ABSTRACT
In this study, an RG-I pectin DNP-W6 was isolated from the stems of Dendrobium nobile Lindl. (Orchidaceae). It contained mannose, glucose, galactose, galacturonic acid and rhamnose in a molar ratio of 0.6: 1.0: 1.5: 1.1: 1.0. Its structural features were revealed by chemical, physical and spectral analysis. The results suggested that DNP-W6 possessed a backbone of a disaccharide of →4)-α-GalAp-(1→2)-α-Rhap-(1→, with approximate 50% substitution at O-4 of the rhamnopyranosyl residues. The side chains were neutral chains including galactosyl, mannosyl and glucosyl chains. The acetyl content was estimated to be 8.9%. The immunological results indicated that DNP-W6 and its derivatives exhibited different immunological activities in vitro, showing that the side chains and acetyl groups had an important effect on the expression of immunological activities.
Introduction
Pectic polysaccharides are a complex family of polysaccharides which occur naturally as structural elements in the primary cell walls and intracellular regions of higher plants [Citation1,Citation2]. The chemical structures can be divided into three main types: homogalacturonan (HG), rhamnogalacturonan-I (RG-I) and rhamnogalacturonan-II (RG-II) [Citation2–5]. HG is a linear chain of (1→4)-α-D-galacturonic acid residues with some of the carboxyl groups methyl esterified [Citation3]. RG-I contains a backbone of the repeating disaccharide [→4)-α-GalAp-(1→2)-α-Rhap-(1→] with substitution at O-4 of the Rhap residues [Citation4]. RG-II is not structurally related to RG-I, since its backbone consists of alternating sequences of Rha and GalA rather than the repeating disaccharide [→4)-α-GalAp-(1→2)-α-Rhap-(1→] [Citation5]. In addition, side chains of RG-II are attached to O-2/O-3 of GalAp and O-4 of the Rhap residues. The research on bioactivity and structure–activity relationship of pectin indicates that the pectic polysaccharides exhibit a variety of biological activities, such as anti-complementary, anti-tumor and immunological activities [Citation6–8]. Moreover, the substituted groups play an important role in the expression of biological activities [Citation9,Citation10]. In the food industry, pectic polysaccharides are used as thickener, stabilizer, gelling agent, emulsifier, flavour fixation agent and texture modifier [Citation1,Citation11,Citation12]. Pectic polysaccharides are widely studied due to their bioactivities and applications, such as pectin from Panax ginseng [Citation13], Centella asiatica [Citation4] and Diospyros kaki Thumb [Citation14], etc. Dendrobium nobile Lindl. (Chinese name ‘Jin-Chai-Shi- Hu’) is a well-known edible and medicinal plant with wide distribution in the southwest and south of China. It is recorded in Chinese Pharmacopoeia [Citation15]. Its stems are used as materials to make herbal tea or a functional beverage for thousands of years in China. In our laboratory, one pectin fraction (DNP-W6) was isolated and purified from D. nobile. In the present paper, we elucidate the structural features and pharmacological activities of DNP-W6, as well as its structure–activity relationship.
Materials and methods
Plant materials and chemicals
The fresh stems of D. nobile were collected from Hejiang County of Sichuan Province in 2007. Voucher specimens were deposited in the herbarium of the Department of Biotechnology and Food Engineering, Hefei University of Technology (No: DNP0002). DEAE–cellulose, Sephacryl S-200, Sephadex G-10, NaBH4, concanavalin (ConA), lipopolysaccharide (LPS) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium brimoide (MTT) were purchased from Sigma–Aldrich (St. Louis, MO, USA). 1-Cyclohexyl-3-(2-morphlinoethy) carbodiimide metho-p-toluenesulphonate (CMC) and trifluoroacetic acid (TFA) came from E. Merck (Darmstadt, Germany). Dextrans were purchased from Fluka (St. Gallen, Switzerland). Other reagents used in this paper were from Chinese sources and of analytical grade.
Analytical methods
The analytical methods used to determine the composition and structure were performed as previously described [Citation7]. Briefly, homogeneity and molecular weight were determined by high performance gel permeation chromatography (HPGPC). Infrared (IR) spectra were recorded using a Perkin Elmer 599B FTIR spectrometer (Waltham, Massachusetts, USA). Gas chromatography (GC) analysis was done on a Shimadzu GC-9A instrument. Gas chromatography mass spectrometry (GC–MS) examination was performed on a Trace GC2010/Trace MS chromatograph. The 1H and 13C nuclear magnetic resonance (NMR) spectra were recorded in D2O on a Bruker Avance DPX-400 instrument. The electrospray ionization mass spectrometry (ESI-MS) analysis of oligosaccharide fractions were performed on a VG Quattro MS/MS spectrometer as described in [Citation16,Citation17].
Isolation and purification of polysaccharides
The extractions of crude polysaccharide DNP-W referred to our previously reported method [Citation7]. The dried D. nobile powder was extracted with distilled water. After precipitation, the supernatant was deproteined, dialyzed (3500 Da molecular weight cut-off) and lyophilized to give the crude polysaccharide DNP-W. DNP-W was subjected to a DEAE–cellulose column and eluted with 0.5 mol/L NaCl. Then, 0.5 mol/L NaCl eluation was further purified on a Sephacryl S-200 column to give DNP-W6. The eluate was monitored by the phenol–H2SO4 method [Citation18].
Chemical analysis
DNP-W6 (10 mg) was hydrolyzed with 2 mol/L TFA (4 mL) at 120 °C for 4 h in a sealed tube. After removing the TFA in vacuum, the hydrolyzate was dissolved in water. One part of the hydrolyzate solution was used for thin layer chromatorgraphy (TLC) analysis. The other portion was reduced with NaBH4 and acetylated with AcOH as previously described [Citation7,Citation19,Citation20]. The resulting alditol acetates were analyzed by GC and GC-MS. In addition, another portion of DNP-W6 (20 mg) was reduced with CMC–NaBH4 to give a carboxyl-reduced polysaccharide DNP-W6R [Citation20]. Then DNP-W6R was hydrolyzed and analyzed as described for DNP-W6. The m-hydroxylbiphenyl method [Citation21] was used to determine the uronic acid content of DNP-W6.
Methylation analysis
The samples of DNP-W6 and DNP-W6R were methylated according to the Ciucanu–Kerek method [Citation22]. After complete methylation, the permethylated polysaccharides were hydrolyzed, reduced and acetylated. The resulting product was analyzed by GC-MS according to the method described by Sweet et al. [Citation23].
Partial hydrolysis with acid
DNP-W6 (500 mg) was treated with 0.15 mol/L TFA at 100 °C for 40 min. The hydrolyzate solution was dialyzed against distilled water (3500 Da molecular weight cut-off). The solution inside the dialysis bag was concentrated and lyophilized to obtain a degraded polysaccharide (DNP-W6P, 210 mg). The exterior solution was collected and lyophilized (DP). DP was applied to a gel filtration chromatograph (Sephadex G-10 column) to give a series of oligomer fractions (DP1, DP2, DP3 and DP4) with increasing molar mass. The oligomer fraction DP4 was further hydrolyzed with 0.2 mol/L TFA at 100 °C for 1 h to give a main degraded product DP5.
De-acetylation and determination of O-acetyl groups
The degree of acetylation (DA) in polysaccharides was assayed by applying a combination of 1H NMR and the method described by Tomoda et al. [Citation3,Citation24,Citation25]. According to the latter method, DNP-W6 (35 mg) was reacted with 0.3 mol/L NaOH (30 mL) at room temperature for 2 h. The solution was neutralized with HCl and dialyzed against distilled water. The resulting product was termed DNP-W6A. Then, DA of the degraded products was determined.
Immunobiological activity assay
The immunobiological activities of polysaccharide fractions were assayed according to the method described previously [Citation7]. Spleen cells were isolated from male BALB/c mice and adjusted to a concentration of 5.0 × 106 cells/mL with RPMI-1640 medium. After cell suspensions (100 µL) were seeded into a 96-well plate in the presence of mitogen ConA (5.0 µg/mL) or LPS (10.0 µg/mL), different dilutions of the polysaccharide samples (25 μg/mL, 50 μg/mL and 100 μg/mL) were added. Then, T and B lymphocyte proliferation was evaluated by the MTT method [Citation26,Citation27].
Data analysis
The data were represented as mean values with standard deviation (± SD) of triple results. Student's t-test for unpaired observations between control and tested samples was used to identify statistical differences.
Results and discussion
Purification and structural characterization
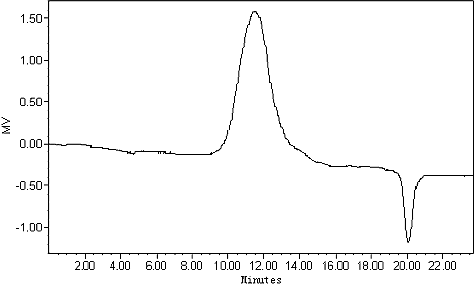
The dried stems of D. nobile were extracted three times with water and the extracts were precipitated with 4 times the volume of 95% ethanol to precipitate the crude polysaccharide DNP-W. After fractionation on DEAE-cellulose chromatography, a fraction was obtained from 0.5 mol/L NaCl eluate. Then it was further purified by Sephacryl S-200 gel filtration chromatography and gave a homogeneous polysaccharide DNP-W6 confirmed by HPGPC (). The average molecular weight of DNP-W6 was estimated to be 6.6 × 105 Da. Its specific rotation [α]D20 is −7.1° (c 0.80, H2O).
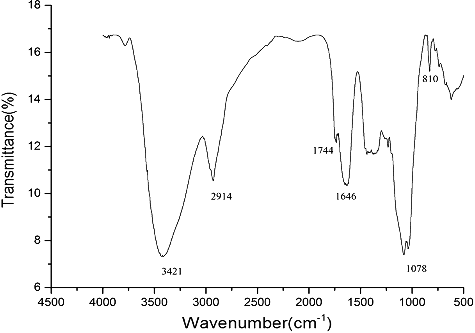
DNP-W6 was free of protein according to the Lowry method and gave no absorption at 280 nm [Citation28]. The IR spectrum of DNP-W6 is shown in . The bands at 3421, 2914 and 1646 cm−1 were due to the hydroxyl stretching vibration, C–H stretching vibration and associated water, respectively [Citation29]. The peaks at 810 cm−1 were assigned to the D-galactopyranose unit. The clear absorption at 1744 cm−1 indicated the presence of uronic acid groups in DNP-W6 [Citation30]. The uronic acid content was calculated to be 18.6% by the m-hydroxybiphenyl method [Citation21].
The sugar components of DNP-W6 were determined by TLC and GC analysis. TLC analysis showed that DNP-W6 contained mannose, glucose, galactose, rhamnose, as well as some amount of galacturonic acid. After DNP-W6 was reduced three times with CMC–NaBH4, a carboxyl-reduced polysaccharide DNP-W6R was obtained. A compared analysis of DNP-W6 and DNP-W6R by GC indicated that DNP-W6 was composed of mannose, glucose, galactose, galacturonic acid and rhamnose in a molar ratio of 0.6: 1.0: 1.5: 1.1: 1.0 (). The ratio of GalA was obtained by computation of the difference in the content of Gal in DNP-W6 and DNP-W6R.
Table 1. Compositional analysis of DNP-W6 and its degraded polymers (ratio).
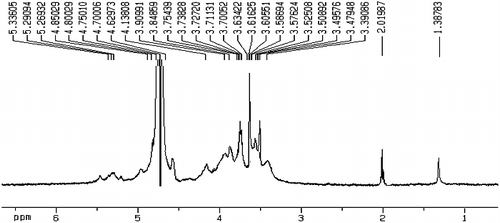
The signal at δ 2.01 ppm in the 1H NMR spectrum () indicated the presence of O-acetyl groups. The content of O-acetyl groups was calculated to be 8.9% according to 1H NMR and the Tomoda method [Citation24].
The linkage types of DNP-W6 were identified by a combination of methylation analysis of DNP-W6 and DNP-W6R. The linkage types of DNP-W6 are shown in . Methylation of DNP-W6 directly showed it did not contain 2,3,6-Me3-Galp, while methylation of the carboxyl-reduced derivative DNP-W6R showed it contained 2,3,6-Me3-Galp. The absence of 2,3,6-Me3-Galp in the analysis of DNP-W6 indicated that the native DNP-W6 contained 1,4-linked GalpA. Obviously, 2,3,6-Me3-Galp came from 1,4-linked GalAp.
Table 2. Linkage analysis of DNP-W6 and its derivatives (ratio).
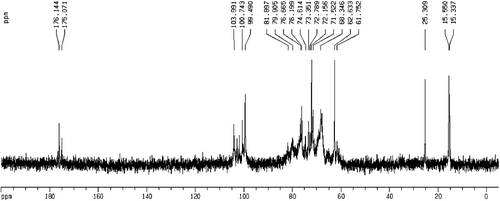
Signals in the 1H NMR and 13C NMR spectra of DNP-W6 were assigned insofar as possible based on the above results and published data [Citation31–33]. The 1H NMR spectrum of DNP-W6 is shown in . The signals at δ 1.38 and δ 2.01 were assigned to a C-methyl proton and an O-acetyl, respevtively. H-1 signals corresponding to the α-Rhap residues (δ 5.29–5.33), the α-GalAp residues (δ 5.26), β-linked Manp residues (δ 4.80–4.85), the β-Galp residues (δ 4.70–4.75) and the β-Glcp residues (δ 4.55–4.62) were also assigned. The 13C NMR spectrum of DNP-W6 is shown in . The signals at δ 15.3, δ 25.3, δ 176.1 and δ 175.1 were assigned to C-methyl, O-acetyl methyl, the carboxyl in the acid form and carboxyl in the acetyl form, respectively. In the anomeric carbon region, signals at δ 103.9–104.3, δ 102.4–102.9, δ 100.7–101.1, δ 100.0 and δ 99.7 could be attributed to C-1 of β-Galp residues, β-Glcp, β-linked Manp, α-GalAp and α-Rhap, respectively.
DNP-W6P, the product of partial acid hydrolysis from DNP-W6, was shown to have the average molecular weight of 2.7 × 105. The composition analysis of DNP-W6P () showed that partial Gal residues, Man residues and Glc residues were removed. But compared with these changes, Rha and GalA residues were largely retained, indicating pectic component RG was the core structure of DNP-W6. The methylation analysis of DNP-W6P () indicated that compared with methylation analysis of DNP-W6, the proportion of 1,6-linked Manp and Galp increased, while 1,3,6-linked Manp and Galp are lost. It suggested that some glycosyl residues were attached to the O-3 of Manp and Galp.
ESI-MS analysis
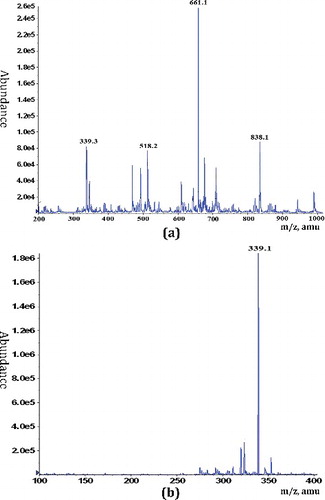
DNP-W6 was treated with TFA to give a series of hydrolytic fractions (DP1, DP2, DP3 and DP4) by Sephadex G-10 column according to increasing molar mass. The composition of DP1 was a mixture of monosaccharides. DP2 and DP3 were very complex and contained much more components. Thus they were not investigated further. DP4 was a main eluate fraction of dialysate. According to the TLC and GLC analyses of DP4, it mainly contained Rha, GalA, Gal, and Glc residues (). ESI-MS experiments of DP4 were carried out. The negative ESI-MS spectrum of DP4 is shown in (a). The main fragment m/z 661.1 was attributed to [Rha2-GalA2-1]−. In addition, mass fragments at m/z 838.1, 518.2 and 339.3 were identified as Rha2-GalA3, Rha-GalA2 and Rha-GalA, respevtively. DP4 was further acid hydrolyzed to give a oligosaccharide mixture DP5. ESI-MS spectrum of DP5 ((b)) showed that it mainly contained m/z 339.1, indicating the presence of [GalA-Rha-1]−. The ESI-MS results revealed that the backbone of DNP-W6 is mainly composed of repeated Rha-GalA units.
Figure 5. Negative ESI-MS of the mixture of DP4 (a) and DP5 (b) oligosaccharides obtained from DNP-W6 by acid hydrolysis.
Based on the above results, it could be concluded that DNP-W6 is a complex pectin. The backbone of DNP-W6 is composed of the average disaccharide of →4)-α-GalAp-(1→2)-α-Rhap-(1→, with approx 50% substitution at O-4 of the Rhap residues. The side chains are linked to the O-4 of Rhap residues, including terminal Manp, β-(1→4)-linked Glcp residues, β-(1→6)-linked Manp residues, terminal Galp, β-(1→3) and β-(1→6)-linked Galp (galactan) residues. The side chains were further substituted at O-3 of mannopyranosyl residues by terminal and β-(1→4)-linked glucopyranosyl residues and at O-3/O-6 by terminal and β-(1→3)-linked galactopyranosyl residues. The acetyl content was estimated to be 8.9%.
Immunological activity and structure
The in vitro immunological activities of DNP-W6 and its derivatives were evaluated and compared (). The pharmacological assay indicated that DNP-W6 could stimulate the proliferation of ConA- or LPS-induced lymphocytes even at the 25 μg/mL level. The activity followed a dose-dependent response curve. The hydrolyzate DNP-W6P enhanced the proliferation of ConA-induced lymphocytes only at 100 μg/mL dose, suggesting that the side chains of DNP-W6 had an effect on the expression of immunological activity. In addition, DNP-W6A, the products of deacetylated DNP-W6, showed the immuno-enhancing activities of ConA- or LPS-induced lymphocytes also only at 100 μg/mL dose, indicating that the acetyl groups played an important role in lymphocytes. The results above suggested carboxyl and acetyl groups played an important role in the properties of DNP-W6.
Table 3. Effects of DNP-W6 and its derivatives on the proliferation of lymphocytes in vitro.
The molecular weight, monosaccharide composition, functional group, branching degree and glycosidic linkage are some of the structural features recognized to contribute to the polysaccharides immunostimulatory activity [Citation34]. The content of uronic acids has been considered to play important roles in biological activity [Citation35–37], and many acid polysaccharides have immunomodulatory activity [Citation36–38]. The existence of functional groups of monosaccharides is a crucial structural feature for immunostimulatory activity [Citation36,Citation37]. However, the structural features of specific polysaccharides may impact the resulting activity in different ways. The effect of the functional group of pectin from D. nobile on the immunological activity has been poorly studied. To the best of our knowledge, this paper investigated, for the first time, the relationship between acetyl groups in polysaccharide from D. nobile and the immunostimulatory activity. The obtained results are useful in enhancing the knowledge of the structure–activity relationship of pectin, as well as for expanding the use of D. nobile.
Conclusions
The present study revealed that a novel polysaccharide (DNP-W6) with an average molecular weight of 6.6 × 105 Da was isolated from Dendrobium nobile Lindl. TLC and GC analysis indicated that DNP-W6 is composed of mannose, glucose, galactose, galacturonic acid and rhamnose in a molar ratio of 0.6: 1.0: 1.5: 1.1: 1.0. Spectral analysis concluded that DNP-W6 is complex pectin. Immunological activity analysis suggested that the side chains, carboxyl and acetyl groups had effect on the expression of immunological activity. These findings are helpful for the potential application of DNP-W6, as well as for expanding the use of D. nobile.
Disclosure statement
The authors declare no conflict of interest.
Additional information
Funding
References
- Renard CMGC, Thibault JF, Voragen, AGJ, et al. Studies on apple protopectin VI: extraction of pectins from apple cell walls with rhamnogalacturonase. Carbohydr Polym. 1993;22(3):203–210.
- Duan J, Zheng Y, Dong Q, et al. Structural analysis of a pectic polysaccharide from the leaves of Diospyros kaki. Phytochemistry. 2004;65(5):609–615.
- Shakhmatov EG, Udoratina EV, Atukmaev KV, et al. Extraction and structural characteristics of pectic polysaccharides from Abies sibirica L. Carbohydr Polym. 2015;123:228–236.
- Wang XS, Liu L, Fang JN. Immunological activities and structure of pectin from Centella asiatica. Carbohydr Polym. 2005;60(1):95–101.
- Zhao J, Zhang F, Liu X, et al. Isolation of a lectin binding rhamnogalacturonan-I containing pectic polysaccharide from pumpkin. Carbohydr Polym. 2017;163:330–336.
- Wu Y, Sun J, Wang Y. Selective estrogen receptor modulator: a novel polysaccharide from Sparganii Rhizoma induces apoptosis in breast cancer cells. Carbohydr Polym. 2017;163:199–207.
- Wang JH, Luo JP, Zha XQ. Structural features of a pectic polysaccharide from the stems of Dendrobium nobile Lindl. Carbohydr Polym. 2010;81(1):1–7.
- Lin L, Xie J, Liu S, et al. Polysaccharide from Mesona chinensis: extraction optimization, physicochemical characterizations and antioxidant activities. Int J Biol Macromol. 2017;99:665–673.
- Kravtchenko T, Penci M, Voragen A, et al. Enzymic and chemical degradation of some industrial pectins. Carbohydr Polym. 1993;20(3):195–205.
- Shao P, Zhu Y, Jin W. Physical and chemical stabilities of β-carotene emulsions stabilized by Ulva fasciata polysaccharide. Food Hydrocolloids. 2017;64:28–35.
- Yin JY, Chen HH, Lin HX, et al. Structural features of alkaline extracted polysaccharide from the seeds of Plantago asiatica L. and its rheological properties. Molecules. 2016 [cited 2017 Jul 31];21(9):1181. DOI: 10.3390/molecules21091181.
- Zou YF, Fu YP, Chen XF, et al. Purification and partial structural characterization of a complement fixating polysaccharide from rhizomes of Ligusticum chuanxiong. Molecules. 2017 [cited 2017 Jul 31];22(2):287. DOI: 10.3390/molecules22020287.
- Luo D, Fang B. Structural identification of ginseng polysaccharides and testing of their antioxidant activities. Carbohydr Polym. 2008;72(3):376–381.
- Shin MS, Lee H, Hong HD, et al. Characterization of immunostimulatory pectic polysaccharide isolated from leaves of Diospyros kaki Thumb.(Persimmon). J Funct Foods. 2016;26:319–329.
- Committee CP. Chinese pharmacopoeia. Beijing, China: China Medical Science Press; 2010. p. 95.
- Reis A, Domingues MRM, Domingues P, et al. Positive and negative electrospray ionisation tandem mass spectrometry as a tool for structural characterisation of acid released oligosaccharides from olive pulp glucuronoxylans. Carbohyd Res. 2003;338(14):1497–1505.
- Ravenscroft N, Cescutti P, Gavini M, et al. Structural analysis of the O-acetylated O-polysaccharide isolated from Salmonella paratyphi A and used for vaccine preparation. Carbohyd Res. 2015;404:108–116.
- Beeley JG. Glycoprotein and proteoglycan techniques. Amsterdam (Netherlands): Elsevier; 1985. p. 397–399. ( Laboratory techniques in biochemistry and molecular biology, vol. 16).
- Wang JH, Zuo SR, Luo JP. Structural analysis and immuno-stimulating activity of an acidic polysaccharide from the stems of Dendrobium nobile Lindl. Molecules. 2017 [cited 2017 Jul 31];22(4):611. DOI: 10.3390/molecules22040611.
- Taylor RL, Conrad H. Stoichiometric depolymerization of polyuronides and glycosaminoglycuronans to monosaccharides following reduction of their carbodiimide-activated carboxyl group. Biochem. 1972;11(8):1383–1388.
- Blumenkrantz N, Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973;54(2):484–489.
- Ciucanu I, Kerek F. A simple and rapid method for the permethylation of carbohydrates. Carbohyd Res. 1984;131(2):209–217.
- Sweet DP, Albersheim P, Shapiro RH. Partially ethylated alditol acetates as derivatives for elucidation of the glycosyl linkage-composition of polysaccharides. Carbohyd Res. 1975;40(2):199–216.
- Tomoda M, Shimizu N, Shimada K, et al. Isolation and structural features of two glucans from the rhizomes of Crinum latifolium. Chem Pharm Bull. 1985;33(1):16–21.
- Liu X, Xie J, Jia S, et al. Immunomodulatory effects of an acetylated Cyclocarya paliurus polysaccharide on murine macrophages RAW264. 7. Int J Biol Macromol. 2017;98:576–581.
- Heeg K, Reimann J, Kabelitz D, et al. A rapid colorimetric assay for the determination of IL-2-producing helper T cell frequencies. J Immunol Methods. 1985;77(2):237–246.
- Sun X, Liu N, Wu Z, et al. Anti-tumor activity of a polysaccharide from blueberry. Molecules. 2015;20(3):3841–3853.
- Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the Folin phenol reagent. J biol Chem. 1951;193(1):265–275.
- Wang JH, Du YQ, Sun HJ, et al. Extraction and preliminary characterization of polysaccharide from Umbilicaria esculenta cultivated in Huangshan Mountain. Biotechnol Biotecnol Equip. 2015;29(4):714–722.
- Song WJ, Pan X, Zhang D. Lead complexation of soluble and bound extracellular polymeric substances from activated sludge: characterized with fluorescence spectroscopy and FTIR spectroscopy. Biotechnol Biotecnol Equip. 2012;26(6):3371–3377.
- Suárez ER, Kralovec JA, Noseda MD, et al. Isolation, characterization and structural determination of a unique type of arabinogalactan from an immunostimulatory extract of Chlorella pyrenoidosa. Carbohyd Res. 2005;340(8):1489–1498.
- Zabłotni A, Perepelov AV, Knirel YA, et al. Structure of the O-polysaccharide of Proteus mirabilis OC (CCUG 10702) from a new proposed Proteus serogroup O75. Carbohyd Res. 2005;340(11):1908–1913.
- De Paula R, Santana S, Rodrigues J. Composition and rheological properties of Albizia lebbeck gum exudate. Carbohydr Polym. 2001;44(2):133–139.
- Ferreira SS, Passos CP, Madureira P, et al. Structure-function relationships of immunostimulatory polysaccharides. Carbohydr Polym. 2015;132:378–396.
- Xu L, Zhang Y, Wang L. Structure characteristics of a water-soluble polysaccharide purified from dragon fruit (Hylocereus undatus) pulp. Carbohydr Polym. 2016;146:224–230.
- Lin M, Xia B, Yang M, et al. Anti-ovarian cancer potential of two acidic polysaccharides from the rhizoma of Menispermum dauricum. Carbohydr Polym. 2013;92(2):2212–2217.
- Chen W, Yuan F, Wang K, et al. Modulatory effects of the acid polysaccharide fraction from one of anamorph of Cordyceps sinensis on Ana-1 cells. J Ethnopharmacol. 2012;142(3):739–745.
- Wu Y, Li Y, Liu C, et al. Structural characterization of an acidic Epimedium polysaccharide and its immune-enhancement activity. Carbohydr Polym. 2016;138:134–142.