ABSTRACT
The present study aims to evaluate the possible hepatoprotective effect of the Red Sea Hyrtios aff. erectus sponge extract against persistent organic pollutants (POPs). The induced hepatotoxicity effects were examined by using different POPs compounds, namely, α-hexachlorocyclohexane, β-hexachlorocyclohexane, γ-hexachlorocyclohexane, PCB 28, PCB 52, aldrin, o,p'-DDE, PCB 101, dieldrin, p,p'-DDE, o,p'-DDD, endrin, PCB 118, p,p'-DDD, p,p'-DDT, PCB 153, PCB 135, PCB 138 and PCB 180, extracted from sediments collected from Lake Mariout. The effects were assessed based on biochemical assays, where BALB/c albino mice (mean weight 28 ± 4 g) exposed to 130.6 mg/100 g b.w./d of POP mixtures were compared to control and induction groups. The POPs toxicity test was carried out on the BALB/c albino mice for one week. The POPs treated group had a significant increase in the levels of transaminases, ALT and AST. There was also a significant increase in serum bilirubin in response to POPs mixture toxicity. In contrast, the protective group revealed a non-significant increase in both aminotransferases and bilirubin level. The results indicate that POPs could act through free radical-induced oxidative stress. Furthermore, the hepatoprotective effect of sponge extract against POPs mixture is due to the presence of polyphenolic compounds in sponge. The present study revealed that polyphenolic compounds play a vital role in diminishing the hepatotoxicity, as confirmed by a decrease in liver toxicity.
Abbreviations
| o,p'-DDD | = | 1,1-Dichloro-2-(p-chlorophenyl)-2-(o-chlorophenyl)ethane |
| p,p'-DDD | = | 1,1-Dichloro-2,2-bis(4'-chlorophenyl)ethane |
| o,p'-DDE | = | 2,2-(2-Chlorophenyl-4'-chlorophenyl)-1,1-dichloroethene |
| p,p'-DDE | = | 1,1-Dichloro-2,2-bis(p-chlorophenyl)ethylene |
| p,p'-DDT | = | 1,1,1-Trichloro-2,2-bis(4-chlorophenyl)ethane |
| α-HCH | = | α-Hexachlorocyclohexane |
| β-HCH | = | β-Hexachlorocyclohexane |
| γ-HCH | = | γ-Hexachlorocyclohexane |
| PCB 28 | = | 2,4,4'-Trichloro-1,1'-biphenyl |
| PCB 52 | = | 2,2',5,5'-Tetrachlorobiphenyl |
| PCB 101 | = | 2,2',4,5,5'-Pentachlorobiphenyl |
| PCB 118 | = | 2,3',4,4',5-Pentachlorobiphenyl |
| PCB 135 | = | 2,2',3,3',5,6'-Hexachlorobiphenyl |
| PCB 138 | = | 2,2',3,4,4',5'-Hexachlorobiphenyl |
| PCB 153 | = | 2,2',4,4',5,5'-Hexachlorobiphenyl |
| PCB 180 | = | 2,2',3,4,4',5,5'-Heptachlorbiphenyl |
Introduction
The marine habitat is a prolific source of bio-active secondary metabolites with the potential to treat various diseases [Citation1,Citation2]. Besides the chemical novelty associated with these compounds, some of them possess novel mechanisms of action as well [Citation3]. The number of identified marine natural compounds has grown by 20% from 2009 to 2013; further, especially effective antimicrobial agents from marine natural products have been raised over the past five years [Citation4].
Several challenges, including sample collection and structure elucidation, have limited the development of this research field [Citation5]. In general, natural molecules isolated from marine environment show higher and more significant bioactivity than those from terrestrial environment [Citation5]. Marine sponges (Porifera) are so far recognized as the most biodiverse and unique marine sources of bioactive natural products, accounting for about 30% of the marine natural products discovered until now [Citation6].
Organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs) are two main categories of organic pollutants (POPs) present as contaminants in the environment. They have been used for many years in different industries and agricultural activities [Citation7,Citation8].
Man-made organochlorines have been considered a serious threat to the long-term health of the marine environment for many years (reviewed in [Citation9]). Although most of these chemicals have a low acute toxicity in mammals, they have been associated with various chronic effects, including immunosuppression, reproductive failure, endocrinological and neurological alterations [Citation10]. Recent studies have shown that these chemicals persist in both the aquatic and terrestrial ecosystems [Citation11]. In Egypt, the highest concentrations of organochlorines have been associated with centers of urbanization [Citation7,Citation8,Citation12].
Despite the accumulating body of data on animal exposure to a single compound, the knowledge of the toxicity of complex mixtures is scarce [Citation13]. The liver is an important organ involved in the metabolism of endogenous or exogenous substances [Citation14]. Most hazardous and xenobiotic substances are metabolized in the liver through detoxification processes involving several activation, conjugation and elimination mechanisms [Citation15]. Exposures to POP compounds (especially pesticides) can be monitored and evaluated based on nervous system damage and neuro-behavioural functions [Citation16–18] or by measuring the level of liver enzymes such as alanine aminotransferase (ALAT) and γ-glutamyltranspeptidase (GGT) as a biological indicator [Citation19–21].
Although plants and plant materials play important roles in biological and pharmacological activities in humans as well as in animals [Citation22], the marine environment continues to be a source of unique and tremendous natural products used in different applications, for example in pharmaceutical and medical biotechnological products. The Red Sea is one of the richest marine environments in biodiversity worldwide [Citation23–28]. Marine sponge are sessile organisms; therefore, their defense mechanisms are largely dependent on the release of powerful compounds as chemical weapons that target predators and invaders but do not act against the own cells of the sponge [Citation1]. This distinctive feature of marine sponge could explain why they are such a rich source of unique biologically active natural products [Citation29].
Since marine natural products have high potential effect in detoxification, they can act as defense chemicals against PCBs, which are metabolically activated in the liver resulting in highly reactive intermediates capable of impairing various cellular functions [Citation30]. Our research group previously demonstrated the hepatotoxicity of persistent organic pollutants (POPs) from the sediment of Lake Mariout [Citation1], in which POP compounds exerted their effect by diminishing the antioxidant capacity. The aim of the present study was to assess whether exposure to a mixture of pesticides elicits early biochemical changes in biomarkers of liver function. The study also aimed to evaluate the potential hepatoprotective effect of marine extracts from sponges against POPs toxicity in BALB/c albino mice.
Materials and methods
Study area
Lake Mariout, a coastal lagoon artificially divided into four main basins, is one of the Egyptian northern delta lakes. It is located in the southern border of Alexandria, Egypt. The lake is mainly polluted by atmospheric deposition [Citation31] and municipal and agricultural wastes rich in pesticides and fertilizers coming predominantly from El Beheira Governorate [Citation7,Citation8].
The Red Sea is recognized as a unique marine environment important for its rich biodiversity, including coral reefs and more than 10 thousands of sea organisms. However, it is subjected to major threats which are related to land-based activities [Citation32–39].
Sediment sampling
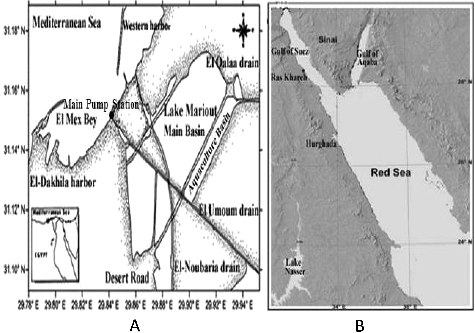
A surface sediment (0–3 cm) sample was collected using a stainless steel grab in 2011 from the main basin before the main pumping station of Lake Mariout (). The sample was freeze-dried and stored in pre-cleaned aluminum containers until analysis.
Extraction and identification of POPs compounds from sediment samples
POPs were extracted from the sediment sample collected from the most polluted area in Lake Mariout (Latitude: 31°07′44.8″ & Longitude: 31°07′44.8″). Pops extraction and determination were done according to UNEP/IOC/IAEA [Citation40], IOC [Citation41] and Shreadah et al. [Citation7].
Sampling and identification of the sponge
A sponge (Hyrtios aff. erectus RMNH POR.8633) sample [Citation1] was collected from Hurghada at the Red Sea (Latitude: 27°11′37.5″ & Longitude: 33°50′48.4″) in 2011 (). The sponge was kindly identified by Dr. Nicole De Voogd, at Naturalis Biodiversity Center, Department of Marine Zoology, RA Leiden, The Netherlands [Citation1]. The voucher specimen is incorporated in the collections of the Zoological Museum of the University of Amsterdam under registration number RMNH POR.8633.
Preparation of sponge hyrtios aff. erectus extract
The sponge extract was prepared and extracted according to Abdel-Moniem et al. [Citation29], where the sponge sample was cut into small parts and extraction was performed using ethyl acetate 3 times (1500 mL). The organic fractions were combined and the solvent was removed at reduced pressure and 35 °C. Residues were re-dissolved for further bioassay. The steps of extraction and isolation of secondary metabolites are shown in Supplementary Figure S1.
Determination of total phenolic and total flavonoid contents in the sponge extract
Total phenolic compounds in the sponge extracts were determined as milligrams of gallic acid equivalent (GAE) in one milliliter of the extract, using a standard curve for gallic acid, by the method of Taga et al. [Citation42]. The total flavonoid content was determined by as milligrams of quercetin equivalent (QE) in one milliliter of the extract, using a standard curve for (+) – querctin by the method of Zhishen et al. [Citation43].
Animals and treatment
A total of 80 adults white male BALB/c mice, weighing from 18 to 25 g, were obtained from the animal house of the Institute of Theodor Bilharz Research Institute (Giza, Egypt). They were kept in plastic cages, each cage containing five animals. The animals were maintained under standard laboratory conditions of temperature (20–26 °C), humidity (20 ± 2%) and light (7:00 a.m. to 7:00 p.m.)/dark (7:00 p.m. to 7:00 a.m.) cycles and were fed on standard rodent chow, with water provided ad libitum. After 1 week of acclimatization to the laboratory environment, the mice were divided into four groups.
Group I: received saline solution subcutaneously for one week and served as a negative control group.
Group II: received 130.6 mg/100 g b.w./day of POPs mixture (α-hexachlorocyclohexane (α-HCH), β-HCH, γ-HCH, PCB 28, PCB 52, aldrin, o,p'-DDE, PCB 101, dieldrin, p,p'-DDE, o,p'-DDD, endrin, PCB 118, p,p'-DDD, p,p'-DDT, PCB 153, PCB 135, PCB 138 and PCB 180), subcutaneously for one week, and served as the induced toxicity group.
Group III: received i.p a dose of 0.7 mg/100 g b.wt/day of H. aff. erectus sponge extract as in group IV, for one week as a protective dose before administration of the pesticide mixture as described in group II. This group served as the protection group.
Group IV: received 0.7 mg/100 g b.w./day i.p of H. aff. erectus sponge extract for one week and served as a positive control group.
At the end of the experiment, mice were anaesthetized using diethyl ether.
Blood sample collection
Heparinized blood samples were collected and kept for 15 min at room temperature. Sera were separated by centrifugation at 3000 rpm for 20 min and stored at −20 °C until use for further analyses.
Biochemical measurements and assessment of liver function tests
Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities were determined according to the methods of Reitman and Frankel [Citation44].
The total protein content was determined by means of the biuret reaction as described by Gornall et al. [Citation45]. The albumin content was determined according to the method of Doumas et al. [Citation46]. The total bilirubin content was determined according to the method described by Walters and Gerarde [Citation47].
Histopathological study
The liver tissues were fixed in formalin and were dehydrated in ascending grades of alcohol. The samples were then cleaned by immersing the tissues in xylene for 1 h (three times), followed by impregnation in melted paraffin, in wax, then incubated in an oven at 60 °C for 1 h. The specimens were embedded in paraffin and were left to solidify at room temperature. Using a rotatory microtone, sections of 5 μm thickness were cut and mounted on clean glass slides. The sections were stained with hematoxylin and eosin (H&E) and examined for any histopathological changes [Citation48].
Statistical analysis
The data are presented as mean values (X) with standard deviation (±SD) for 7 animals in each group. Comparisons between the means of various treatment groups were analyzed using the least significant difference (LSD) test. Differences were considered significant at P < 0.05. All statistical analyses were performed using the statistical software SPSS, version 11.5.
Results and discussion
POPs from sediments of lake mariout
POP compounds in general and PCBs specifically are widely used in various industries, although their chemical and physical stability and lipophilic character make them dangerous environmental hazards, highly permanent and accumulating in soils and aquatic biota [Citation7,Citation8,Citation12]. Soil is considered the major source of PCBs in the environment after the ban on their industrial production [Citation7,Citation8,Citation12,Citation49]. Organochlorines are of particular importance not only because of their pronounced toxicity to aquatic life, but also because they are lipid (fats) soluble and because they are very slowly biodegradable. The slow rate of PCB biodegradation is often exceeded by the rate of their release, resulting in their accumulation in the environment [Citation7,Citation8,Citation12,Citation49]. The concentrations of different PCBs extracted from the sediment sample of Lake Mariout showed the presence of 18 types of pesticides ( and ). A total of 11 OCPs and 7 PCBs were identified and quantified. OCPs and PCBs were found to be ubiquitous pollutants in the aquatic environment of Lake Marriout, reflecting the local usage and input of these pollutants [Citation50]. The total concentrations of OCPs and PCBs in sediments ranged from 0.04 to 64.82 ng/g and 0.7 to 280.28 ng/g, respectively. Among OCPs, β-HCH, γ-HCH, aldrin, endrin, p,p'-DDE, p,p'-DDT, p,p'-DDE and dieldrin were the most abundant compounds. The component of PCB congeners included tri-, tetra-, penta- and hexa-chlorinated biphenyls. The contamination levels of PCBs and HCHs can be categorized as moderate to high compared to other urbanized regions worldwide. According to sediment quality guidelines, β-HCH, p,p'-DDT and o,p'-DDT would be more relevant OCP species for the eco-toxicological risk in Lake Marriout. The maximum permissible levels of organochlorine pollutants postulated by the National Academy of Engineering and National Academy of Sciences are 1000–5000 ng/g for PCBs and 100 ng/g for cyclodienes (wet weight).
Table 1. Concentrations of different POPs compounds in the sediment sample.
The results from the present study ( and ) revealed the presence of high concentrations of PCBs in the Lake Main Basin. Among other PCBs, the highest concentration was that of PCB 101(3.342 μg/g; dry weight), whereas the lowest one referred to β-hexachlorocyclohexane (0.002 μg/g; dry weight). This could be attributed to the discharge of huge amounts of sewage and agricultural wastewaters [Citation49,Citation50] revealing a high accumulation rate with a slow rate of PCBs biodegradation. In a similar study, POPs concentrations in sediments of Lake El-Burrullus ranged from 4.6 to 213.9 ng/g with an average of 47.2 ng/g; dry weight. The maximum concentration of PCBs was 214 ng/g in the area affected by the discharge of a great amount of sewage and agricultural wastes from drain 8 into the Lake annually [Citation8], which agrees well with many previous studies [Citation7,Citation8,Citation12,Citation49]. The recommended levels by the Swedish Food Regulation are 5000 ng.g–1 for DDTs, 2000 ng/g for PCBs and 200 ng/g for HCB [Citation51]. The US Food and Drug Administration (FDA) tolerance limit is 2000 ng/g wet weight for total PCBs in fish and shell fish [Citation52]. The present study indicated that all measured concentrations of pesticides and PCBs were much lower than those reported as permissible levels.
Total phenolic and flavonoid compounds in the sponge extract
The biochemical profile of the sponge extract showed 6.52-fold higher content of total flavonoids (1.02 mg QE/mL) than total phenolics (0.17 mg GAE/mL). Many supplements have been used to protect the liver against damage, including administration of antioxidants such as ββ-carotene, vitamin C and vitamin E [Citation53–58]. The phenolic compounds in general and especially flavonoids are a large group of naturally occurring compounds that are found in terrestrial plants and are daily consumed through human diet. Flavonoids receive much interest for their potential pharmacological properties because of their antioxidant properties [Citation29,Citation53]. The antioxidant activity of phenolic compounds has been demonstrated by their ability to inhibit enzymes such as lipoxygenase, cyclooxygenase [Citation59], along with chelating metal ions [Citation60], and scavenging free radicals [Citation61]. In spite of their role as free-radical scavengers, there was no clear report on the antioxidant effect of most of the phenolic compounds in PCBs-induced rats or mice. The results from the present study suggested that the polyphenolic compounds (phenolics and flavonoids), especially flavonoids, present in high concentrations in the extract could have potential protective effects against POPs-induced toxicity in mice, in agreement with previous reports [Citation53].
Mortality rate
The mortality rates for each group were determined in our experiment. In group II (induction group), three of 20 animals died by day 4: two of them died within the first 72 h following induction with POPs mixtures, and another one died within the next 24 h. In group IV (protection group), two mice died within 24 and 72 h, respectively (after marine sponge extract and BCBs administration). There were no differences (no deaths) in the other two groups, group I (negative control) and group III (positive control).
Effect of sponge extract on the activities of ALT and AST enzymes
It is well documented that POPs are environmental toxicants associated with many severe health effects, through wide diffusion in the environment, bioconcentration in the food chain and bioaccumulation in the biosphere [Citation11]. POPs-induced toxicity is associated with the production of free radicals. The liver, on the other hand, plays a key role in the maintenance of the homeostasis of the organism. Traditional liver assay gives information about the integrity of hepatocytes, such as serum transaminases (ALT and AST– aminotransferases), with ALT being the standard clinical biochemistry marker of liver diseases [Citation53–55]. The results for the activity of serum ALT and AST are shown in . The activity of serum ALT (87.68 ± 3.38 U/L, P < 0.001) and AST (161.91 ± 8.37 U/L, P < 0.001) in mice treated with the POPs mixture (group II) were significantly increased compared to those in the negative control group (group I) mice (48.83 ± 3.42 and 94.58 ± 6.21), respectively. In contrast, the group pretreated with 0.7 mg/100 g b.w/day of sponge extract (group III) for 7 days had a significantly lower ALT (75.76 ± 4.29) and AST activity (111.45 ± 5.68) compared to group II, i.e. the induction group (P < 0.001). The levels of both ALT and AST in group IV (positive control) showed non-significant increase and/or decrease when compared to their corresponding values in group I ().
Table 2. Effect of sponge extract on the activities of serum ALT and AST.
In this study, the observed increase in aminotransferase enzyme activities (AST and ALT) in the induction and pretreatment groups might be due to an increase in the cell-membrane permeability or cell-membrane damage of hepatocytes. The increase in the AST and ALT activities was most probably caused by PCBs. Notably; this increase in the pretreated group was not significant, reflecting the protective effect of the sponge extract. Such an effect may be due to an oxidative impairment that occurs when the generation of reactive oxygen species overrides the ability of the antioxidant system to neutralize them, leading to an increase in oxidative processes and a decrease in antioxidant defenses [Citation53]. The accumulation of volatile metabolites and other free radicals in hepatic cellular components may increase the cellular degeneration in the liver [Citation54]. Karakilcik et al. [Citation56] also reported that the high levels of these enzymes are indicators of hepatic damage both in human and experimental animals.
The exact mechanism of action underlying the hepatotoxicity of POPs is not very well known. It has been suggested that POPs are transformed through redox pathways into primary and secondary metabolites responsible for the hepatotoxic effect of POPs [Citation57]. These substituted or intermediary metabolites may react with free amino groups of proteins, thus rendering them dysfunctional [Citation59]. Evidence supporting these findings was reported in our previous paper [Citation2] through the oxidative stress associated with POPs compounds and their effect on the antioxidant capacity.
Effect of sponge extract on the levels of total protein, albumin and bilirubin
The level of total protein was significantly decreased in the group treated with the POPs mixture, i.e. the induction group, compared to that in the negative control group (group I) by about 42.3% (). The albumin value in the induction group, i.e. group II (1.68 ± 0.46), showed a significant decrease (P < 0.001) by 30.5% compared to group I, i.e. the negative control group (2.42 ± 0.15).
Table 3. Effect of sponge extract on the serum levels of total protein, albumin, and total bilirubin of induced toxicity groups of mice.
Administration of sponge extract prior to POPs mixture injection (group III) resulted in a significant decrease in the total bilirubin content compared to group II by 34.4% (0.81 ± 0.08 versus 1.23 ± 0.23 g/dL at P = 0.003). The levels of total protein were significantly decreased in the POPs mixture treated group, i.e. induction group, compared to their corresponding values in the negative control group (group I) by about 42.3%. In addition, the albumin value (1.68 ± 0.46 g/dL) in group II (induction group) was significantly lower (P < 0.001) by 30.5% compared to that in group I, i.e. the negative control group (2.42 ± 0.15). In contrast to group II (induction group), the administration of sponge extract prior to POPs mixture injection in group III led to a significant decrease in the total bilirubin content compared to group II by 34.4% (0.81 ± 0.08 versus 1.23 ± 0.23 g/dL; P = 0.003).
The decrease in the serum total proteins and total albumin in POPs-treated mice (induction group) may be attributed to changes in the intracellular protein synthesis and the level of oxidative enzymes in the liver [Citation62,Citation63]. This is in agreement with the suggestion that the reduction in the protein content may be due to a decrease in the level of serum globulin [Citation63,66]. Moreover, the reduction in the serum albumin level following treatment with PCBs may be due to the decrease in the formation of protein in the liver and/or from the alimentary tract [Citation54]. Moreover, we observed an increase in the bilirubin level in the group induced with PCBs, which is in agreement with Selvakumar et al. [Citation53]. This was probably due to the hemolysing property of PCBs, since, in the protected-treated group, the total bilirubin level was within a normal level compared to the negative control group.
Histological findings
In this study, histological parameters served as another endpoint for assessment of the hepatotoxicity induced by POPs, in addition to the biochemical alterations. The results from the histopathological examination of mice liver are shown in (A–D). Group I (negative control) and group III had normal liver with no remarkable pathological changes ((A,C)). In group II (treated with the POPs mixture, 130.6 mg/100 g b.w./d for 7 days) there was dilatation in the central and portal veins with newly formed bile ductules and oedema. Inflammatory cells infiltration in the portal area and focal haemorrhage were noticed in the hepatic parenchyma (B1 and B2). In contrast, the control (group I) and sponge extract treated group (group IV) showed normal liver architecture with no remarkable pathological changes ((A,D)). Some mice in group IV (protected group treated with sponge extract prior to treatment with POPs) showed near normal hepatic architecture ((C)).
Figure 3. Hepato-histopathological effect of Hyrtios aff. erectus sponge extract on POPs induced toxicity in mice of different groups. Note: Light microscopy; H&E × 400.
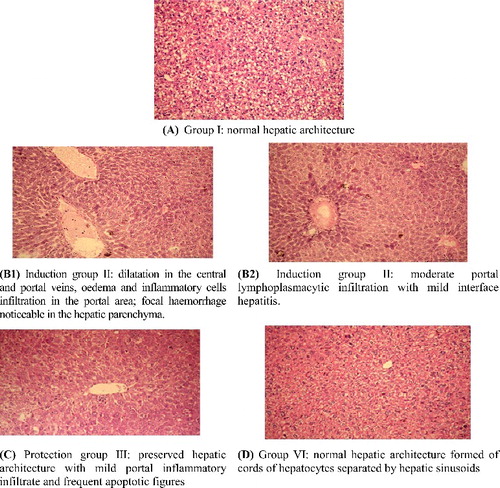
Both the histpathological and biochemical indicators demonstrated that the destructive effect of POPs compounds was ameliorated in the protected group as compared to the induction group. We suggest that this protective effect was attributable to the polyphenolic compounds in the sponge extract. Few studies have focused on the alternative approach to protect against POPs-induced hepatotoxicity using antioxidants derived from marine natural products [Citation1,Citation29,Citation64,Citation65,66]. The results from the present study corroborate our suggestion that the Hyrtios aff. erectus sponge extract is an ideal antioxidant and hepatoprotective agent [Citation1]. Further studies are, however, required to purify and identify the active compounds and investigate other possible underlying mechanisms, such as regulation of different signalling pathways in liver cells.
Conclusions
This study confirmed the protective effect of the extract from the marine sponge Hyrtios aff. erectus against POPs-induced hepatotoxicity. The mechanism underlying the hepatoprotective effect is most likely related to increased total antioxidant capacity due to high polyphenolic content in the extract, which could diminish the oxidative stress. However, the hepatoprotective effects should be retested with pure compounds responsible for these activities. Further studies are required to explore other possible mechanisms as well.
Supplemental_Data.pdf
Download PDF (357.4 KB)Acknowledgments
The authors thank Dr. El Sayed A. E. Hamed and Dr. Khalid Elhaddad from the National institute of Oceanography and Fisheries, Alexandria Branch, for helping in obtaining the sponge samples.
Disclosure statement
The authors declare no conflict of interest.
References
- Abd El-Moneam NM, Shreadah MA, El-Assar SA, et al. A protective role of antioxidants capacity of Hyrtios aff. Erectus sponge extract against mixture of persistent organic pollutants (POPs)-induced hepatic toxicity in mice liver: biomarkers and ultrastructural study. Environ Sci Pollut Res. 2017;24(27):22061–22072.
- Abd El-Moneam NM, El-Assar SA, Shreadah MA, et al. Isolation, identification and molecular screening of Pseudomonas sp. metabolic pathways NRPs and PKS associated with the Red Sea sponge, Hyrtios aff. Erectus, Egypt. J Pure Appl Microbiol. 2017;11(3):1299–1311.
- Montaser R, Luesch H. Marine natural products: a new wave of drugs ? Future Med Chem. 2011;3:1475–1489.
- Choudhary A, Naughton LM, Montánchez I, et al. Current status and future prospects of marine natural products (MNPs) as antimicrobials. Mar Drugs. 2017 [cited 2018 Feb 06];15(9):E272. DOI:10.3390/md15090272
- Li K, Chung-Davidson YW, Bussy U, et al. Recent advances and applications of experimental technologies in marine natural product research. Mar Drugs. 2015;13(5):2694–2713.
- Blunt JW, Copp BR, Keyzers RA, et al. Marine natural products. Nat Prod Rep. 2014;31:160–258.
- Shreadah MA, Said TO, Othman IM, et al. Polychlorinated biphenyls and chlorinated pesticides in sediments along the semi-closed areas of Alexandria, Egypt. J Environl Protection. 2012;3(2):141–149.
- Shreadah MA, Said TO, Othman M, et al. OCPs and PCBs in seawater from Egyptian Mediterranean coast of Alexandria. Develop Anal Chem. 2014;1:19–24.
- Maurya PK, Malik DS. Bioaccumulation of xenobiotics compound of pesticides in riverine system and its control technique: A critical review. J Industr Pollut Control. 2016;32(2):580–594.
- Longnecker MP, Klebanoff MA, Brock JW, et al. Polychlorinated biphenyl serum levels in pregnant subjects with diabetes. Diabetes Care. 2001;24(6):1099–1101.
- Said TO, Shreadah MA, Mansour MA, et al. OCPs, PCBs and THCs in Sparus auratus Species from the Egyptian Mediterranean coast. Open J Mar Sci. 2017:7(2):317–326.
- Said TO, El-Moselhy KM, Rashad AM, et al. Organochlorine Contaminants in water, sediment and fish of Lake Burullus, Egyptian Mediterranean Sea. Bull Environ Contam Toxicol. 2008;81(2):136–146.
- Pelletier G, Masson S, Wade MJ, et al. Contribution of methylmercury, polychlorinated biphenyls and organochlorine pesticides to the toxicity of a contaminant mixture based on Canadian Arctic population blood profiles. Toxicol Lett. 2009;184(3):176–185.
- Kshirsagar AV, Bang H, Bomback AS, et al. A simple algorithm to predict incident kidney disease. Arch Int Med. 2008;168(22):2466–2473.
- Sheweita SA, Newairy AA, Mansour HA, et al. Effect of some hypoglycemic herbs on the activity of phase I and II drug-metabolizing enzymes in alloxan-induced diabetic rats. Toxicol. 2002;174:131–139.
- Bazylewicz-Walczak B, Majczakowa W, Szymczak M. Behavioral effects of occupational exposure to organophosphorous pesticides in female greenhouse planting workers. Neurotoxicol. 1999;20:819–826.
- Rom WN. Environmental and occupational medicine. 4th ed. Philadelphia (USA): Lippincott Williams and Wilkins; 2007. p. 1884.
- Stephens R, Spurgeon A, Calvert IA, et al. Neuropsychological effects of long-term exposure to organophosphates in sheep dip. Lancet. 1995;345:1135–1139.
- Altuntas I, Delibas N, Demirci M, et al. The effects of methidathion on lipid peroxidation and some liver enzymes: Role of vitamins E and C. Arch Toxicol. 2002;76:470–473.
- Altuntas I, Delibas N, Sutcu R. The effects of organophosphate insecticide methidathion on lipid peroxidation and antioxidant enzymes in rat erythrocytes: Role of vitamins E and C. Hum Exp Toxicol. 2002;21:681–685.
- Di Lorenzo L, Corfiati M, Bulfaro D, et al. Aspartate aminotransferase (AST) to alanine aminotransferase (ALT) ratio in health surveillance of workers exposed to vinyl chloride monomer: Preliminary results. G Ital Med Lav Ergon. 2003; Suppl (3):109–111.
- Thompson MA, Aberg JA, Hoy JF, et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the international antiviral society-USA panel. JAMA. 2012;308(4):387–402.
- Shriadah MA, Okbah MA, El-Deek MS. Trace metals in the water columns of the Red Sea and the Gulf of Aqaba, Egypt. Water Air Soil Pollut. 2004;153:115–124.
- Abdel-Halim A, Aboel-Khair EM, Fahmy MA, et al. Environmental assessment on the Aqaba Gulf coastal waters; Egypt. Egyptian J Aquatic Res. 2007;33(1):1–14.
- Abdel-Halim AM, Abdel Nabi MA, Abdel Fattah LM, et al. Environmental studies on the Aqaba Gulf coastal waters during 2011–2013. J Environ Protect. 2016;7:1411–1437.
- Abo-El khair EM, Abdel Halim AM, Fahmy MA, et al. Environmental impact assessment of Northern Red Sea regions during 2005 – 2007. Egyptian J Aqu Res. 2008;34(2):20–30.
- Abo-el-Khair EM, Abdel Halim AM, Shriadah MA, et al. Environmental conditions of the Suez Gulf and the Red Sea Coastal Waters, Egypt. In: Proceedings of the 8th International Conference on the Mediterranean Coastal Environment; 2007 Nov 13–17; Alexandria, Egypt. Mediterranean Coastal Foundation Medcoast; 2007. p. 517–526.
- Abo-El-Khair EM, Abdel Fattah LM, Abdel-Halim AM, et al. Assessment of the hydrochemical characteristics for the coastal waters of the Suez Gulf during 2011–2013. J Environ Protect. 2016;7:1497–1521.
- Abdel-Monem N, Abdel-Azeem AM, El Ashry ESH, et al. Assessment of secondary metabolites from marine-derived fungi as antioxidant. J Med Chem. 2013;3(3):60–73.
- Hernández HG, Tse MY, Pang SC, et al. Optimizing methodologies for PCR-based DNA methylation analysis. Biotechniques. 2013;55(4):181–197.
- GESAMP. Impact of oil and related chemicals and wastes on the marine environment. London (UK): International Maritime Organization; 1993. (Reports and Studies; GESAMP 50).
- Okbah MA, Shata MA, Shriadah MA. Geochemical forms of trace metals in mangrove sediments-Red Sea (Egypt). Chem Ecol. 2005;21:23–36.
- Gurguess SM, Shreadah MA, Fahmy MA, et al. Assessment of water quality in the Red Sea using in situ measurements and remote sensing data. Egyptian J Aqu Res. 2009;35(2):1–13.
- Shreadah MA, Said TO, El Zokm GM, et al. Physico-chemical characterititics of the surficial sediments along the Egyptian Red Sea coasts. Egyptian J Aqu Res. 2008;34(4):16–34.
- Shreadah MA, Said TO, Abd El Ghani SA, et al. Alkyllead and alkyltin species in different fishes collected from the Suez Gulf, Egypt. Egyptian J Aqu Res. 2008;34(4):64–73.
- Shreadah MA, Masoud MS, Said TO, et al. Application of IR, X-ray, TGA and DTA to determine the mineral composition of the sediments and study of reaction kinetics along the Egyptian Red Sea coasts. Egyptian J Aqu Res. 2008;34(2):83–95.
- Masoud MS, Said TO, El-Zokm GM, et al. Speciation of Fe, Mn and Zn in surficial sediments from the Egyptian Red Sea coasts. Chem Speciation Biodiv. 2010;22(4):257–269.
- Masoud MS, Said TO, El- Zokm GM, et al. Assesment of Heavy Metals Contamination in Surface Sediments of the Egyptian Red Sea coasts. Australian J Basic Applied Sci. 2012;6:44–58.
- Fahmy MF, Abdel Fattah LM, Abdel-Halim AM, et al. Evaluations of the coastal water quality of the Egyptian Red Sea during 2011-2013. J Environ Protection. 2016;7(12):1810–1834.
- UNEP/FAO/ IAEA IOC/. Sampling of selected marine organisms and sample preparation for the analysis of chlorinated hydrocarbons. Nairobi (Kenya): UNEP; 1991. (Reference methods for marine pollution studies; No. 12 Rev. 2)
- IOC. Chlorinated biphenyls in open waters: sampling extraction, clean-up and instrumental determination. Paris (France): UNESCO; 1993. (IOC Manual and guides; No. 27).
- Taga MS, Miller E, Pratt D. Chia seeds as a source of natural lipid antioxidants. J Am Oil Chemists’ Soc. 1984;61(5):928–931.
- Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64(4):555–559.
- Reitman S, Frankel S. Glutamic – pyruvate transaminase assay by colorimetric method. Am J Clin Path. 1957;28:56–63.
- Gornall AC, Bardawill CJ, David MM. Determination of serum proteins by means of the biuret reaction. J Biol Chem. 1949;177:751–767.
- Doumas BT, Watson WA, Biggs HG. Albumin standards and the measurement of serum albumin with bromcresol green. Clinica Chimica Acta. 1971;31(1):87–96.
- Walters MI, Gerarde H. An ultramicromethod for the determination of conjugated and total bilirubin in serum or plasma. Microchem J. 1972;15(2):231–243.
- Griffith J, Farris E. The osseous system. In: Griffith J, Farris E, editors. The rat in laboratory investigation. Philadelphia (USA): JB Lippincott; 1942. p. 418–419.
- Shreadah MA, Said TO, El-Sharkawi FM, et al. POPs in sediments of the Eastern and Western coast of the Egyptian Mediterranean sea; A comparative study. Develop Anal Chem. 2016;3:1–11.
- Khalil Mkh, El Zokm GM, Fahmy MA, et al. Geochemistry of some major and trace elements in sediments of Edku and Mariut Lakes, North Egypt. World Appl Sci J. 2013;24(3):282–294.
- SFR, Foreign Substances in Food. The National Food Administration SLVFS: 1. Uppsala (Sweden): Swedish Food Regulation; 1983.
- National Academy of Sciences and National Academy of Engineering, environmental ecological research services. Washington DC (USA): NAS-NAE; 1972. p. 1–62.
- Selvakumar C, Maheshwari U, Suganthi S, et al. Oxidant-antioxidant disturbance in men classified as obese according to the preliminary WHO guidelines for Asians. J Stress Physiol Biochem. 2012;8(1):172–181.
- El-Gharieb MA, El-Masry TA, Emara AM, et al. Potential hepatoprotective effects of vitamin E and Nigella sativa oil on hepatotoxicity induced by chronic exposure to malathion in human and male albino rats. Toxicol Environ Chem. 2010;92(2):391–407.
- Binukumar BK, Gill KD. Chronic exposure to pesticides- neurological, neurobehavioral and molecular targets of neurotoxicity. In: Stoytcheva M, editor. Pesticides in the modern world – effects of pesticides exposure. Rijeka (Croatia): InTech; 2010.
- Karakilcik AZ, Zerinm M, Arslanm O, et al. Effects of vitamin C and E on liver enzymes and biochemical parameters of rabbits exposed to aflatoxin B1. Vet Hum Toxicol. 2004;46:190–192.
- Venkataraman T, Valdes M, Elsby R, et al. Loss of DExD/H box RNA helicase LGP2 manifests disparate antiviral responses. J Immunol. 2007;178(10):6444–6455.
- Teppema LJ, Nieuwenhuijs D, Sarton E, et al. Antioxidants prevent depression of the acute hypoxic ventilatory response by subanaesthetic halothane in men. J Physiol. 2002;544:931–938.
- Potapovich AI, Kostyuk VA. Comparative study of antioxidant properties and cytoprotective activity of flavonoids. Biochemistry (Mosc). 2003;68(5):514–519.
- Yokozawa T, Satoh A, Cho EJ. Ginsenoside-Rd attenuates oxidative damage related to aging in senescence-accelerated mice. J Pharm Pharmacol. 2004;56(1):107–113.
- Guardia T, Rotelli AE, Juarez AO, et al. Anti-inflammatory properties of plant flavonoids. Effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Farmaco. 2001;56(9):683–687.
- Abella V, Santoro A, Scotece M, et al. Non-dioxin-like polychlorinated biphenyls (PCB 101, PCB 153 and PCB 180) induce chondrocyte cell death through multiple pathways. Toxicol Lett. 2015;234(1):13–19.
- Williams GM, Iatropoulos MJ. Alteration of liver cell function and proliferation: differentiation between adaptation and toxicity. Toxicol Pathol. 2002;30(1):41–53.
- Bhushan B, Saxena PN, Saxena N. Biochemical and histological changes in rat liver caused by cypermethrin and beta-cyfluthrin. Arh Hig Rada Toksikol. 2013;64:57–67.
- Fan X, Bai L, Zhu L, et al. Marine algae-derived bioactive peptides for human nutrition and health. J Agric Food Chem. 2014;62(38):9211–9222.
- Abdel-Monem NM, Abdel-Azeem AM, El-Ashry EH, et al. Pretreatment hepato- protective effect of the marine fungus derived from sponge on hepatic toxicity induced by heavy metals in rats. BioMed Res Int. 2013 [cited 2018 Feb 06];2013:510879. [15 p.] DOI:10.1155/2013/510879.