the addition of YE and peptone, 1468 IU/mL nisin and 7.8 g/L lactic acid were obtained when 10 g/L YE was supplemented with 10 g/L peptone. A further increase in YE and peptone supplementation shows similar productions of nisin and lactic acid after reaching the maximum. Therefore, 10 g/L YE and 10 g/L peptone were the optimum among the tested values.
The medium composition has been investigated from many aspects for lactic acid production, including the addition of various concentrations of nutrients in the form of YE and peptone. For example, MRS medium, which contains YE and peptone, was superior to YE only. The addition of nutrients and higher nutrient concentrations generally had a positive effect on the lactic acid production [Citation34]. The effects of 11 nitrogen sources on lactic acid production through SSF were tested by Nguyen et al. [Citation25], revealing that the strain preferred organic nitrogen sources for L-lactic acid production. Furthermore, lactic acid production was markedly influenced by the type of nitrogen source. Yeast extract, peptone, skim milk, beef extract and casein hydrolysate increased the lactic acid production compared to the other nitrogen sources such as corn-steep liquor, urea, etc.; and 10 g/L YE and 10 g/L peptone yielded the highest lactic acid concentration [Citation25]. The same concentrations of 10 g/L YE and 10 g/L peptone for highest nisin and lactic acid production were observed in this work.
SSF
An SSF experiment was performed with 3% corn stover, cellulase loading of 25 FPU/g, initial pH of 6.0 and 10 g/L YE supplemented with 10 g/L peptone. presents the SSF kinetic profiles of nisin titer and lactic acid production during the cultivation. The maximum nisin titer and lactic acid concentration were 1,635 IU/mL and 15.2 g/L, respectively.
Figure 5. Kinetic profiles of nisin and lactic acid production by Lactococcus lactis subsp. lactis ATCC 11 454 during the SSF course.
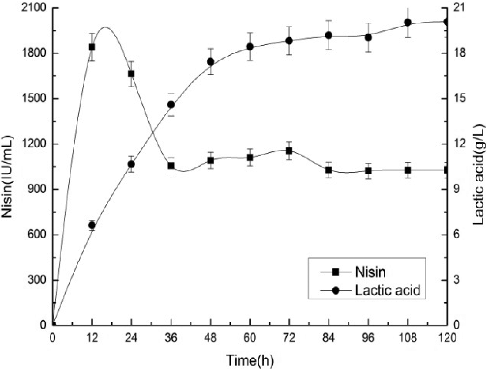
The low-cost medium containing the condensed corn soluble or cheese whey have been employed for nisin production by Lactococcus lactis subsp. lactis ATCC 11 454 in Wolf-Hall's work. The highest nisin titer of 795 IU/mL was obtained in the medium of cheese whey with pH control [Citation35]. Fermented barley extract obtained from a barley shochu by-product and its ethanol fractions have been evaluated as a medium for nisin production by Lactococcus lactis subsp. lactis ATCC 11 454 in the work of Furuta et al. [Citation4]. They obtained a maximum nisin titer of 1520 IU/mL with optimal medium [Citation4]. The nisin titer of 1,635 IU/mL obtained in our study is higher than the values reported in these works. This suggests that SSF with corn stover is an economically feasible and efficient process to produce nisin and lactic acid simultaneously.
Conclusion
In this study, nisin and lactic acid were produced from corn stover through SSF. The nisin titer and lactic acid concentration were 1635 IU/mL and 15.2 g/L, respectively after optimization of the conditions for nisin and lactic acid production. The results indicated that SSF with corn stover is a promising bioprocess for the economic production of nisin and lactic acid simultaneously. The product mixture of the process including nisin, lactic acid, cellulase and polysaccharides could be used as a potential additive of ruminant animal feed.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Cheigh CI, Choi HJ, Park H, et al. Influence of growth conditions on the production of a nisin-like bacteriocin by Lactococcus lactis subsp. lactis A164 isolated from kimchi. J Biotechnol. 2002;95:225–235.
- Li C, Bai J, Cai Z, et al. Optimization of a cultural medium for bacteriocin production by Lactococcus lactis using response surface methodology. J Biotechnol. 2002;93:27–34.
- Liu C, Liu Y, Chen S. Effects of nutrient supplements on simultaneous fermentation of nisin and lactic acid from cull potatoes. Appl Biochem Biotechnol. 2005;121:475–483.
- Furuta Y, Maruoka N, Nakamura A, et al. Utilization of fermented barley extract obtained from a by-product of barley shochu for nisin production. J Biosci Bioeng. 2008;106:393–397.
- Carvajal-Zarrabal O, Nolasco-Hipolito C, Bujang KB, et al. Production of nisin Z using Lactococcus lactis IO-1 from hydrolyzed sago starch. J Ind Microbiol Biotechnol. 2009;36:409–415.
- Reis JA, Paula AT, Casarotti SN, et al. Lactic acid bacteria antimicrobial compounds: characteristics and applications. Food Eng Rev. 2012;4:124–140.
- Lv W, Cong W, Cai Z. Improvement of nisin production in pH feed-back controlled, fed-batch culture by Lactococcus lactis subsp. lactis. Biotechnol Lett. 2014;26:1713–1716.
- Van't Hul JS, Gibbons WR. Neutralization/recovery of lactic acid from Lactococcus lactis: effects on biomass, lactic acid, and nisin production. World J Microb Biotechnol. 1997;13:527–532.
- Liu C, Liu Y, Liao W, et al. Simultaneous production of nisin and lactic acid from cheese whey. Appl Biochem Biotechnol. 2004;113:627–638.
- Liu X, Chung YK, Yang ST, et al. Continuous nisin production in laboratory media and whey permeate by immobilized Lactococcus lactis. Process Biochem. 2005;40:13–24.
- Abdel-Rahman MA, Tashiro Y, Sonomoto K. Lactic acid production from lignocellulose-derived sugars using lactic acid bacteria: overview and limits. J Biotechnol. 2001;156:286–301.
- Zhao K, Qiao Q, Chu D, et al. Simultaneous saccharification and high titer lactic acid fermentation of corn stover using a newly isolated lactic acid bacterium Pediococcus acidilactici DQ2. Bioresource Technol. 2013;35:481–489.
- Xia Y, Zhang P, Sun J, et al. Engineering wild-type robust Pediococcus acidilactici strain for high titer l- and d-lactic acid production from corn stover feedstock. J Biotechnol. 2016;217:112–121.
- Liu G, Sun J, Zhang J, et al. High titer L-lactic acid production from corn stover with minimum waste water generation and techno-economic evaluation based on Aspen plus modeling. Bioresource Technol. 2015;198:803–810.
- Hu JL, Lin YX, Zhang ZT, et al. High-titer lactic acid production by Lactobacillus pentosus FL0421 from corn stover using fed-batch simultaneous saccharification and fermentation. Bioresource Technol. 2016;214:74–80.
- Lee SS, Mantovani HC, Russell JB. The binding and degradation of nisin by mixed ruminal bacteria. FEMS microbial Ecol. 2002;42:339–345.
- Yang WZ, Beauchemin KA, Rode LM. A comparison of methods of adding fibrolytic enzymes to lactating cow diets. J Dairy Sci. 2000;83:2512–2520.
- Titi HH, Tabbaa MJ. Efficacy of exogenous cellulase on digestibility in lambs and growth of dairy calves. Livest Sci. 2004;87:207–214.
- Sluiter A, Hames B, Ruiz R, et al. Determination of structural carbohydrates and lignin in biomass. United States: National Renewable Energy Laboratory Golden; 2008. Lap–008.
- Wolf CE, Gibbons WR. Improved method for quantificationof the bacteriocin nisin. J Appl Microbiol. 1996;80:453457.
- Zhao X, Song Y, Liu D. Enzymatic hydrolysis and simultaneous saccharification and fermentation of alkali/peracetic acid-pretreated sugarcane bagasse for ethanol and 2, 3-butanediol production. Enzyme Microb Tech. 2011;49:413–419.
- De Arauz LJ, Jozala AF, Mazzola PG. Nisin biotechnological production and application: a review. Trends Food Sci Technol. 2009;20:146–154.
- Zhang MJ, Wang F, Su RX, et al. Ethanol production from high dry matter corncob using fed-batch simultaneous saccharification and fermentation after combined pretreatment. Bioresource Technol. 2010;101:4959–4964.
- Almarsdottir AR, Sigurbjornsdottir MA, Orlygsson J. Effect of various factors on ethanol yields from lignocellulosic biomass by Thermoanaerobacterium AK17. Biotechnol Bioeng. 2012;109:686–694.
- Nguyen CM, Kim JS, Nguyen TN, et al. Production of L-and D-lactic acid from waste Curcuma longa biomass through simultaneous saccharification and cofermentation. Bioresource Technol. 2013;146:35–43.
- Lin Y, Tanaka S. Ethanol fermentation from biomass resources: current state and prospects. Appl Microbiol Biot. 2006;69:627–642.
- Pessani NK, Atiyeh HK, Wilkins MR. Simultaneous saccharification and fermentation of Kanlow switchgrass by thermotolerant Kluyveromyces marxianus IMB3: the effect of enzyme loading, temperature and higher solid loadings. Bioresource Technol. 2011;102:10618–10624.
- Chen K, Zhang H, Miao Y, et al. Simultaneous saccharification and fermentation of acid-pretreated rapeseed meal for succinic acid production using Actinobacillus succinogenes. Enzyme Microb Tech. 2011;48:339–344.
- Nakasaki K, Adachi T. Effects of intermittent addition of cellulase for production of L-lactic acid from wastewater sludge by simultaneous saccharification and fermentation. Biotechnol Bioeng. 2003;82:263–270.
- Vuyst LD, Vandamme EJ. Bacteriocins of lactic acid bacteria: microbiology, genetics and applications. London: Blackie Academic & Professional; 1994.
- Yang R, Johnson MC, Ray B. Novel method to extract large amounts of bacteriocins from lactic acid bacteria. Appl Environ Microb. 1992;58:3355–3359.
- Huot E, Barrena-Gonzalez C, Petitdemange H. Comparative effectiveness of nisin and bacteriocin J46 at different pH values. Lett Appl Microbiol. 1996;22:76–79.
- Liu W, Zheng H, Wu Z, et al. Effects of pH profiles on nisin fermentation coupling with foam separation. Appl Microbiol Biotechnol. 2010;85:1401–1407.
- Karin H, Ba¨rbel HH. Factors affecting the fermentative lactic acid production from renewable resources. Enzyme Microb Technol. 2000;26:87–107.
- Wolf-Hall CE, Gibbons WR, Bauer NA. Development of a low-cost medium for production of nisin from Lactococcus lactis subsp. lactis. World J Microb Biotechnol. 2009;25:2013–2019.
