ABSTRACT
Rosa alba L., also known as the white oil-bearing rose, has been cultivated in relatively small areas of Europe – predominantly in the Rose Valley of Bulgaria. An increasing number of studies in vitro and in vivo, including clinical studies, have demonstrated the therapeutic efficacy of rose oils in recent years. However, little is known about the cytotoxic and genotoxic potential of the phytocomplex oil extract derived from Rosa alba L. The aim of the present work was to investigate the cytotoxicity and clastogenic effects of Rosa alba L. essential oil and its main constituent – geraniol, as well as citral A, associated with allergies and contact dermatitis. We used classical cytogenetic methods and comet assay. 1-Methyl-3-nitro-1-nitrosoguanidine was used as a standard mutagen. The data showed that R. alba L. essential oil (in concentrations up to 1000 μg/mL) did not exhibit a statistically significant cytotoxic effect. The essential oil did not significantly increase the level of mitotic disturbances (micronuclei and aneuploidy effects) and had no significant effect on the induction of chromatid aberrations, compared to the untreated control sample. Only geraniol and citral A (in the concentrations used) increased significantly the percentage of migrated DNA in the comet tail, compared to the whole oil extract. This study gives a reason to believe that R. alba L. oil has potential as a supplementary component in some therapeutic strategies. It would be harmless to normal cells and tissues, but with potential for additive or synergistic cytotoxicity against cancer cells.
Introduction
Roses are a source of essential oils for perfumes, cosmetics and scents and have a large impact for the pharmaceutical industry. In recent years, an increasing number of studies in vitro and in vivo, including clinical studies, have demonstrated a therapeutic efficacy of rose oils: antidepressive effects, psychological relaxation, improvement of sexual dysfunction, antioxidant, antimicrobial, antifungal and anti-inflammatory effects, probiotic, stool regulating and smooth muscle relaxing actions, lipid-lowering, antiobese and antiulcerogenic effects [Citation1–9]. Rose oils are recommended not only for inhaled and topical application (as it is in aroma therapy and dermatology), but also for oral administration in physiologically relevant doses. Some recent studies report the anticancer activity of rose oils and their potential to be used as supplementary ingredients in therapy [Citation10–13]. They are focused on the whole phytocomplex rather than on single constituents of the rose oil extract. However, the side-effects are rarely taken into account, which makes it difficult to predict the translational potential of those data for the clinical practice.
Rosa alba L., also known as the white oil-bearing rose, has been cultivated in relatively small areas of Europe in the last century – predominantly in the Rose Valley of Bulgaria [Citation14,Citation15]. The essential rose oils of R. alba L. and R. damascena Mill. are among the most expensive products on the world markets. The cytotoxicity of oil extracts from R. damascena Mill. is most widely investigated. However, little is known about the genotoxic potential of rose oils, as well as about the cytotoxic effects of phytocomplex oil extract derived from R. alba.
The aim of the present work was to investigate the cytotoxic and clastogenic effects of R. alba. essential oil using classical cytogenetic methods and comet assay on a cell model.
Materials and methods
Extraction of rose oil
Petals of R. alba were used. The plants were cultivated in the experimental plantation of the Institute of Rose and Essential Oil Plants (Kazanlak, Bulgaria). The plant material was collected in the morning in May/June 2014. The rose oil was distilled immediately by water-steam distillation of a semi-industrial processing line in the same institute. The process parameters of the distillation were as follows: 10 kg raw material for charge; 1:4 hydro-module; rate of 8%–10%; duration of 150 min. The aromatic water was re-distilled in the same apparatus. The essential oil of each charge was the sum of the primary and the secondary oil in their natural ratio. Total oil was a mixture of distillates collected over 15 days, i.e. the time of the collection campaign of R. alba. Finally, it was dried with sodium sulphate, filtered and stored appropriately.
Gas chromatography-mass spectrometry (GC-MS) of total R. alba oil
GC-MS analysis was carried out on a 7890 A gas chromatograph interfaced with a 5975 C mass selective detector (Agilent Technologies, Santa Clara, CA, US). Separations were performed using a 30 m × 0.25 mm (inner diameter) HP-5 ms silica-fused capillary column coated with 0.25 µm film of poly(dimethyl siloxane) as a stationary phase (Agilent Technologies, Santa Clara, CA, US). The flow rate of the carrier gas (helium) was maintained at 1.0 mL/min. The injector and the transfer line temperature were kept at 250 °C. The following oven temperature program was used: 40 °C for 3 min, then 5 °C per min – to 300 °C for 5 min; run time 60 min. The injections were carried out in a split mode with a split ratio of 10:1. The mass spectrometer scanned from 50 to 550 m/z. The injection volume was 1 µL.
Constituents were identified on the basis of the retention index (RI). RI was determined with reference to a homologous series of n-alkanes (C10–C34), under identical experimental conditions, MS Library search (NIST/EPA/NIH software, version 2.1), as well as by comparison with the fragmentation pattern of the mass spectra with data published in the literature [Citation16]. The percentage compositions of the samples were computed from the GC peak areas.
Chromosome aberration assay
Cells isolated from seeds of the reconstructed karyotype MK 14/2034 of Hordeum vulgare were used as a model of normal cells. Seeds were presoaked for 1 h in tap water and germinated for 19 h in Petri dishes on moist filter paper at 24 °C. They were treated with 50 µg/mL 1-methyl-3-nitro-1-nitrosoguanidine (MNNG; Sigma-Aldrich, Steinheim, Germany) for 1 h, as a standard mutagen. Different concentrations of the R. alba essential oil (250, 500 and 1000 µg/mL) were also added independently to the seeds for 1 h. For evaluation of chromosome aberrations, the seeds were exposed after recovery times (18 to 30 h at 3 h intervals) for 2 h at 24 °C to a 0.025% colchicine solution (Merck, Darmstadt, Germany) saturated with α-bromonaphthaline prior to fixation in ethanol:glacial acetic acid (3:1). For scoring of micronuclei, colchicine treatment was omitted. Fixed root tips were hydrolyzed in 1 N HCl at 60 °C for 9 min, Feulgen-stained, macerated in 4% pectinase in distilled water for 14 min and squashed on slides.
The first post-treatment mitosis was analysed to determine the percentage of metaphases with chromosome aberrations (chromatid and iso-chromatid breaks, chromatid translocations, intercalary deletions and duplication/deletions on the basis of at least 500 cells at 18, 21, 24, 27 and 30 h after treatment), 1000 cells for frequency of micronuclei (MN) (30 h after treatment) and 3000 cells for mitotic index (MI) (24 h after treatment) per experiment and variant [Citation17].
Comet assay
Cells isolated from leaves of H. vulgare were used as a model of normal cells. The seeds were germinated for 4 days in Petri dishes on moist filter paper and grown for 10 days in an aerated wet-chamber at 24 °C. The leaves were treated in 2 mL tubes in the dark at 24 °C with: R. alba essential oil (250, 500 and 1000 µg/mL); geraniol and citral A analytical grade (Sigma–Aldrich) (500 µg/mL, respectively); or MNNG (50 µg/mL), for 24 h. Immediately after exposure, nuclei were isolated from leaves by careful slicing with a razor blade in 150 µL, 1 × TBE [90 mmol/L Tris-borate, 2 mmol/L ethylenediaminetertaacetic acid (EDTA), pH 8.4], on ice (in a dark place). For each condition, 30 µL of the cell suspension were mixed with 30 µL of 1% low-melting point agarose (Gibco, Gaithersburg, USA) at 42 °C and dropped on slides precoated with 0.5% mild-melting point agarose. Each drop was covered with a 22 mm × 22 mm cover-slip and the slides were stored on ice. After removal of the cover-slips, the nuclei were denatured in 300 mmol/L NaOH, 5 mmol/L EDTA, pH 13.5 for 10 min, at room temperature. The slides were washed three times with cold 1 × TBE buffer for 5 min and subjected to electrophoresis in ∼1500 mL 1 × TBE, at 15-17 mA and 1 V/cm (31 V), at room temperature, for 6 min. After electrophoresis, the agarose slides were placed in 70%, 90% and 96% ethanol for 5 min each, before air drying and staining with acridine orange (1 µL/mL).
Comets were examined from slides under 25-fold magnification, using a fluorescence microscope (Jenalumar Zeiss, Minchin, Germany; at 520 nm wavelength) equipped with a filter for acridine orange and a digital camera (Canon, Power Shot A95, Tokyo, Japan). The COMET image analysis system CASP (Comet Assay Software Project, www.casp.of.pl) was used for measurement of DNA content in the head and tail of each comet. Per experimental point, usually, six microgels from three independent experiments were evaluated, with each providing a median value of ‘% DNA in tail’ of minimum 200 comets.
Statistical analysis
Statistical analysis of comet assays, chromosome aberrations and micronuclei was carried out using the two-tailed Fisher's exact-test for group comparison of the different treatment variants [Citation18]. An adapted formula was used for comparison of the upper limit of confidence interval of the expected and observed chromosome aberrations in individual loci and evaluation of aberration ‘hot spots’ in barley [Citation19,Citation20].
Results and discussion
Chromatographic analysis
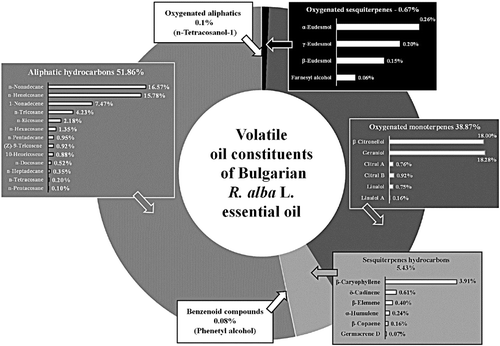
The first step in this study was chromatographic analysis of the volatile oil constituents of Bulgarian R. alba essential oil. The chromatogram fingerprint () is typical of Rosaceae family, the main constituent being geraniol (18.28%) [Citation14–16].
Genotoxic potential of total R. alba essential oil
Next, we explored the potential of R. alba essential oils to act as a genotoxic agent (at certain concentrations) based on comet formation in the cell nucleus after induction of single-strand (SSB) and/or double-strand breaks (DSB), in comparison with MNNG as a standard mutagen. We used the alkaline/neutral (A/N) protocol of the comet assay, which is sensitive to detect preferential SSBs and DSBs of DNA [Citation17,Citation18,Citation21]. SSBs in themselves are not anticipated to generate chromosomal aberrations. The chromosome aberrations obtained after treatment with alkylating agents (after S-phase of cell mitosis), could be a result of DSBs and they may arise as a result of unrepaired SSBs. Additional O6MeG-T mispairs can eventually result in highly toxic DSBs [Citation22]. The results from our study described below support these assumptions.
shows good agreement between the results obtained by the comet assay and classical chromosome aberrations assay in the treated samples. R. alba essential oil did not affect significantly the amount of DNA in the comet tail (A) or the frequency of chromosome aberrations (B), compared to the control (non-treated) cells. In MNNG-treated cells, both parameters increased significantly.
Figure 2. Genotoxic potential of R. alba L. essential oil in reconstructed karyotype MK 14/2034 of barley, analysed by: (A) A/N comet assay (leaf cells); (B) chromosome aberration assay (root tip meristem cells). Samples were treated with 250 µg/mL (RA250), 500 µg/mL (RA500) and 1000 µg/mL (RA1000) R. alba essential oil or 50 μg/mL MNNG (MNNG50); control: non-treated sample.
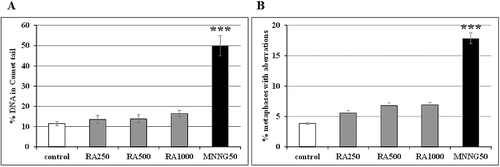
R. alba essential oil did not affect the mitotic index (MI) either, which indicates a lack of cytotoxicity (). In a concentration of 250 μg/mL, the oil extract slightly decreased the frequency of micronuclei formation (MN), but the value was not statistically significant versus the control value. In the concentrations of 500 and 1000 μg/mL, the values of MN in oil-treated cells were the same as those in the non-treated cells, which indicates a lack of genotoxicity. In contrast, MNNG caused significant cytotoxic and genotoxic effects: MI decreased (∼34%) and MN increased (∼4-fold) compared to the control ().
Table 1. Cytotoxic and genotoxic potential of R. alba L. essential oil detected by chromosome aberration assay.
Compared to the standard karyotype of H. vulgare, the reconstructed karyotype MK 14/2034 with all chromosomes distinguishable allows a much higher resolving power in investigations of aberration location and has proved to be a useful analytical tool. Heterochromatic chromosome regions have been shown to be sites of aberration clustering [Citation19]. The underlying causes of aberration clustering, however, still remain an area of dispute. We detected induction of aberration hot spots in oil-treated and MNNG-treated samples (). Seven aberration hot spots were observed after treatment with MNNG and four of them were located within heterochromatin ((A)). For comparison, 1000 µg/mL of the R. alba essential oil induced five aberration hot spots: two ones were found in heterochromatic regions and three ones, in the telomeric regions of chromosomes 2, 43 and 71 of the reconstructed karyotype ((B)). Lower concentrations of the R. alba essential oil (250 and 500 µg/mL) caused fewer aberration hot spots: three and two, respectively (data not shown).
Figure 3. Induction of aberration hot spots by MNNG (A) and R. alba essential oil (B) in the barley reconstructed karyotype MK14/2034. Samples were treated with 50 μg/mL MNNG (MNNG50) and 1000 µg/mL R. alba essential oil (RA1000); Chr: chromosome.
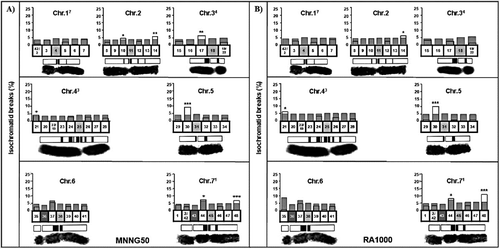
We found that R. alba essential oil induces specific ‘aberration hot spots’ in barley, which are not limited to heterochromatin-containing segments only, but also involve telomere segments. It was observed that, similar to previous observations with other cytotoxic/genotoxic agents [Citation20], only isochromatid breaks appeared with aberration clustering, unlike the chromatid breaks, translocation, interstitial deletions and duplication/deletions.
It is widely accepted that MNNG does not generate DNA breaks directly, but mediates these only during repair. Menke et al. [Citation21] suggest that MNNG-induced chromatid-type aberrations within the heterochromatin apparently originate from recombination misrepair of damage within large tandem repeat arrays during the S-phase of mitosis. For example, MNNG causes different types of chromatid aberrations with breakpoints, preferentially located within the heterochromatic regions of the Vicia faba genome [Citation21].
Genotoxic potential of individual constituents/genotoxic potential of geraniol and citral A
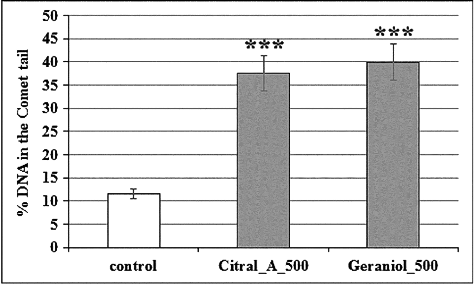
In the next stage of the study, we investigated the genotoxic potential of two monoterpenes: geraniol, which is a main constituent of the R. alba essential oil, and citral A (geranial), which has been associated with allergies and contact dermatitis in the cosmetics industry […]. We used them at a high concentration (500 µg/mL) with the aim to assess the cytotoxic and genotoxic effects and to demonstrate any harmful effect of these components when used in excessive concentrations. Geraniol represents 18.28% of the R. alba essential oil and citral A, 0.76%; they are the most important components due to their pharmacological effects [Citation8]. In general, the essential oils and their purified constituents are harmless at low concentrations. However, several studies have reported cytotoxic and genotoxic effects of citral A in human cells, when applied in high doses [Citation23,Citation24]. Geraniol can induce cell cycle arrest and apoptosis in cultured cancer cells and modulate the expression of various cell cycle regulators in vitro and in vivo [Citation25]. Our results correspond well with these data: in a concentration of 500 µg/mL, geraniol and citral A led to a total stop of mitotic activity. Thus, in this case chromatid aberration induction was not observed.
We observed that geraniol and citral A significantly increased the frequency of DNA comet tail formation, similar to the standard mutagen MNNG (). The effect of both monoterpenes was concentration-dependent (data not shown). Recently, Hagag et al. [Citation12] have reported selective cytotoxic and genotoxic effects of R. damascena essential oil (10 μg/mL) on leukemia lymphocytes, without any cytotoxicity and genotoxicity on normal blood cells. According to our previous studies, the results obtained in plant-based test systems largely correlate with those in mammalian cells and whole animal models. Thus, our results give us a reason to believe that R. alba oil has potential as a supplementary component in some therapeutic strategies, for example, in anticancer therapy. It would be harmless to normal cells and tissues, but with potential for additive or synergistic cytotoxicity against cancer cells.
Conclusions
This study demonstrated that R. alba L. essential oil did not exhibit statistically significant cytotoxic effects up to 1000 μg/mL on root tip meristem cells isolated from Hordeum vulgare. The oil extract did not increase significantly the level of mitotic disturbances (micronuclei and aneuploidy effects) and had no significant effect on the induction of chromosome aberrations, compared to the negative, untreated control. However, geraniol and citral A were cytotoxic and genotoxic when applied in a high dose (500 μg/mL). Both monoterpenes increased significantly the percentage of migrated DNA in the comet tail, from isolated live cells compared to the control and total oil extract. These investigations into the cytotoxic and genotoxic potential of Bulgarian rose essential oil (or some of its individual constituents) could be useful in health research on the pharmacological capacity and activity of natural plant compounds. The R. alba essential oil and its constituents are promising to be further tested in cancer cell lines for its/their potential as adjuvant anticancer therapy.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Chrubasik C, Roufogalis BD, Muller-Ladner U, et al. A systematic review on the Rose canina effect and efficacy profiles. Phytother Res. 2008;22:725–733.
- Mahboubi M. Rosa damascena as holy ancient herb with novel applications. J Tradit Complement Med. 2015;6:10–16.
- Mohebitabar S, Shirazi M, Bioos S, et al. Therapeutic efficacy of rose oil: a comprehensive review of clinical evidence. Avicenna J Phytomed. 2017;7:206–2013.
- Gonzalez-Manan D, D'Espessailles A, Dossi CG, et al. Rosa mosqueta oil prevents oxidative stress and inflammation through the upregulation of PPAR-α and NRF2 in C57Bl/6 J mice fed a high-fat diet. J Nutr. 2017;147:579–588.
- Dossi CG, Cadagan C, San Martin M, et al. Effects of Rosa mosqueta oil supplementation in lipogenic markers associated with prevention of liver steatosis. Food Funct. 2017;8:832–841.
- Boskabady MH, Shafei MN, Saberi Z, et al. Pharmacological effects of Rosa damascena. Iran J Basic Med Sci. 2011;14:295–307.
- Gochev V, Dobreva A, Girova T, et al. Antimicrobial activity of essential oil from Rosa alba. Biotechnol Biotechnol Equip. 2010;24:512–515.
- Mileva M, Kusovski V, Krastev D, et al. Chemical composition, in vitro antiradical and antimicrobial activities of Bulgarian Rosa alba L. essential oil against some oral pathogens. Int J Curr Microbiol App Sci. 2014;3:11–20.
- Talib WH, Mahasneh AM. Antimicrobial, cytotoxicity and phytochemical screening of Jordanian plants used in traditional medicine. Molecules. 2010;15:1811–1824.
- Gehrcke M, Giuliani LM, Ferreira LM, et al. Enhanced photostability, radical scavenging and antitumor activity of indole-3-carbinol-loaded rose hip oil nanocapsules. Mater Sci Eng C Mater Biol Appl. 2017;74:279–286.
- Shokrzadeh M, Habibi E, Modanloo M. Cytotoxic and genotoxic studies of essential oil from Rosa damascena Mill. Med Glas (Zenica). 2017;14(2):152–157.
- Hagag HA, Bazaid SA, Abdel-Hameed SS, et al. Cytogenetic, cytotoxic and GC-MS studies on concrete and absolute oils from Tais rose, Saudi Arabia. Cytotechnology. 2014;66:913–923.
- Rosso R, Corasaniti MT, Bagetta G, et al. Exploitation of cytotoxicity of some essential oils for translation in cancer therapy. Evid Based Complement Alternat Med. 2015 [cited 2017 Dec 11];2015:397821. DOI:10.1155/2015/397821
- Kovatcheva N, Zheljazkov VD, Astatkie T. Productivity, oil content, composition, and bioactivity of oil-bearing rose accessions. HortScience. 2011;46:710–714.
- Rusanov K, Kovacheva N, Rusanova M, et al. Flower phenotype variation, essential oil variation and genetic diversity among Rosa alba L. accessions used for rose oil production in Bulgaria. Sci Hort. 2013;161:76–80.
- Adams RP. Identification of essential oil components by gas chromatography/mass spectrometry. 4th ed. Carol Stream (IL): Allured Publishing; 2007.
- Jovtchev G, Stergios M, Schubert I. A comparison of N-methyl-N-nitrosourea-induced chromatid aberrations and micronuclei in barley meristems using FISH techniques. Mutat Res. 2002;517:47–51.
- Jovtchev G, Menke M, Schubert I. The comet assay detects adaptation to MNU-induced DNA damage in barley. Mutat Res. 2001;493:95–100.
- Rieger R, Michaelis A, Schubert I, et al. Non-random intrachromosomal distribution of chromatid aberrations induced by X-rays, alkylating agents and ethanol in Vicia faba. Mutat Res. 1975;27:69–79.
- Jovtchev G, Gateva S, Stergios M, et al. Cytotoxic and genotoxic effects of paraquat in Hordeum vulgare and human lymphocytes in vitro. Environ Toxicol. 2010;25:294–303.
- Menke M, Meister A, Schubert I. N-Methyl-N-nitrosourea-induced DNA damage detected by the comet assay is Vicia faba nuclei during all interphase stages is not restricted to chromatid aberration hot spots. Mutagenesis. 2000;15:503–506.
- Margison GP, Santibanez-Koref MF. O6-alkylguanine DNA alkyltransferase: role in carcinogenesis and chemotherapy. BioEssays. 2002;24:255–266.
- Sinha S, Jothiramajayam M, Ghosh M, et al. Evaluation of toxicity of essential oils palmarosa, citronella, lemongrass and vetiver in human lymphocytes. Food Chem Toxicol. 2014;68:71–77.
- Queiroz RM, Takiya CM, Guimaraes LP, et al. Apoptosis inducing effects of Melissa officinalis L. essential oil in glioblastoma multiforme cells. Cancer Invest. 2014;32:226–235.
- Kim SH, Bae HC, Park EJ, et al. Geraniol inhibits prostate cancer growth by targeting cell cycle and apoptosis pathways. Biochem Biophys Res Commun. 2011;407:129–134.