 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The drug preparations from dried flower of Carthamus tinctorius L. (CTL) are widely used as an adjuvant medication in cardiovascular diseases in China. In this research, Safflower yellow extract (SYE), a CTL drug in market, was studied through components analysis and two animal experiments with different dosages to assess its pharmacodynamic effects. The whole constituents of SYE were characterized through ultra-fast liquid chromatography–diode array detector–quadrupole time-of-flight tandem mass spectrometry. Cerebral arteriole thrombus was induced in C57BL/6J mouse by laser irradiation in vivo using two-photon laser-scanning microscopy. The time of thrombus formation and vessel diameter were calculated to evaluate the antithrombotic effect. In the model of blood stasis syndrome, rats were injected with adrenaline injection before and after ice-bath to induce hemorheology disorders. The results identified 29 constituents in SYE and hydroxysafflor yellow A was the main component (19.8%). In the mouse model, SYE inhibited thrombus formation in a dose-dependent manner and postponed the occlusion time in brain arteriole at dosages of 26.0 and 52.0 mg/kg. In the rat model of blood stasis syndrome, SYE significantly decreased the whole blood viscosity, suppressed red blood cell aggregation and platelet aggregation in high-dose (p < 0.01). The activated partial thromboplast in time was prolonged at a dosage of 7.0 mg/kg (p < 0.05) as well. In conclusion, SYE administration could inhibit the thrombus formation and beneficially influence the blood rheologyin blood stasis syndromes at relevant low dosages.
ABBREVIATIONS
| ADR | = | adverse drug reactions |
| APTT | = | activated partial thromboplastin time |
| CTL | = | Carthamus tinctorius L. |
| EAI | = | erythrocyte aggregation index |
| ERI | = | erythrocyte rigidity index |
| FITC | = | fluorescein isothiocyanate-dextran |
| HSYA | = | hydroxysafflor yellow A |
| MPAR | = | maximum platelet aggregation rate |
| PT | = | prothrombin time |
| RCEI | = | red corpuscle electrophoresis index |
| SYE | = | safflower yellow extract |
| TPLSM | = | two-photon laser-scanning microscopy |
| UFLC-DAD-Q-TOF-MS/MS | = | ultra-fast liquid chromatography–diode array detector–quadrupole time-of-flight tandem mass spectrometry |
| WBV | = | whole blood viscosity |
Introduction
The dried flower of Carthamus tinctorius L. (CTL) is a traditional herbal medicine employed in China since dynasty Han (150 A.D., recorded in Synopsis of Golden Chamber by Zhang Zhongjing) and mainly used in therapies of dysmenorrhea or blood stasis syndrome [Citation1]. Nowadays, CTL drug preparations are extensively applied in clinical treatments of coronary heart disease, acute ischemic stroke, hypertension or cerebro-vascular disease as an adjuvant therapy [Citation2].
Modern phytochemistry studies have identified over 104 compounds from CTL, including quinochalones, flavonoids, alkaloid, aromatic glycosides, etc. Safflower yellow is are presentative mixture extracted from CTL, which exerts important effects [Citation3] and contains safflower yellow A, hydroxysafflor yellow A, safflower yellow B, etc. Among them, hydroxysafflor yellow A (HSYA) is the most popular substance. It has been demonstrated that HSYA can antagonize the platelet activating factor (PAF) from binding its receptors [Citation4], which reflects an anti-coagulation capability and exerts protective effects on ischemia [Citation5]. In addition, HSYA shows other pharmacological properties such as vasodilatation effects [Citation6], antioxidant activity [Citation7] and even anticancer activity by inhibiting angiogenesis [Citation8]. As a result, Chinese Pharmacopoeia includes the content determination of HSYA in quality control of CTL and relevant medicines.
Nevertheless, medicines prepared from HSYA or CTL are excessively applied and even abused in ischemia and cardiovascular diseases in clinical therapy, which might cause serious adverse reactions. It is necessary to investigate the whole components of CTL medicines and medicine doses for safety consideration.
In this research, we selected Safflower yellow extract (SYE), a medicine prepared as a lyophilized powder for injection on the market, to study the effects of different dosages on mouse (13.0, 26.0 and 52.0 mg/kg) and rat (1.5, 3.0, 4.5 mg/kg). A whole components analysis was carried out through Ultra-fast liquid chromatography–diode array detector–quadrupole time-of-flight tandem mass spectrometry (UFLC-DAD-Q-TOF-MS/MS). A mouse model of cerebral arterial thrombosis was generated through laser irradiation in vessel using two photon laser-scanning microscopy (TPLSM), which could induce noninvasive thrombus and image thrombus formation in real time [Citation9]. SYE was administrated in three different doses to observe the inhibitory effect against cerebral acute thrombosis. Additionally, SYE was applied in low dosages in rats of blood stasis syndrome to evaluate its improvement in blood circulation [Citation10]. Hemorheologyparameters were analyzed, such as whole blood viscosity, erythrocyte aggregation, erythrocyte rigidity, etc.
Materials and methods
Components analysis based on UFLC-Q-TOF-MS/MS and content determination of HSYA on HPLC
SYE was supplied by the YouCare Pharmaceutical Group Company in China in the form of a lyophilized powder within safflower yellow extract and mannitol.
The components analysis was performed by Shimadzu UFLC system (Shimadzu Co, Japan) and Triple TOF 5600 mass spectrometer (AB Sciex, USA) with a Phenomenex C18 column (2.1 mm × 100 mm, 2.6 μm) at 40.0 °C. The mobile phase was comprised of solvent A (0.1% formic acid in acetonitrile) and solvent B (0.1% formic acid in water) at a flow rate of 0.3 mL/min. The mobile phase gradient was 2% A (0.0 min)–11% A (10.0 min)–11% A (15.0 min)–15% A (16.0 min)–30% A (22.0 min). The ionization source conditions of the mass spectrometer were as follows: the ion spray voltage was set to 5500 V; ion source gas 1, gas 2 and curtain gas were set to 55, 55 and 35 psi, respectively; source temperature was set to 550.0 °C. The MS data were collected from those mass-to-charge ratio between 100 and 1500 Da in both positive and negative ionization modes and analyzed by using PeakView (version 1.2.0.3, AB Sciex, USA).
The reference substance of HSYA (17.72 mg) was dissolved in 10 mL volumetric flask and diluted quantitatively with ultrapure water to obtain a stock solution with a concentration of 1.772 mg/mL, considering the purity. Then we quantitatively transferred 0.2, 0.4, 0.8, 1.2, 1.6 and 2.0 mL of Standard stock solution to six 10-mL volumetric flasks, diluted with water to volume and mixed. The quantification of HSYA was performed on a 250 mm × 4.6 mm, 5 μm particle, Dikma DiamonSil C18 column at 30 °C with Dionex Ultimate 3000 HPLC System. The mobile phase was comprised of solvent A (acetonitrile) and solvent B (0.05 mol/L sodium dihydrogen phosphate in water, pH 4.0) with gradient programme 0–30 min (10%–25% A). The flow rate was 1.0 mL/min and the injection volume was 20 μL.
Animals and ethical statement
Thirty-six male C57BL/6J mice and forty-eight male Sprague–Dawley (SD) rats were obtained from Guangdong Medical Experimental Animal Center (Certification No: SCXK-(Yue) 2013-0002). The animals were raised in the specific-pathogen-free laboratory with the following conditions: temperature 23 ± 1 °C, relative humidity of 50 ± 1%, and a 12/12-hour light/dark cycle. The animal experimental protocol (Permission No. 20142000 226) was approved by the Animal Ethics Committee of the School of Life Sciences, SunYat-sen University. All procedures performed in this study involving animals were in accordance with the ethical standards of the institution or practice.
Cerebral arteriole thrombus induced by laser irradiation in mouse model in vivo
Materials and reagents
SYE was dissolved in 0.9% sodium chloride solution(SCI, JiangXi Kelun Pharmaceutical Co., China) for injection with appropriate concentrations. Heparin sodium injection (HSI) 2 mL:12,500 units (Changzhou Qianhong Bio-pharma Co., China) was diluted in appropriate concentrations with SCI. Fluorescein isothiocyanate-dextran (FITC, 2000 KD, Sigma, Germany) was dissolved in SCI with a concentration of 5% for imaging under TPLSM.
Animal preparation and surgical procedures
Thirty-six male C57BL/6J mice (20–24 g, 8 weeks) were divided into six groups of six mice each: control group, SCI group, SYE-Low group (dosage of 13.0 mg/kg), SYE-Mid group (26.0 mg/kg), SYE-High group (52.0 mg/kg) and HSI group (0.2 mL/kg).
The method of Zhang et al. [Citation11] was used. The mouse was anesthetized with chloral hydrate (4.2%, 10.0 mL/kg) and placed on the heating pad at 37.0 °C. An incision was made on the midline of scalp for surgery. A cranial window was produced over the left parietal cortex, grinded by a micro-drill. The whole procedure was performed carefully, avoiding internal bleeding, in order to acquire a clear microscopic field. After the surgery, a metal plate was glued upon the cranial window to fix animal on TPLSM (Leica DM6000, Germany). Then, the mouse was injected intravenously with FITC (10.0 mL/kg) for arteriole thrombosis model [Citation12].
Microscopy of thrombus formation in mouse brain arteriole
The animals in the drug groups were injected intra-peritoneally with SYE or HIS, respectively, 30 min before the following occlusion process. The SCI group was given an equal volume of SCI solution.
The image of cerebral vessel throughout the cranial window was first obtained with a 10× magnification air objective and transferred to a water-immersion one (40× magnification) for high-resolution. Arterioles with a diameter of18 ± 3 μm and 8 ± 3 μm/ms in blood velocity were selected as target vessels for occlusion. The target arteriole was placed in the center of the field and magnified 20 times. A single photon mode was used to irradiate the vessel to induce endothelium injures. The laser was set at 540 nm with 90% intensity of energy (maximum power 3.5 W) and started irradiating until the vessel was completely occluded.
The modeling process was recorded by Leica Las AF (version 2.6.0) to calculate the irradiation time necessary for occlusion [Citation13] as well as the diameter changes of the arteriole. The change ratio of vessel diameter could reflect the blood flow volume in arterioles and imply the risk of thrombosis to some degree. The ratio of the diameter in the target arteriole was calculated using the formula given below. The arteriole was kept for observation for 15 min to make sure there was no recanalization.
In the formula, diameter (max) represents the maximum value of the diameter when the arteriole dilated and diameter (min) represents the minimum value when the arteriole constricted. Diameter (baseline) means the diameter value of the arteriole in normal condition before irradiation.
Statistical analysis
The time necessary for occlusion and the ratio of vessel diameter were presented as mean values (n = 6) with standard deviation(±SD). Statistical analysis was performed using t test with SPSS 18.0 (SPSS Inc., Chicago, IL, USA) with results exported through GraphPad Prism (GraphPad Software, Inc., version 6.01).
Hemorheology disorders induced by blood stasis syndrome in rat model
Materials and reagents
SYE was dissolved in SCI solution for injection. Low-molecular-weight heparin calcium injection (LHCI) was purchased from GlaxoSmithKline Co., China. Extract of Ginkgo biloba leaves injection (EGBLI) was purchased from YouCare Pharmaceutical Group CO, China. Adrenaline hydrochloride injection (Shanghai Harvest Pharmaceutical Co., China) was diluted to 0.4 mg/mL with SCI.
Animal preparation and operation for blood stasis model
Fifty-six male SD rats (180–220 g, 8 weeks) were divided into seven groups of eight each: normal group, model group, SYE-Low group (SYE 1.5 mg/kg/d), SYE-Mid group (SYE 3.0 mg/kg/d), SYE-High group (SYE 4.5 mg/kg/d), EGBLI group (1.0 mL/kg/d) and LHCI group (50 μL/kg/d). The EGBLI and LHCI groups were set as positive controls. The LHCI group was hypodermically injected with LHCI, while the EGBLI and SYE groups were dosed by intramuscular injection. The rats in the normal group and the model group were given no drug treatment, but were intramuscularly injected with SCI of the same volume as the drug groups.
Rats were dosed consecutively once daily for 11 days. On the 10th day, 30 min after dosing, the rats were subcutaneously injected with adrenaline (0.8 mg/kg) except those of the normal group, which accepted the same volume of SCI. After two hours, the rats of the model and the drug groups (LHCI, EGBLI and SYE groups) were kept in ice-cold water (0–2 °C) for 5 min to induce blood stasis syndrome. Two hours later, the rats were injected with SCI or adrenaline as previous procedures again and fasting for 12 h. On the last day, the rats were anesthetized with chloral hydrate (10%, 3.5 mL/kg) and blood was drawn from the abdominal aorta [Citation14].
Data analysis
Blood plasma samples were collected and examined through standard operating procedures. The data of blood rheology and coagulation function for analysis included whole-blood viscosity (WBV), erythrocyte aggregation index (EAI), erythrocyte rigidity index (ERI), red corpuscle electrophoresis index (RCEI), activated partial thromboplastin time (APTT), prothrombin time (PT) and maximum platelet aggregation rate (MPAR). All data were presented as mean values ±SD (n = 8) and evaluated by t tests in SPSS 18.0 (SPSS Inc., Chicago, IL, USA).
Results and discussion
To ensure the quality of SYE samples used in this study, twenty-nine constituents, including seven quinochalones and fifteen flavonoids, were identified. Moreover, to better understand the pharmacodynamic effects of SYE, two animal experiments were designed in this research including inhibitory effect on anti-thrombosis through TPLSM and improvement of blood stasis syndrome evaluated by the hemorheology indices.
The ion chromatograms of SYE in positive and negative ionization modes are shown in . Twenty-nine constituents of SYE were identified or tentatively characterized in positive and negative ionization modes ((B,C)) via UFLC-DAD-Q-TOF-MS/MS. Among them, six constituents were confirmed by retention time and MS data of reference substances and 23 constituents were characterized by MS data and related studies ( and ) [Citation3,Citation15–17]. The characterized constituents included six alkaloids, one organic acid, seven quinochalones and fifteen flavonoids. Among them, the chromatogram through UPLC-DAD ((A)) showed that hydroxysafflor yellow A was the most abundant component in SYE. The linearity was y = 345.51x − 0.3949 (r2 = 0.9999) with a range of 0.03544–0.3544 mg/mL, and the content of hydroxysafflor yellow A (HSYA) was 19.80%.
Figure 1. UPLC-DAD (A) and ion chromatograms of SYE in positive (B) and negative (C) ionization models.
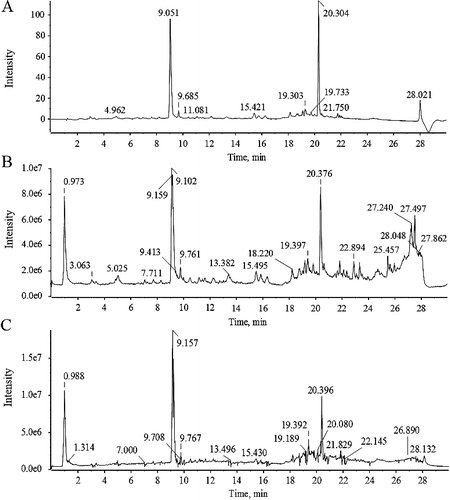
Table 1. Identification details of the components in SYE using UFLC-Q-TOF-MS/MS technique.
Figure 2. Chemical structural formulas of the identified constituents in SYE. Six constituents were confirmed by retention time and MS data of reference substances, and 23 constituents were characterized by MS data and related studies.
The thrombus model of cerebral arterioles was developed in C57BL/6J mice with laser irradiation of low intensity. The clots were firstly attached in the downstream vessel wall of the injured area and finally adhered on the injured intimal layer after constant laser irradiation. This process, induced by laser irradiation, had demonstrated that the mechanisms of clot formation were primary endothelial and basement membrane injuries [Citation18]. The thrombus exerted by laser thermal injury was combined with platelets and fibrin [Citation19], whose formation steps could be similarly described by platelet functions and coagulation cascade at a functional level [Citation20]. As a result, the analysis of thrombus growth or measurement of blood flow velocity became conventional methods to assess the thrombolysis effects of drugs.
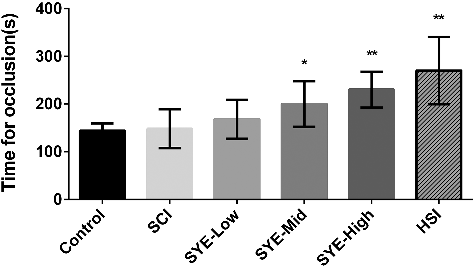
In this research, the arteriole within limited range of vessel diameter and flow velocity was selected to develop thrombus excluding the error interferences such as different initial flow velocity or endothelial injury level. The time necessary for arterioles occlusion in mice with drug pre-treatments would be a representative factor to evaluate the antithrombotic effect (). SYE inhibited thrombus formation in a dose-dependent manner, which postponed the occlusion time in the SYE-Mid and SYE-High groups (200 ± 43 s, p = 0.0376 and 230 ± 33 s, p < 0.01) with statistical difference compared to the control group (144 ± 14 s). Furthermore, the high dose of SYE showed a similar capability of anti-thrombosis to the HSI (270 ± 63 s).This result demonstrated that HSYA has strong inhibiting effects against platelet adhesion [Citation21].Other flavonoids such as quercetin were also considered to possess inhibitory potencies for platelet aggregation [Citation22,Citation23] as well. The pharmacodynamic effects of HSYA and flavonoids through inhibiting the platelet aggregation could explain the results that the time for occlusion in arteriole was prolonged in the SYE groups.
Figure 3. Time for arteriole occlusion of thrombus induced by laser irradiation in mice vessels. SYE was applied at three doses of 13, 26 and 52 mg/kg body weight by intraperitoneal injection on C57BL/6J mice. After 30 min, thrombus formation was induced in brain arterioles by laser irradiation and the time necessary for arteriole occlusion was measured. SYE was compared with heparin sodium injection (0.2 mL/kg). The graph depicts the mean values ± SD (n = 6); *p < 0.05 and **p < 0.01 as compared with the control.
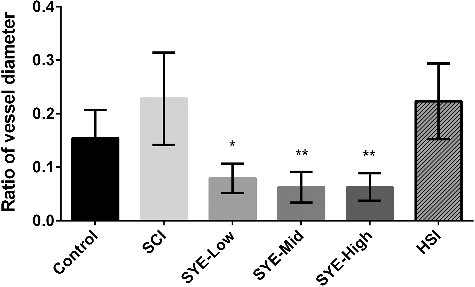
In addition, as a reaction to irradiation stimulation, the arteriole persisted in constricting or dilating and resulted in diameter changes (). The ratio of vessel diameter could reflect the blood flow volume. The results showed that the SYE groups of all three dosages indicated lower ratios (0.079 ± 0.027, 0.070 ± 0.033 and 0.063 + 0.026) compared to the control, SCI and HSI group (0.154 ± 0.053, 0.228 ± 0.086 and 0.223 ± 0.071) with significant differences (). As HSYA could reduce the vascular tension through activating the voltage-gated K+ channel and relaxing the vascular smooth muscle [Citation6], this might be the reason for the smaller diameter changes in the SYE groups and for keeping the arteriole with a relatively stable blood flow. The results implied that SYE could maintain the stable blood flow volume and resist thrombosis more mildly than the heparin sodium did.
Figure 4. Arteriole diameter measurement during laser irradiation in the control (A) and SYE (B) group with high dosage. A brain arteriole of C57BL/6J mice with diameter of 18 ± 3 μm was selected as the target vessel for occlusion and was zoomed in at 20× magnification under TPLSM.
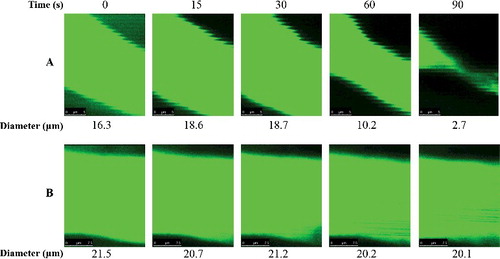
Figure 5. Ratio of vessel diameter. SYE was applied at three doses of 13, 26 and 52 mg/kg body weight by intraperitoneal injection on C57BL/6J mice. After 30 min, thrombus formation in brain arteriole was induced by laser irradiation and the diameter changes were measured and calculated as the ratio of diameter change to the initial diameter. The graph depicts the mean values ±SD (n = 6); *p < 0.05 and **p < 0.01 as compared with the control.
Additionally, SYE was employed in rats with blood stasis syndrome to study the ameliorative effects. The rats were placed in ice-cold water during the time interval between two injections of epinephrine, which could produce hemorheology disorders [Citation24]. The results of the model group showed that hemorheology abnormalities appeared, such as blood hyper-coagulability and rises of blood viscosity, indicating that the blood stasis syndromes were developed by an appropriate method. Several representative hemorheology indices were detected and statistically analyzed among all groups.
WBV is a reflection of the intrinsic resistance of blood to flow in vessels, influenced by red blood cells, platelets and plasma. The result of WBV included five different shear rates: WBV at 5/s, 30/s, 50/s, 150/s and 200/s ((A)). Each shear rate in the model group showed significant increases compared to the normal group (p < 0.01), suggesting an abnormality condition in body blood circulation. Increased viscosity might cause ischemia or thrombosis in pathologic conditions. Furthermore, the risk of major cardiovascular events (e.g. cardiovascular death or acute myocardial infarction) usually increased with elevations in WBV, which means that elevated WBV might cause early heart diseases [Citation25], whereas in the SYE group, WBV decreased remarkably, especially in high dose (4.5 mg/kg/d) as well as in the LHCI group. The SYE of medium dose (3.0 mg/kg/d) induced an effect similar to EGBLI in most shear rates. The EAI, ERI and RCEI values are presented in (B). Both EAI and RCEI showed an aggregation tendency among red blood cells, reflecting the blood viscosity as well. ERI represents the deformability of the red blood cells. All three indices decreased significantly in the SYE-High and LHCI group compared with the model group (p < 0.01), whereas they were not affected in SYE of low-dose. The results implied that, in medium and high doses, SYE could ameliorate blood circulation and reduce the risk of stasis syndrome even better than EGBLI.
Figure 6. Hemorheology parameters measurements: WBV (A) at different shear rates; EAI, ERI and RCEI (B); APTT and PT (C); MPAR (D). SYE was applied at three doses of 1.5, 3.0 and 4.5 mg/kg body weight by intramuscular injection on SD rats. The graph depicts mean values ± SD (n = 8); #p < 0.05 and ##p < 0.01 as compared with the normal, *p < 0.05 and **p < 0.01 as compared with the model.
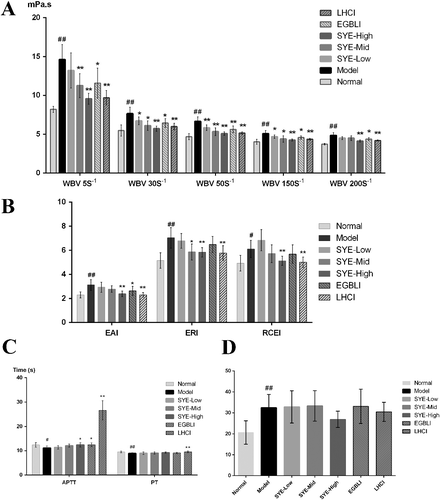
APTT, PT and MPAR denote the capability of blood coagulation. Data analysis showed that APTT and PT were shorter in the model group compared with the normal group (p = 0.029 and p < 0.01), whereas they increased significantly in the LCHI group compared with model group (p < 0.01 in both). SYE had the APTT value prolonged only in high dosage (p = 0.028). Although no significant differences appeared in PT and MPAR, SYE showed potential effects in a dose-dependent manner to some degree, which indicated that the dose of SYE could be increased properly to achieve a better effect in anticoagulation function. These results also explained the antithrombotic effects of SYE.
Noteworthy, three doses of SYE in the rat model were all lower than the clinical dose (7.0 mg/kg/d in rat equally). For safety considerations, injections of traditional Chinese medicine (TCM) were required to be used in rational doses in case of anaphylaxis or other adverse drug reactions (ADR). Taking Safflower injection (one of CTL drugs in market) as an example, the ADR monitor center declared that over three thousand ADR cases were reported in 2012, including 154 serious ones. Among them, overdose of Safflower injection was the main reason blamed for the severe cases. Consequently, safflower injection became one of the main monitored drugs of TCM [Citation26]. Thus, medicines prepared from CTL were supposed to be avoided in patients in high dosage who were children, pregnant women or those with coagulation dysfunction. In this research, we proved that SYE exerted significant anti-thrombotic effect in high dosages (26.0 and 52.0 mg/kg in mice) and circulation promotion in relative low dosage (4.5 mg/kg/d). The results suggested that, to acquire a pre-protective effect or for long-term medication, lower dosages of SYE could also achieve satisfactory outcomes.
Conclusions
In summary, the present research conducted a whole components analysis on safflower yellow extract (SYE) and identified 29 substances. The examination of the pharmacodynamic effects of SYE in a mouse model of cerebral arteriole thrombosis and rat model of blood stasis syndrome confirmed that SYE administration could inhibit the thrombus formation in mouse brain arterioles and could influence beneficially the blood rheology in a rat model of blood stasis at relevant low dosages. Especially in low-dosage medication, SYE still caused improvement of the hemorheology disorders. These results suggested that the dosage of SYE should be taken into consideration in clinic medication to acquire different therapeutic outcomes.
Disclosure statement
The authors declare that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Additional information
Funding
References
- Meng C, Zhao PW, Sun YL. Advances in pharmacological function of Carthamus tinctorius and its essential constitutes. Global Trad Chin Med. 2012;5(7):556–560.
- Yang L, Wang L, Yao M, et al. The effectiveness and safety of edaravone combined with safflower yellow injections for the treatment of acute cerebral infarction: a meta-analysis. Chin J Clin Res. 2013;26(4):313–316.
- Zhou X, Tang L, Xu Y, et al. Towards a better understanding of medicinal uses of Carthamus tinctorius L. in traditional Chinese medicine: a phytochemical and pharmacological review. J Ethnopharmacol. 2014;151(1):27–43.
- Yang XW, Li YH, Zhang H, et al. Safflower Yellow regulates microglial polarization and inhibits inflammatory response in LPS-stimulated Bv2 cells. Int J Immunopathol Pharmacol. 2016;29(1):54–64.
- Liu Y, Lian Z, Zhu H, et al. A systematic, integrated study on the neuroprotective effects of hydroxysafflor yellow A revealed by (1) H NMR-based metabonomics and the NF-kappaB pathway. Evid Based Complement Alternat Med. 2013.
- Bai Y, Lu P, Han C, et al. Hydroxysafflor yellow A (HSYA) from flowers of Carthamus tinctorius L. and its vasodilatation effects on pulmonary artery. Molecules. 2012;17(12):14918–14927.
- Wang Y, Zhang C, Peng W, et al. Hydroxysafflor yellow A exerts antioxidant effects in a rat model of traumatic brain injury. Mol Med Rep. 2016;(14):3690–3696.
- Yang F, Li J, Zhu J, et al. Hydroxysafflor yellow A inhibits angiogenesis of hepatocellular carcinoma via blocking ERK/MAPK and NF-kappaB signaling pathway in H22 tumor-bearing mice. Eur J Pharmacol. 2015;754:105–114.
- Whinna HC. Overview of murine thrombosis models. Thromb Res. 2008;122:S64–S69.
- Liu H, Zhang W-J, Long C-F, et al. Protective effects of traditional Chinese herbal formula Compound Xueshuantong Capsule (CXC) on rats with blood circulation disorders. Biotechnol Biotechnol Equip. 2017;31(4):846–854.
- Zhang Q, Lan Y, He X-f, et al. Allopurinol protects against ischemic insults in a mouse model of cortical microinfarction. Brain Res. 2015;1622:361–367.
- Li P, Su W, Yun S, et al. Toward a scientific understanding of the effectiveness, material basis and prescription compatibility of a Chinese herbal formula Dan-hong injection. Sci Rep. 2017;7:46266.
- Murphy TH. Two-photon imaging of neuronal structural plasticity in mice during and after Ischemia. Cold Spring Harb Protoc. 2015;2015(6):941–952.
- Liu H, JP L, Li P, et al. Core bioactive components promoting blood circulation in the Traditional Chinese Medicine Compound Xueshuantong Capsule (CXC) based on the relevance analysis between chemical HPLC fingerprint and in vivo biological effects. PLoS One. 2014;9(11):e112675.
- Jin Y, Zhang XL, Shi H, et al. Characterization of C-glycosyl quinochalcones in Carthamus tinctorius L. by ultraperformance liquid chromatography coupled with quadrupole-time-of-flight mass spectrometry. Rapid Commun Mass Spectrom. 2008;22(8):1275–1287.
- Fan L, Guo D-A. Stability and degradation of hydroxysafflor yellow A and anhydrosafflor yellow B in the safflower injection studied by HPLC-DAD-ESI-MSn. J Chin Pharm Sci. 2011;20(1).
- Shang E, Zhu Z, Liu L, et al. UPLC-QTOF-MS with chemical profiling approach for rapidly evaluating chemical consistency between traditional and dispensing granule decoctions of Tao-Hong-Si-Wu decoction. Chem Cent J. 2012;6(1):143.
- Perez P, Alarcon M, Fuentes E, et al. Thrombus formation induced by laser in a mouse model. Exp Ther Med. 2014;8(1):64–68.
- Kamocka MM, Mu J, Liu X, et al. Two-photon intravital imaging of thrombus development. J Biomed Opt. 2010;15(1):016020.
- Bochenek ML, Schütz E, Schäfer K. Endothelial cell senescence and thrombosis: ageing clots. Thromb Res. 2016;147:36–45.
- Li Y, Shi Y, Sun Y, et al. Restorative effects of hydroxysafflor yellow A on hepatic function in an experimental regression model of hepatic fibrosis induced by carbon tetrachloride. Mol Med Rep. 2017;15(1):47–56.
- Wright B, Moraes LA, Kemp CF, et al. A structural basis for the inhibition of collagen-stimulated platelet function by quercetin and structurally related flavonoids. Br J Pharmacol. 2010;159(6):1312–1325.
- Yu L, Hu G-Q, Du X, et al. Inhibition of cerebral ischemia/reperfusion injury-induced apoptosis: nicotiflorin and JAK2/STAT3 pathway. Neural Regen Res. 2017;12(1):96.
- Li HX, Han SY, Wang XW, et al. Effect of the carthamins yellow from Carthamus tinctorius L. on hemorheological disorders of blood stasis in rats. Food Chem Toxicol. 2009;47(8):1797–802.
- Cowan AQ, Cho DJ, Rosenson RS. Importance of blood rheology in the pathophysiology of atherothrombosis. Cardiovasc Drugs Ther. 2012;26(4):339–48.
- Wang K, Yang L. Analysis of 192 cases of traditional Chinese medicine and its adverse drug reaction. China Health Standard Manage. 2016;7:164–166.
