ABSTRACT
Antenatal post-traumatic stress disorder (PTSD can leave offspring with life-long mental and behavioural disorders. This study investigated the epigenetic mechanism of the effects of Jin Kui Shen Qi Wan (JKSQW) in preventing antenatal PTSD. Thirty-six pregnant rats were randomized into Control, PTSD and JKSQW. Offspring characteristics were investigated with open-field test (OFT), elevated pull maze (EPM), gene expression profile chip test and methylated DNA immunoprecipitation sequencing. In the OFT test, the PTSD offspring showed lower OFT scores than the Control and JKSQW groups (p < 0.05). The PTSD group's EPM performance, OE (open arm entry) + CE (closed arm entry), OE% and OT% (open arm time) were also lower than those of either the Control or the JKSQW group (p < 0.05). In the gene expression profile chip test, 54 genes showed dysregulation through PTSD intervention and were normalized by JKSQW. Endocytosis (Epn3, Folr1 and Hspa1l) and MAPK signaling (Fgf17, Hspa1l, Ntf3 and Pla2g5) pathways were annotated. Combining those genes and methylation sequencing, Ntf3 was identified in both tests. Compared with the Control, the PTSD offspring showed Ntf3 hypermethylation (Log2FC = 1.022) at 225679501∼225680000 on Chromosome 4 and downregulation in the gene expression profile. The Ntf3 methylation level in JKSQW was restored nearly to the Control level (Log2FC = −1.082 in JKSQW vs. PTSD). In conclusion, the reduction in the offspring's behavioural performance might constitute experimental evidence of kidney deficiency due to Ntf3 hypermethylation, which could be prevented by JKSQW.
Introduction
Post-traumatic stress disorder (PTSD) is clinically characterized by recurrent flashbacks, psychological distress and distressing memories or dreams about a past traumatic event. These symptoms present after exposure to traumatic events, e.g. earthquakes, car accidents, sexual violence, war, operations, etc. [Citation1] Epidemiological research papers on trauma and PTSD in several populations have been reported. The incidence rate ranges from 9.5% to 52%, with PTSD presenting in 9.5% of cancer patients, 20% of intensive care, 12% of combat soldiers and 27% of firefighters [Citation2–Citation4]. PTSD is more prevalent in females. The rates of PTSD, both among patients with serious mental illness and among women with substance use disorders, greatly exceed the rate in the general population [Citation5,Citation6]. Behrendt and Moritz [Citation7] reported that circumcised women showed a significantly higher prevalence of PTSD (30.4%) and other psychiatric syndromes (47.9%) than uncircumcised women. Childbirth is considered as a traumatic experience that can lead to PTSD. PTSD is pervasive in women of childbearing age. The cascade of behavioural health and neuroendocrine changes commonly associated with PTSD may adversely affect perinatal health. PTSD is mostly related to anxiety and sadness during a past pregnancy or childbirth, previous difficult delivery experiences, a preference for cesarean section, emotional crises during pregnancy, higher expected intensity of pain, increased fear of childbirth and depression during pregnancy [Citation8]. It has been reported [Citation9] that antenatal depressive symptoms, state anxiety and perinatal psychoform and somatoform dissociation were identified as PTSD symptom risk factors three months post-partum. The hypothalamic–pituitary–adrenal (HPA) axis plays an important role in the stress regulatory system, and the dysfunction of HPA axis activity is a well-characterized feature in PTSD [Citation10]. The HPA axis secretes glucocorticoids in response to stress stimulation, which are essential in maintaining biological and psychological homeostasis and adaptation to chronic stressors [Citation11].
Antenatal maternal effects are regarded as an influential factor in offspring development in various species. Prenatal PTSD in Sprague–Dawley pregnant rats negatively affected the neuro-developmental outcomes in the offspring, including delayed cognitive development and behavioural problems [Citation12]. There is a growing body of evidence supporting the role of complex interactions between traumatic stress and various genetic factors in PTSD [Citation13]. Epigenetic alteration is reported to underlie and underpin the interactions and mediate the impact of environmental and stressful traumatic stimulation on gene expression and regulation. Kwapis et al. [Citation14] discussed the role of epigenetic mechanisms in the formation, storage, updating and extinction of fear memories in PTSD and concluded that epigenetic mechanisms might provide a novel target for pharmaceutical and other treatments to reduce unpleasant memories contributing to PTSD. Kaminsky et al. [Citation15] reported that epigenetic variation at SKA2 mediated the vulnerability to suicidal behaviours and PTSD through the dysregulation of the HPA axis in response to stress. Labonte et al. [Citation16] reported that traumatic events could induce DNA methylation alterations in certain promoters of hGR, with transcriptional modifications correlated with a hypoactive HPA axis in PTSD. Antenatal PTSD during pregnancy can leave lifelong mental disorders and behavioural problems to the offspring. Previous research showed that the effect may result from the trans-generational epigenetic programming of genes operating in the HPA axis, such as hGR [Citation17].
Although PTSD is not yet a recognized disorder in Chinese Medicine, its symptoms are likely to be relieved by treatment with Chinese Medicine [Citation18]. In Chinese Medicine, prenatal stressors such as those implicated in PTSD are considered as factors that impair kidney Qi (kidney energy). The kidneys are regarded as the origin and resource of congenital constitution, which governs reproduction and development [Citation12]. JKSQW is a typical herbal formula supplementing kidney Qi, which helps in the recovery of the physiological functions of the kidneys. A growing body of research on the roles of Chinese Medicine in the treatment of PTSD is emerging. Qiu et al. [Citation19] reported that ‘Free and Easy Wanderer Plus’ – a traditional Chinese herbal medicine – could significantly reverse the behavioural deficits resulting from single prolonged stress (SPS)-induced PTSD in rats, and that the anti-PTSD effects of the medicine were associated with allopregnanolone biosynthesis. Zhang et al. [Citation12] found that maternal shaking (earthquake simulation) during pregnancy negatively affected the physical and nervous system development, with specific alterations to neuro-hormone production and gene expression, resulting in negative outcomes for the offspring, which could be reversed by JKSQW.
To our knowledge, plenty of reports have focused on the dysregulation of the HPA axis genes caused by site-specific methylation; however, only a few studies have covered the whole-genome methylation dysregulation related to maternal PTSD. JKSQW is an effective medicine for treating the symptoms of PTSD; however, no reports have covered the underlying epigenetic mechanism. Therefore, we hypothesized that antenatal PTSD could negatively influence the offspring, which could be prevented and treated by JKSQW, and the underlying mechanism could be attributed to certain methylation dysregulation.
Materials and methods
Animals
Forty non-pregnant female (230∼280 g) and 46 male (222g∼280 g) Sprague–Dawley rats were involved in this study. The rats were allowed one week to adapt to the conditions (22 °C, 12-hour light/dark cycles and water and food ad libitum). After the adaptation period, the male rats mated with the female rats, whose pregnancy was confirmed by observing vaginal plugs. Then, 36 pregnant rats were randomly selected and grouped in accordance with the random number table: Control (n = 12), PTSD (n = 12), and JKSQW (n = 12). Each set of 12 rats was housed in three cages, four animals in each cage in each group. All rats were induced with equivalent stress during pregnancy. No difference in body weight was detected 7 days after pregnancy and before intervention (Control: 397.886 ± 21.112 g, PTSD: 392.691 ± 12.482 g; JKSQW: 400.980 ± 14.238 g, ANOVA, p = 0.720, t = 0.345, df = 2, F = 0.720, p = 0.495) among the three groups. All the offspring were housed with their mothers until the 30th day postpartum [Citation12].
The experimental protocol was reviewed and approved by the Experimental Animal Care and Use Committee of Chengdu University of Traditional Chinese Medicine. Care was taken to minimize discomfort and the number of animals used throughout experiments. The rats were euthanised by decapitation.
PTSD simulation
The SPS model was used to mimic PTSD. The SPS model replicates the specific neuroendocrinological abnormalities observed in PTSD patients [Citation20]. The pregnant SD rats in the PTSD group were restrained for 2 h and immediately afterwards underwent an uninterrupted 20-minute forced swim in water (T = 25 °C, 40 cm depth of water) in a plastic tub (diameter 50 cm, height 70 cm), 6 rats at a time. The intervention was performed between 8 and 14 days after pregnancy. No rat was found to have died or become very weak during the process.
JKSQW feed
JKSQW powder (Tong Ren Tang Technologies, Co., Ltd.) 0.5∼0.6 g/d was added to the ordinary feed in this group until delivery [Citation11]. A JKSQW tablet consists of Radix Rehmanniae Preparata (Shu Di Huang), Cortex Moutan Radicis (Mu Dan Pi), Fructus Corni Officinalis (Shan Zhu Yu), Rhizoma Dioscoreae Oppositae (Shan Yao), Rhizoma Alismatis Orientalis (Ze Xie), Sclerotium Poriae Cocos (Fu Ling), Radix Aconiti Lateralis Preparata (Zhi Fu Zi) and Cortex Cinnamomi Cassiae (Rou Gui).
Open field test (OFT)
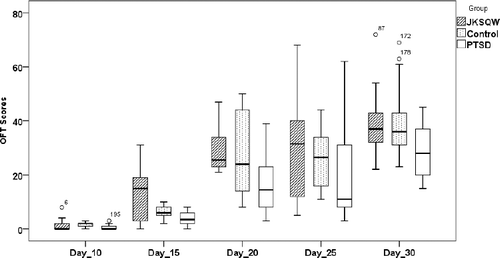
A square board (120 cm × 120 cm) was painted with yellow and white squares (15 cm × 15 cm). Eighteen offspring from each group, 10-day, 15-day, 20-day, 25-day and 30-day-old were randomly selected and placed in the center of the board. We counted how many squares the offspring crawled across in 2 min. Each additional score number was given only when all four paws of the offspring were in one square [Citation12].
Elevated plus maze (EPM)
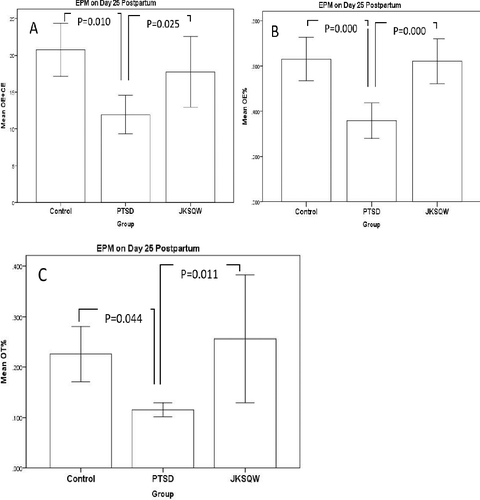
The EPM test was conducted with two open arms (50cm*10 cm) and two closed arms with high walls (50cm*10cm*40 cm) and a central area (10*10 cm) 60 cm above the floor. The illumination above the central platform was around 20 lx. The behaviours on the maze were recorded by a video camera and were analyzed with Super Maze (XR-Xmaze, Shanghai Xinxin, Shanghai, China). Observation included OE (open arm entry), OT (open arm time), CE (closed arm entry), CT (closed arm time), OE% (OE/(OE+CE) *100%), and OT% (OT/(OT+CT) *100%) [Citation20].
Gene expression profile chip experiment
The blood samples were taken from the femoral artery, 10 samples for each group. The test was conducted as described in our previous studies [Citation12,Citation21], including three steps, i.e. Step1.RNA extraction and purification, Step2. RNA amplification and labeling, and Step 3. data acquisition.
Methylated DNA immunoprecipitation sequencing (MeDIP-Seq)
The sequencing used the same samples as the profiling experiment: blood samples from the femoral artery, 10 samples per group. Total DNA was extracted and purified by DNeasy Blood & Tissue Kit (Qiagen, #69 506). After fragmentation, covaris shearing generated dsDNA fragments with 3' or 5' overhangs. The overhangs were converted into blunt ends using an End Repair Mix. The products of the ligation reaction were purified on a gel and the unligated adapters were removed. A sequencing library with an appropriate size range was selected for cluster generation. Enrichment was immediately followed by the application of the Magnetic Methylated DNA Immunoprecipitation kit (Diagenode). The size of the DNA library templates was checked by an Agilent Technologies 2100 Bioanalyzer, and the concentration by a Qubit® 2.0 Fluorometer. The cluster was generated and hybridized with primers on the cBot System following the cBot User Guide (Illumina Inc.). Sequencing was performed using a flow cell (Flow Cell V3, single end 1 × 50 nt, Illumina Inc.) with the clusters on an Illumina HiSeq 2500. The data were analyzed as follows: Raw Reads → Preprocess Reads → Mapping Genome → BAM/SAM → DMR Detection → Relation Gene, Relation CpG Island [Citation22].
Statistical analysis
The behavioural test data were analyzed using SPSS (Statistical Package for the Social Sciences) version 19.0. A Generalized Estimating Equations (GEE) test was used to analyze group differences on the repeated observation of OFT and analysis of variance (ANOVA) to EPM. Alpha was set to 0.05 for all analyses.
Results and discussion
OFT and EPM
Offspring were observed 10, 15, 20, 25 and 30 days after birth. The GEE test showed a statistically significant difference in the repeatedly measured OFT scores (Wald χ2 = 30.514, df = 1, p < 0.05), and the PTSD offspring showed lower OFT scores than those of the Control and JKSQW group (). ANOVA analysis revealed that, in the comparison of OE (open arm entry) + CE (closed arm entry), OE% and OT% (open arm time), the PTSD results were all statistically poorer than those of the Control and JKSQW group (p < 0.05) ().
In this study, the findings showed that the negative impacts derived from maternal PTSD were passed on to the offspring, with lowered OFT scores and EPM performance, which are validated by behaviour tests [Citation23]. In the OFT, the PTSD offspring showed lower scores, but JKSQW intake by mothers until the end of the pregnancy normalized the performance of the offspring in this test. Previous research has reported that the PTSD experience of juvenile stress could reduce the performance in the OFT Our previous studies found that prenatal earthquake simulation on pregnant rats lowered the offspring's OFT performance [Citation21,Citation22]. In EPM, the PTSD offspring produced poor results for OE+CE, OE% and OT%, which could all be corrected with JKSQW. Estanislau and Morato [Citation23] reported that prenatal stress had more pronounced anxiogenic effects than maternal separation, as judged by reduced exploration of the open arms of the elevated plus-maze. Estanislau and Morato [Citation24] reported that decreases in the percentage of entries into and the time spent in the open arms were observed in 60-day-old prenatally stressed female rats. From the perspective of Chinese Medicine, once the parental kidney is injured from prenatal PTSD, the consequent effects could be handed down to the offspring, resulting in the reduction of locomotion and exploration [Citation12,Citation25]. JKSQW is a representative herbal formula to supplement the kidney Qi, which helps in the recovery of the physiological functions of the kidney, i.e. motor abilities, and OFT and EPM performance [Citation21].
Gene expression profile
In the comparison of the PTSD offspring vs. the Control group, the gene expression profile showed that 70 genes were upregulated and 42 genes were downregulated 2-fold or more increase or a decrease to 0.5 or less of the expression level, respectively (p < 0.05). In the comparison of JKSQW vs. Control, 37 984 genes were found not to be differentially expressed (p > 0.05), of which 54 genes were found to be differentially expressed in the comparison of PTSD vs. Control (). Therefore, these 54 genes showed dysregulation through PTSD intervention and were normalized by JKSQW.
Table 1. Fifty-four differentially expressed genes due to PTSD and their normalization JKSQW.
KEGG annotation
With Kyoto Encyclopedia of Genes and Genomes (KEGG) annotation, the differentially expressed genes were identified to be associated with two significant pathways, i.e. endocytosis and MAPK signaling, were identified (p < 0.05, q < 0.05, enrichment test P-value < 0.05, hits = 3). The endocytosis pathway involved Epn3, Folr1 and Hspa1l, and the MAPK signaling pathway involved Fgf17, Hspa1l, Ntf3 and Pla2g5 from the 54 genes above.
Methylation sequencing and combination with gene expression profile chip test
In the comparison of the methylation sequencing outcome between PTSD and Control, 10 797 statistically significant loci were obtained, including 114 216 CF and 5296 differentially expressed genes, among which 943 genes showed hypermethylation (fold change > 2) and 1775 genes showed hypomethylation. Meanwhile, in the comparison of PTSD vs. JKSQW, 14 887 loci were obtained, including 177 091 CF and 891 genes with hypermethylation and 730 genes with hypomethylation. Combining the methylation sequencing and gene expression profile chip test findings, the gene Ntf3 was identified in both tests. In comparison with the Control group, Ntf3 showed hypermethylation (Log2FC = 1.022) at 225679 501∼225680 000 on Chromosome 4 in PTSD, and its expression was downregulated in the PTSD offspring. The methylation level of Ntf3 in JKSQW was normalized to almost the same level as in the Control group (Log2FC = −1.082 in JKSQW vs. PTSD). Meanwhile, the locus 225696 294∼225696 500 was also found to be hypermethylated (Log2FC = 1.043). However, this locus of methylation dysregulation was not found in the comparison of PTSD vs. Control (p > 0.05).
Prenatal PTSD causes predisposition to cognitive and emotional disturbances and is a risk factor for the development of nervous system and locomotion retardation. For instance, Palacios-García et al. [Citation26] found that prenatal stress downregulated Reelin expression by methylation of its promoter and induced adult behavioural impairments in rats. PTSD has been proved to contribute to genome-wide significant polymorphism, which was associated with differential epigenetic regulation [Citation27]. Our findings demonstrated that 54 significantly differentially expressed genes were involved in trans-generation PTSD stimulation, which could be normalized by JKSQW. Thus it is reasonable to assume that the therapeutic effect of JKSQW lies in treating the negative impacts of prenatal stress in dysregulating gene expression, or the underlying mechanism of supplementing ‘kidney deficiency,’ as it is called in Chinese Medicine. Interestingly, the MAPK signaling and endocytosis pathways were involved in the alterations. Our findings are in accordance with previous reports [Citation28–Citation32]. Microarray-based profiling of the hippocampus and frontal cortex showed the involvement of signal transduction cascades affected by prenatal stress including (MAPK) signaling, neurotrophic factor signaling, glutamatergic and GABAergic neurotransmission, phosphodiesterase/cyclic nucleotide signaling, etc. [Citation28]. Endocytosis is the process whereby outer material is engulfed into a cell through the cell membrane. Endocytosis plays multiple roles in PTSD. Glutamate, a major excitatory neurotransmitter involved in synaptic transmission in the brain, is released from the presynaptic terminal and binds to AMPA receptors that mediate synaptic transmission [Citation29]. The number of synaptic α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptors is controlled via exocytosis and endocytosis and lateral diffusion [Citation30]. Bai et al. [Citation31] reported that D-serine might enhance fear extinction by increasing GluA2-containing AMPA receptor endocytosis, and that D-serine may be a potential therapeutic agent against learning and memory disorders. Lin et al. [Citation32] found that β-adrenergic receptor activation and GABAA receptor endocytosis were involved in the amygdala for the reinstatement of fear memories. As mentioned above, epigenetic regulation bridges environmental and genetic factors and underlies the trans-generation effect. Methylation is one of the major mechanisms of gene expression regulation.
Our genome methylation sequencing findings showed extensive dysregulation of methylation resulting from prenatal PTSD. JKSQW could also extensively alter the methylation profile. Evidence has been released regarding Chinese herbal medicine's possible intervention in gene methylation. For instance, Sohn et al. [Citation33] found that V. rotundifolia was the best candidate for the demethylation of ZIC1, a tumour suppressor gene that is silenced through promoter hypermethylation in colorectal cancer. Su et al. [Citation34] found that Radix Angelicae Sinensis (Dang Gui) and Z-Ligustilide were able to demethylate the Nrf2 promoter CpGs, resulting in the re-expression of Nrf2 and Nrf2 target genes, which might therefore contribute to the anticancer effect of Radix Angelicae Sinensis.
Among the genes whose dysregulation was corrected with JKSQW in PTSD offspring, Ntf3 was screened. Hypermethylation underlay the downregulation of Ntf3 expression. JKSQW normalized its expression through its hypomethylation effect. Ntf3 is a neurotrophic factor in the NGF (nerve growth factor) family of neurotrophins, which promotes the survival and differentiation of existing neurons, and encourages the growth and differentiation of new neurons and synapses [Citation34,Citation35]. Ntf3 plays a role similar to that of NGF and BDNF (brain derived neurotrophic factor) in the neurotrophic process [Citation36]. Recent reports have suggested that Ntf3 gene may play a certain role in the progression of ADHD (attention-deficit hyperactivity disorder), Hirschsprung and other central nervous system disorders [Citation37]. However, to our knowledge, no literature on the effect of Ntf3 in prenatal maternal PTSD exists.
The herbal formulas in Chinese Medicine have been involved in the Chinese health care system for over 2,000 years. The field of genetics, including the use of ‘omic’ technologies, is an evolving area of science that has emerging applications in the modern research of herbal medicine. Related omics studies include pharmacogenomics, proteomics and metabolomics. Chinese Herbal Medicine may benefit from omics studies, and this new field may be termed ‘herbomics’ [Citation38]. Hsieh et al. [Citation39] reported that 3,294 Chinese Medicine medicinals contained 48,491 chemicals that interact with epigenetics-related proteins and that 29.8% of the medicinals were epigenome- and miRNA-modulating, primarily, via interactions with the Polycomb group and methyl CpG-binding proteins. Furthermore, 99% of 200 government-approved Chinese Medicine formulas interact with the epigenome or miRNA. Sun et al. [Citation40] reported that an arsenic-containing Chinese herbal formula resulted in significant genome-wide demethylation in the treatment of MDS (myelodysplastic syndromes). JKSQW was reported to cause genome-wide gene expression dysregulation in the treatment of prenatal stress or ‘kidney xu’ (a pattern name used in Chinese Medicine to define a group of symptoms related to kidney disorders) [Citation12]. To our knowledge, this is the first report on the methylation regulation effect of JKSQW. This finding may provide new perspectives for understanding the effect of JKSQW in assisting kidney function.
There are still certain limitations to the present study. Ntf3 has not been validated by RT-PCR yet. Samples from the brain region, e.g. the hippocampus, might be more specific and persuasive in demonstrating the epigenetic mechanism of influences from prenatal PTSD. This genetic and molecular alteration of Ntf3 will be more deeply studied in our next project. Another limitation is that no positive control, such as a biochemical drug or psychological therapy, was employed, which might reduce the reliability of the finding that JKSQW prevented PTSD. In future clinical research, other modalities will be included to validate the effectiveness of JKSQW.
Conclusions
Prenatal maternal simulation of PTSD during pregnancy could negatively alter the behavioural performance in the offspring, as shown by their reduced OFT and EPM scores, which might constitute experimental evidence of kidney deficiency resulting from prenatal damage. JKSQW could successfully rectify the kidney deficiency and improve the offspring's OFT and EMP performance. Fifty-four differentially expressed genes contributed to the alterations. Furthermore, the hypermethylation of Ntf3 might play a key role in inducing kidney deficiency through maternal PTSD, which could be treated with JKSQW.
Acknowledgments
This study was supported by the National Science Funds of China under grant number 81373710 and Fund of Chengdu University of TCM under grant number ZRQN1541.
Disclosure statement
The authors declare no conflict of interests.
Additional information
Funding
References
- Osório C, Jones N, Jones E, et al. Combat experiences and their relationship to post-traumatic stress disorder symptom clusters in UK military personnel deployed to Afghanistan. Behav Med. 2017;10:1–10.
- Wu X, Wang J, Cofie R, et al. Prevalence of posttraumatic stress disorder among breast cancer patients: a meta-analysis. Iran J Public Health. 2016;45(12):1533–1544.
- Hoge CW, Riviere LA, Wilk JE, et al. The prevalence of post-traumatic stress disorder (PTSD) in US combat soldiers: a head-to-head comparison of DSM-5 versus DSM-IV-TR symptom criteria with the PTSD checklist. Lancet Psychiatry. 2014;1(4):269–277.
- Greene T, Neria Y, Gross R. Prevalence, detection and correlates of PTSD in the primary care setting: A systematic review. J Clin Psychol Med Settings. 2016;23(2):160–180.
- Gillikin C, Habib L, Evces M, et al. Trauma exposure and PTSD symptoms associate with violence in inner city civilians. J Psychiatr Res. 2016;83:1–7.
- Gradus JL, Leatherman S, Curreri A, et al. Gender differences in substance abuse, PTSD and intentional self-harm among veterans health administration patients. Drug Alcohol Depend. 2017;171:66–69.
- Behrendt A, Moritz S. Posttraumatic stress disorder and memory problems after female genital mutilation. Am J Psychiatry. 2005;162(5):1000–1002.
- Polachek IS, Dulitzky M, Margolis-Dorfman L, et al. A simple model for prediction postpartum PTSD in high-risk pregnancies. Arch Womens Ment Health. 2016;19(3):483–490.
- Haagen JF, Moerbeek M, Olde E, et al. PTSD after childbirth: A predictive ethological model for symptom development. J Affect Disord. 2015;185:135–143.
- Lehrner A, Bierer LM, Passarelli V, et al. Maternal PTSD associates with greater glucocorticoid sensitivity in offspring of Holocaust survivors. Psychoneuroendocrinology. 2014;40:213–220.
- Ryan KK, Mul JD, Clemmensen C, et al. Loss of melanocortin-4 receptor function attenuates HPA responses to psychological stress. Psychoneuroendocrinology. 2014;42:98–105.
- Zhang XG, Zhang H, Tan R, et al. Mechanism of earthquake simulation as a prenatal stressor retarding rat offspring development and Chinese medicine correcting the retardation: hormones and gene-expression alteration. Evid Based Complement Alternat Med. 2012 [cited 2017 Feb 25];2012:670362. DOI: 10.1155/2012/670362
- Boks MP, van Mierlo HC, Rutten BP, et al. Longitudinal changes of telomere length and epigenetic age related to traumatic stress and post-traumatic stress disorder. Psychoneuroendocrinology. 2015;51:506–512.
- Kwapis JL, Wood MA. Epigenetic mechanisms in fear conditioning: implications for treating post-traumatic stress disorder. Trends Neurosci. 2014;37(12):706–720.
- Kaminsky Z, Wilcox HC, Eaton WW, et al. Epigenetic and genetic variation at SKA2 predict suicidal behavior and post-traumatic stress disorder. Transl Psychiatry. 2015; [cited 2017 Feb 25];5:e627. DOI: 10.1038/tp.2015.105
- Labonte B, Azoulay N, Yerko V, et al. Epigenetic modulation of glucocorticoid receptors in posttraumatic stress disorder. Transl Psychiatry. 2014 [cited 2017 Feb 25];4:e368. DOI: 10.1038/tp.2014.3
- Rodgers AB, Morgan CP, Bronson SL, et al. Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J Neurosci. 2013;33(21):9003–9012.
- Qiu ZK, He JL, Liu X, et al. Developing a traditional Chinese medicine diagnostic structure for post-traumatic stress disorder. J Ethnopharmacol. 2017;198:324–330.
- Qiu ZK, Zhang GH, He JL, et al. Free and Easy Wanderer Plus (FEWP) improves behavioral deficits in an animal model of post-traumatic stress disorder by stimulating allopregnanolone biosynthesis. Neurosci Lett. 2015;602:162–166.
- Han F, Ding J, Shi Y. Expression of amygdala mineralocorticoid receptor and glucocorticoid receptor in the single-prolonged stress rats. BMC Neurosci. 2014 [cited 2017 Feb 25];15:77. DOI: 10.1186/1471-2202-15-77
- Zhang XG, Zhang H, Lin L, et al. Genes underlying positive influence of prenatal environmental enrichment and negative influence of prenatal earthquake simulation and corrective influence of Chinese herbal medicine on rat offspring: Irf7 and Ninj2. Afr J Tradit Complement Altern Med. 2014;11(2):367–376.
- Zhang XG, Zhang H, Liang XL, et al. Epigenetic mechanism of maternal post-traumatic stress disorder in delayed rat offspring development: dysregulation of methylation and gene expression. Genet Mol Res. 2016 [cited 2017 Feb 25];15(3): gmr.15039009. DOI: 10.4238/gmr.15039009
- Estanislau C, Morato S. Prenatal stress produces more behavioral alterations than maternal separation in the elevated plus-maze and in the elevated T-maze. Behav Brain Res. 2005;163(1):70–77.
- Estanislau C, Morato S. Behavior ontogeny in the elevated plus-maze: prenatal stress effects. Int J Dev Neurosci. 2006;24(4):255–262.
- Kolasani A, Xu H, Millikan M. Determination and comparison of mineral elements in traditional Chinese herbal formulae at different decoction times used to improve kidney function–chemometric approach. Afr J Tradit Complement Altern Med. 2011;8(5 Suppl):191–197.
- Palacios-García I, Lara-Vásquez A, Montiel JF, et al. Prenatal stress down-regulates Reelin expression by methylation of its promoter and induces adult behavioral impairments in rats. PLoS One. 2015; [cited 2017 Feb 25];10(2):e0117680. DOI: 10.1371/journal.pone.0117680
- Almli LM, Stevens JS, Smith AK, et al. A genome-wide identified risk variant for PTSD is a methylation quantitative trait locus and confers decreased cortical activation to fearful faces. Am J Med Genet B Neuropsychiatr Genet. 2015;168B(5):327–336.
- Van den Hove DL, Kenis G, Brass A, et al. Vulnerability versus resilience to prenatal stress in male and female rats; implications from gene expression profiles in the hippocampus and frontal cortex. Eur Neuropsychopharmacol. 2012;23(10):1226–1246.
- Vermetten E, Zhohar J, Krugers HJ. Pharmacotherapy in the aftermath of trauma; opportunities in the golden hours. Curr Psychiatry Rep. 2014 [cited 2017 Feb 25];16(7):455. DOI: 10.1007/s11920-014-0455-y
- Krugers HJ, Hoogenraad CC, Groc L. Stress hormones and AMPA receptor trafficking in synaptic plasticity and memory. Nat Rev Neurosci. 2010;11(10):675–681.
- Bai Y, Zhou L, Wu X, et al. D-serine enhances fear extinction by increasing GluA2-containing AMPA receptor endocytosis. Behav Brain Res. 2014;270:223–227.
- Lin HC, Tseng YC, Mao SC, et al. GABAA receptor endocytosis in the basolateral amygdala is critical to the reinstatement of fear memory measured by fear-potentiated startle. J Neurosci. 2011;31(24):8851–8861.
- Sohn SH, Cho K, Bae H. Screening for herbal medicines that affect ZIC1 gene methylation in colorectal cancer. Mol Cel Toxico. 2013;9(3):211–218.
- Su ZY, Khor TO, Shu L, et al. Epigenetic reactivation of Nrf2 in murine prostate cancer TRAMP C1 cells by natural phytochemicals Z-ligustilide and Radix angelica sinensis via promoter CpG demethylation. Chem Res Toxicol. 2013;26(3):477–485.
- Halepoto DM, Bashir S, A L-Ayadhi L. Possible role of brain-derived neurotrophic factor (BDNF) in autism spectrum disorder: current status. J Coll Physicians Surg Pak. 2014;24(4):274–278.
- Calabrese F, Rossetti AC, Racagni G, et al. Brain-derived neurotrophic factor: a bridge between inflammation and neuroplasticity. Front Cell Neurosci. 2014 [cited 2017 Feb 26];8:430. DOI: 10.3389/fncel.2014.00430
- Park S, Kim BN, Kim JW, et al. Neurotrophin 3 genotype and emotional adverse effects of osmotic-release oral system methylphenidate (OROS-MPH) in children with attention-deficit/hyperactivity disorder. J Psychopharmacol. 2014;28(3):220–226.
- Sarris J, Ng CH, Schweitzer I. ‘Omic’ genetic technologies for herbal medicines in psychiatry. Phytother Res. 2012;26(4):522–527.
- Hsieh HY, Chiu PH, Wang SC. Epigenetics in traditional Chinese pharmacy: a bioinformatic study at pharmacopoeia scale. Evid Based Complement Alternat Med. 2011 [cited 2017 Feb 26];2011:816714. DOI: 10.1093/ecam/neq050
- Sun SZ, Ma R, Hu XM, et al. Karyotype and DNA-methylation responses in myelodysplastic syndromes following treatment with traditional Chinese formula containing arsenic. Evid Based Complement Alternat Med. 2012 [cited 2017 Feb 26];2012:969476. DOI: 10.1155/2012/969476