ABSTRACT
Bulgarian yogurt is usually associated with good health and longevity. This study aimed to analyse the current microbial content of homemade Bulgarian yogurt. Identification by 16S rDNA sequencing revealed that out of 76 isolated strains, 53% belonged to Lactobacillus delbrueckii subsp. bulgaricus, 14% to other lactobacilli, and 32% to lactic acid cocci (Streptococcus thermophilus, Pediococcus acidilactici, Enterococcus faecium and Leuconostoc). All isolates inhibited the growth of pathogenic bacteria (10% of them by the putative action of bacteriocins); 18 isolates were able to produce extracellular exopolysaccharides (EPS), whereas 24% of them demonstrated extremely high proteolytic activity. Remarkably, 10 Lb. bulgaricus strains produced prebiotic galactooligosaccharides (GOS). High-resolution accurate mass spectrometry (HRAMS) analysis revealed production of tri- and tetrasaccharides containing atypical β(1→4) glycosidic bonds, reported for the first time for Lb. bulgaricus. Based on the beneficial features, along with good technological behaviour, we recommend several isolates as highly promising for Bulgarian yogurt starters.
1. Introduction
The genuine Bulgarian yogurt has always attracted great interest because of its proven beneficial effects on consumers. The first historical pieces of evidence of yogurt-making in Bulgarian lands are from 4000 years B.C. when the Thracian people domesticated the sheep. Later, in the 5th–7th century, yogurt became the usual food of Slavs and ancient Bulgarians as did bread. The first pieces of evidence for the probiotic action of Bulgarian yogurt date back to the 16th century, when the French King Francois I was cured of chronic diarrhea by a simple yogurt diet [Citation1]. The beneficial effect of Bulgarian yogurt was widely investigated during the last century. The famous Russian biologist Elie Metchnikoff, developing his theory about the prolongation of life [Citation2], was the first who proposed that the daily yogurt consumption engenders the longevity of Bulgarians. The agent causing yogurt fermentation was shown for the first time by the Bulgarian microbiologist Stamen Grigoroff [Citation3], who found a rod-shaped bacterium called “Bacterium bulgaricum – Grigoroff, today known as Lactobacillus delbrueckii ssp. bulgaricus (Lb. bulgaricus).
According to the FAO/WHO definition (1984), yogurt refers to milk fermented by symbiotic starters containing Lb. bulgaricus and Streptococcus thermophilus, the final product containing no less than 107 viable CFU per gram of yogurt. The main beneficial effects of yogurt are due to the organic acids and antimicrobial substances produced by the starter cultures. The strains have antimicrobial effects against pathogens in the gastrointestinal tract [Citation4], alleviate lactose intolerance and diarrhea [Citation5,Citation6], stimulate the immune system [Citation7], prevent colon cancer and produce bioactive peptides that can control the blood pressure [Citation8]. A number of studies have shown that Lb. bulgaricus strains possess activity against Clostridium difficile, Enterobacteriaceae, Str. mutans and Pseudomonas aeruginosa [Citation9]. Bacteriocin-like agents of seven isolates of Lb. bulgaricus were reported to be active even against antibiotic-resistant Helicobacter pylori strains [Citation10]. Recently, a new advantage of Lb. bulgaricus over conventional probiotic bacteria based on the stereoisomer of lactic acid produced was discovered. Toyoda et al. [Citation11] showed that the survival of cultured neurons defective in Parkinson's disease is enhanced by D-lactate. Since D-lactate is produced by Lb. bulgaricus, the consumption of large amounts of yogurt for treatment and prevention of Parkinson's disease was strongly recommended. Today, 110 years after the discovery of Lb. bulgaricus, yogurt consumption of around the world is steadily increasing. The latest report by the International Dairy Federation (IDF, 2016) showed that the average yogurt consumption has increased globally over the past 5 years, as the top 3 markets by value are China, the USA and Japan [Citation12]. The increasing consumption of original Bulgarian type yogurt in China is additionally expanding the yogurt market of the biggest Chinese dairy manufacturers, such as Bright Dairy & Food Co. Ltd., whose sales exceeded 20 billion RMB in 2016.
In response to this demand, the aim of the present work was to analyse the current microbial status of homemade yogurts in Bulgaria and to isolate starter cultures with promising properties. Several regions in Bulgaria are known to contain endemic yogurt microflora: Tran, the Western, Central and Eastern Balkan Mountains, Thracian and Rose Valleys, Rila, Pirin and the Rhodope Mountains. Based on the unique yogurt microbial diversity in these locations, plenty of novel promising isolates were explored. Here, we report the characterisation of strains combining excellent technological behaviour with beneficial properties.
2. Materials and methods
2.1. Samples collection, strains and growth conditions
Seventy-seven samples of different cow, sheep, goat, and buffalo yogurts prepared by ancient national recipes were collected by visiting a number of remote villages, museum-villages, monasteries; and High-mountain dairy farms in Bulgaria. The samples were collected over a two-year period 2015–2016.
The test-microorganisms used in the antibacterial activity assay were Escherichia coli ATCC®25 922™, Klebsiella pneumoniae G31 NBIMCC 8650, Vibrio cholerae Non-O1 NBIMCC 8716 and Listeria monocytogenes ATCC®19115™.
Lactic acid rod-shaped bacteria were cultivated in MRS medium; lactic acid cocci in M17 medium, at 42 °C, or 37 °C; at anaerobic conditions, using Anaerocult® A mini (Merck KGaA, Darmstadt, Germany). E. coli ATCC®25 922™ and K. pneumoniae were grown in LB medium, V. cholerae Non-O1 in Nutrient broth, and L. monocytogenes ATCC®19115™ in BHI medium, at 37 °C, on a water bath rotary shaker GFL 1092 (Gesellschaft für Labortechnik mbH, Burgwedel, Germany) with agitation of 100 rpm. All growth media were supplied by Carl Roth GmbH, Karlsruhe, Germany, and when needed, broths were solidified by the addition of 1.5% (w/v) agar (HiMedia Laboratories, Mumbai, India).
For analysis of the metabolite profiles, two different approaches were applied: (i) cultivation in skimmed milk containing 4.1% (w/v) lactose, for 24–48 h at optimal temperature, followed by yogurt fractionation by centrifugation, and analysis of the soluble metabolites; (ii) cultivation in Lactose medium, containing 10 g/L of bacto peptone, 5 g/L yeast extract, 8 g/L meat extract, 5 g/L sodium acetate, 2 g/L K2HPO4, 2 g/L ammonium citrate dibasic, 0.1 g/L magnesium sulfate, 0.1 g/L manganese sulphate and 40 g/L lactose.
For analysis of the exopolysaccharide (EPS) production, MRS derivative media containing glucose, lactose or sucrose (2 g/L) were used.
2.2. High-performance liquid chromatography (HPLC) analysis
Liquid products of microbial metabolism, as well as the remaining sugars, were detected by Perkin Elmer HPLC system Series 10 (Waltham, Massachusetts, USA), with an RI detector. An HPLC column Aminex HPX-87H at 65 °C with mobile phase 5 mmol/L H2SO4 at a flow rate 0.6 mL/min (BioRad Laboratories, CA, USA) was used for lactic acid, succinic acid and ethanol determination. Glucose, galactose, lactose and galactooligosaccharides (GOS) were separated by a BioRad column HPX-87C at 85 °C (water as the mobile phase with a flow rate of 0.6 mL/min). For the quantification of GOS (trisaccharides) as standard was used raffinose [Citation13]. All standard substances were purchased from Merck KGaA, Darmstadt, Germany.
2.3. Liquid chromatography/mass spectrometry (LC/MS) analysis
2.3.1. Chromatographic conditions
Analyses were carried out on a Q Exactive® mass analyzer equipped with a TurboFlow® LC system and IonMax II® electro spray ionization module (Thermo Scientific Co, USA) Atlantis T3, 3.5 μm (100 × 2.1 mm) column (Waters Co, USA) using the following mobile phase: A – 20 mmol/L ammonium acetate in water; B – buffer A/acetonitrile (1/9 v/v) at a flow rate of 300 μL/min and gradient: 0% B for 180 s; 0%–60% B for 150 s; 60% B for 30 s; 60– 0% B for 60 s and 0% B for 5 min. The injection volume was 10.0 μL.
2.3.2. Mass spectrometric conditions
The analysis was carried out using a Q Exactive hybrid quadrupole-Orbitrap Mass spectrometer (Thermo Scientific Co, USA) equipped with a heated electrospray ionization module IonMax® (Thermo Scientific Co, USA). Full-scan spectra over the m/z range of 100–2000 were acquired in negative ion mode at resolution settings of 140 000. All MS parameters were optimised for sensitivity to the target analytes using the instrument control software program. Q Exactive parameters were: spray voltage 4.0 kV, sheath gas flow rate 32, auxiliary gas flow rate 10 L/min, spare gas flow rate 3 L/min, capillary temperature 280 °C, probe heater temperature 300 °C and S-lens RF level 50. Parallel reaction monitoring (PRM) mode was used for qualification of the polysaccharides. Optimised values of the collision energy were HCD 30%. The selected ions with m/z 341.11 for disaccharides, m/z 503.16 for tri-saccharides and m/z 665.21 for tetrasaccharides were used in parallel reaction monitoring. Data acquisition and processing were carried out with Xcalibur 2.4® software package (Thermo Scientific Co, USA). Calculations for theoretical m/z values were made by Mass Frontier 5.1 Software program (Thermo Scientific Co, USA).
2.4. L(+) and D(−) lactic acid estimation
The ratio of L(+) to D(−) lactic acid was estimated by a UV method using an L/D-lactic acid kit (R-Biopharm AG, Darmstadt, Germany).
2.5. Protease activity assay
The extracellular protease activity was assayed by Protease Colorimetric Detection Kit PC0100 (Sigma-Aldrich) using 25 μL cell-free supernatants (after 48 h cultivation of the strains) and 1% Hammarsten casein as a substrate (Merck, Darmstadt, Germany). One unit protease activity releases 1 μmoL tyrosine for 1 min at the assay conditions 37 °C, pH 7.5. The presented data were aligned to Anson units (AU).
2.6. Exopolysaccharides production assay
Ten millilitres of cell-free supernatants of 24-h cultures were boiled for 10 min in a 100 °C water bath. The precipitated protein fractions were removed by centrifugation for 15 min at 10 000 rpm (Boeco M-240 centrifuge; Boeckel & Co KG, Hamburg, Germany). The remaining clear supernatants were mixed well with 3 volumes of 96% ethanol, incubated at −20 °C for 18 h, and centrifuged at 10 000 rpm for 30 min, at 4 °С. The obtained pellet of EPS was washed twice with 50% ethanol, dried and resuspended in 1 mL Milli-Q® water (Merck). The quantitative assay of extracellular exopolysaccharides was done as described by Dubois et al. (1956) [Citation14] with some modifications. One milliliter of sample with appropriate dilution was mixed with 25 μL phenol (80%) and 2.5 mL H2SO4 (96%) and incubated at room temperature for 30 min. The estimation of the released sugars was in accordance with the standard curve of fructose solution, at a wavelength of 495 nm (UV/VIS Helios Omega Spectrophotometer, Thermo Scientific Inc., Waltham, USA).
2.7. Antibacterial activity assay
The assay used the agar well diffusion method as described previously [Citation15]. The antagonistic activity of 100 μL cell-free supernatants of the strains, acidic or neutralized to pH 6 and treated with catalase, was evaluated. The test microorganisms' cultures were grown for 24 h and diluted to 106 CFU/mL. The wells were 10 mm in diameter; the antagonistic activity was estimated according to measurement of a sterile halo around the agar well in millimeters.
2.8. DNA isolation, polymerase chain reaction (PCR) and sequence analysis
Total genomic DNA was isolated from 24-hour-old cultures grown in appropriate media, using a GeneJET Genomic DNA Purification Kit (Thermo Fisher Scientific Inc., Waltham, USA). For 16S rRNA gene amplification, we used the eubacterial primer pair: 27F: 5’ AGAGTTTGATCCTGGCTCAG 3’ and 1492R: 5’ AAGGAGGTGATCCAGCC 3’, and Phusion High-Fidelity Master Mix (Thermo Fisher Scientific Inc.), in a total volume of 50 μL, template DNA 2 ng/μL, and final concentrations of primers 10 pmol/μL (Macrogen Inc.). The PCR amplification was done by QB-96 Satellite Gradient Thermal Cycler (LKB Vertriebs GmbH, Vienna, Austria) under the following temperature profile: 95 °C for 5 min, 35 cycles consisting of 94 °C for 1 min, 55 °C for 1 min, 72 °C for 2 min, followed by final elongation at 72 °C for 5 min.
Sequencing of the obtained PCR fragments was done by Macrogen Inc. (Amsterdam, The Netherlands). Sequence alignment was done by Chromas LITE, version 2.1 and CAP3; the comparison with GenBank data was done by BLAST and ClustalW. The phylogenetic trees were built by the neighbour-joining method using MEGA 7 software. The sequences are deposited in GenBank of NCBI with accession numbers from MG437344 to MG437394 and MG438466 to MG438490.
3. Results and discussion
3.1. Lactic acid bacteria (LAB) strains isolation and identification
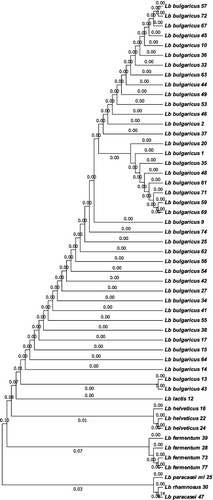
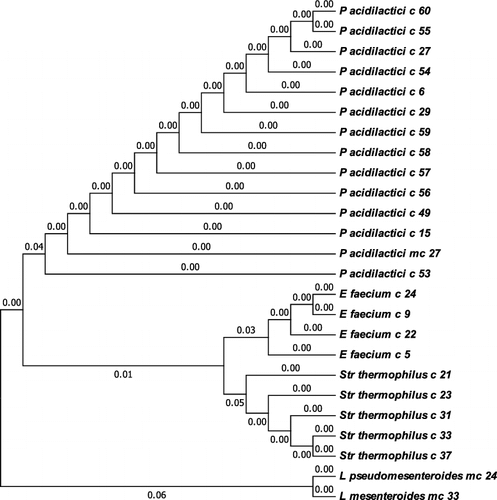
Bulgarian yogurt (kiselo mlyako) is one of the most popular types of yogurt. Owing to its health effects well-known worldwide, it has become a hallmark of Bulgaria. That is why several Bulgarian institutions are engaged in studying the biodiversity of LAB, isolation of pure cultures and maintaining LAB strains’ collections: the National Bank for Industrial Microorganisms and Cell Cultures, the private dairy companies Lactina and Genesis, the Institute of Microbiology at the Bulgarian Academy of Sciences, and the government-owned company LB Bulgaricum Plc., which owns the richest selection of symbiotic Lb. bulgaricus/Str. thermophilus starter cultures. However, many strains in these collections were isolated from various dairy sources besides yogurt: white and yellow hard cheeses, katak [Citation16,Citation17], plant leaves [Citation18], fermented foods and beverages [Citation19,Citation20]. In addition, part of the yogurt strains, formerly assigned to Lb. bulgaricus by morphological and biochemical criteria were later re-classified as Lb. lactis or Lb. helveticus by DNA fingerprinting [Citation21]. More than a century after the discovery of Lb. bulgaricus, the present study is an attempt at a new comprehensive investigation of Bulgarian yogurt microflora. A rich, representative collection of Bulgarian yogurts from remote locations was created, thus covering the whole territory of the country and ensuring the uniqueness of the samples. Seventy-six distinct LAB strains were obtained from 77 homemade yogurt samples collected in 60 different locations in Bulgaria. The strains were isolated using selective media (MRS and M17), the phenotypic criteria for their selection being Gram-positive staining, a catalase-negative reaction of the colonies and the ability to produce lactic acid. As a result, we isolated 52 different strains with rod-shaped morphology and 24 strains of lactic acid cocci.
Genetic identification based on 16S ribosomal RNA gene sequencing identified six different species of lactobacilli (). Besides Lb. delbrueckii subsp. bulgaricus (Lb. bulgaricus, 40 strains), the studied Bulgarian yogurts contained Lb. delbrueckii subsp. lactis (Lb. lactis), Lb. helveticus, Lb. paracasei, Lb. rhamnosus and Lb. fermentum. One of the rod-shaped LABs was assigned to Weissella confusa. Among the lactic acid cocci, 13 strains were representatives of Pediococcus acidilactici, 5 ones of Str. thermophilus, 4 ones of Enterococcus faecium and 2 ones of the genus Leuconostoc ().
Considering the distribution by region, the yogurts with the largest LAB biodiversity were those from the Rhodope Mountains, where the majority of the samples contained two or three LAB strains of different morphology. However, the proto-cooperation between Lb. bulgaricus and Str. thermophilus strains was best preserved in the yogurts from the Central and Western Balkans. The Rhodope yogurts were a rich source of Lb. bulgaricus with probiotic properties, co-existing with P. acidilactici strains. As it was expected, the majority of the artisanal yogurts contained Lb. bulgaricus strains (40 strains). Other Lactobacillus species found in the Bulgarian yogurt samples were Lb. lactis (one strain) and Lb. helveticus (3 strains), as also observed by Dimitrov et al. [Citation17]. Lb. fermentum is completely atypical for the Bulgarian yogurt; however, its presence in homemade Chinese yogurt has been reported [Citation22]. Further, to the best of our knowledge, this is the first report of the species Lb. paracasei and Lb. rhamnosus as an accompanying microflora of yogurt. Notably, the “classic” symbiotic partner of Lb. bulgaricus in Bulgarian yogurt, Str. thermophilus, was present in only 5 out of the 77 analysed samples. The less pretentious, but not less beneficial species P. acidilactici was an accompanying microflora in 13 homemade yogurts.
3.2. Metabolite profiling of the novel LAB strains
The ability of the strains to produce liquid metabolites was estimated using skimmed milk, or Lactose medium. All Lb. bulgaricus and two Str. thermophilus strains coagulated milk and are considered main starters, unlike the accompanying strains. All Lb. bulgaricus strains were able to curd milk for a short time and degrade high amounts of lactose (). The isolates that were strong acidifiers, forming more than 10 g/L lactic acid in milk for 24 h were Lb. bulgaricus strains 1, 2, 20, 32, 37, 44, 56, 57, 59 and 62. Lb. bulgaricus strains 9, 41, 43, 46 and 74 were moderate acidifiers (producing 4–5 g/L lactic acid for 24 h) and did not synthesize excessive amounts of lactic acid even after 48 h of fermentation. The highest amount of lactic acid was produced by Lb. helveticus 16 (up to 22.05–25.61 g/L in milk for 24 and 48 h, respectively). In addition to lactic acid, the Lb. bulgaricus strain 37 produced 0.1 g/L succinic acid. The Lb. bulgaricus strains accumulated galactose in the course of milk fermentation, ranging from 4.7 g/L to 13.5 g/L and were not able to convert it due to the mechanism of lactose transport – anti-port requiring one exported galactose residue for every entering lactose molecule. Galactose is twice as sweet as lactose and may enhance the sweet taste of the yogurt. Part of the accompanying lactobacilli was hetero-fermentative. The Lb. fermentum strains produced 5–6.75 g/L ethanol.
Table 1. Liquid metabolites formed by Lb. delbrueckii ssp. bulgaricus strains after 24 h fermentation of skimmed milk (4.1% lactose) and Lactose medium (4% lactose).
As the majority of the lactic acid cocci did not coagulate milk, lactose conversion was assayed in Lactose medium (). All Lb. bulgaricus and Lb. lactis strains produced 100% D(-) lactic acid stereoisomer; Str. thermophilus 100% L(+); Lb. helveticus, Lb. paracasei and Lb. rhamnosus predominantly the L(+) form.
Table 2. Liquid metabolites formed by the strains of accompanying microflora after 24 h fermentation of Lactose medium (4% lactose).
3.3. Extracellular exopolysaccharides (EPS) production
Today's consumers prefer yogurt with a sweet taste and creamy texture. That is why an important technological feature of the starter cultures is the capability of the strains to produce extracellular EPS that give higher viscosity and a thicker texture to the product and are desirable because the manufacturer could avoid the addition of stabilizers of animal or plant origin to natural yogurts [Citation23]. Among the LAB isolates, we selected the strains forming viscous, mucoid colonies. Eighteen strains belonging to various morpho-types were evaluated to be EPS-producing strains. The quantitative estimation showed that six Lb. bulgaricus strains produced 60–154 mg/L EPS, as the highest EPS amount was synthesized by the strains 63 and 69 (). Other strains capable of0 forming EPS were Lb. helveticus 22, Lb. rhamnosus 30 and Lb. fermentum 28 and 77. These strains produced amounts of EPS similar to those of Lb. bulgaricus (between 120 and 153 mg/L) and similarly preferred lactose than glucose as a carbon source. Three of the strains produced the largest amount of EPS from sucrose. The EPS production by P. acidilactici, Str. thermophilus and L. mesenteroides was strain-specific, the majority of the strains producing amounts of extracellular EPS higher than 120 mg/L in media containing lactose or sucrose. W. confusa produced a low amount of EPS from glucose. The Lb. bulgaricus strains produced EPS in the medium containing lactose and preferred it to MRS. This is in agreement with Patel et al. [Citation24], who reported that EPS of Lb. bulgaricus are usually heteropolysaccharides formed of glucose and galactose (and rarely rhamnose) units. Since the reported yields of EPS ranged from 50 to 350 mg/L for Str. thermophilus, and 60–150 mg/L for Lb. bulgaricus, isolate Lb. bulgaricus 69 (154 mg/L) may be noted as a very good producer of EPS (). It is noteworthy that the best EPS producers among the strains of the adjunct microflora were Lb. fermentum 77 (producing 153 mg/L EPS from sucrose) and L. mesenteroides mc 33 (139.6 mg/L from lactose).
Table 3. Extracellular exopolysaccharides (EPS) production of novel LAB isolates in media containing different carbohydrates: glucose, lactose or sucrose (2 g/L).
Table 4. Antimicrobial activity* of the newly isolated Lactobacillus strains against pathogenic test-microorganisms.
3.4. Proteolytic activity
LABs utilize milk proteins as a primary source of essential amino acids. Proteinase enzymes hydrolyze more than 40% of the peptide bonds of α- and β-caseins, resulting in the formation of more than 100 different oligopeptides during milk fermentation. Part of these peptides (phosphopeptides) accelerate the mineral absorption (calcium, phosphorous and iron); other peptides produced by Lb. bulgaricus strains are known to possess angiotensin-converting enzyme (ACE)-inhibitory activity and participate in blood pressure regulation [Citation8], or to be engaged in antimicrobial activity [Citation12].
The majority of the newly isolated Lactobacillus strains possessed high proteolytic activity with casein as a substrate, with values exceeding 250 AU/mL. Lb. bulgaricus strains 63, 71 and 69 showed the highest activity, reaching 308.4, 318 and 329.4 AU/mL, respectively (A). These values are in agreement with the observations of other authors [Citation25,Citation26] who showed the high specificity of Lb. delbrueckii ssp. bulgaricus towards proline-containing substrates.
Figure 3. Extracellular proteolytic activity of Lactobacillus strains (A) and Lactic acid cocci (B) isolated from homemade Bulgarian yogurt.
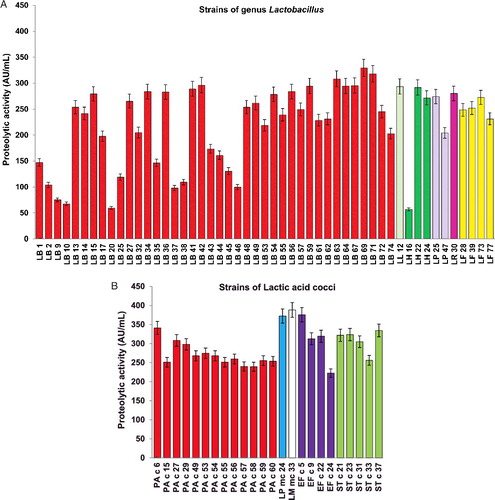
Considering our results, lactic acid cocci were even more proteolytic than the lactobacilli (B). The strains that showed exclusively high values were Leuconostoc mc 24 and mc 33 (372.4 and 388.4 AU/mL), as well as Ent. faecium c 5 (375.7 AU/mL). Among the streptococci, Str. thermophilus c 37 stood out with 334.5 AU/mL. Since only the extracellular protease activity was assayed, the high values of proteolytic activity displayed by Str. thermophilus strains are probably due to the presence of cell-envelope proteases, which are commonly released in the culture medium, as described by Chang et al. [Citation27]. The high protease activity of Str. thermophilus c 37 and c 31 allowed the strains to grow and produce lactic acid more rapidly in milk. The key role of proteolysis in the acidification capacity of this bacterium was recently underlined by Cui et al. [Citation28] and Hafeez et al. [Citation29].
The high proteolytic activity is probably associated with the putative bacteriocin production by Lb. bulgaricus strain 63 and P. acidilactici c 27. Hence, the high proteolytic activity of the starters ensures their biological activity and is a part of the proto-cooperative relations between the starters. The remaining activity in the presence of protease inhibitors (PMSF or EDTA) suggested that the proteolytic activity of Lb. bulgaricus 63 was due to the production of a mix of serine and metal type of proteases (data not shown).
3.5. Antimicrobial activity
Most of the novel isolates were able to inhibit the growth of pathogenic bacteria. The low pH caused by lactic acid production is the most powerful antimicrobial factor against Gram-negative pathogens such as E. coli, K. pneumoniae, and V. cholerae, the causative agent of cholera. The strains whose culture liquids formed the largest zones of pathogens’ growth inhibition are shown in and . The cell-free supernatants of Lb. bulgaricus strains 44, 46 and 63 preserved their bactericidal effect even when neutralised to pH 6.0 and catalase-treated, thus suggesting the presence of bacteriocins. The same applies to P. acidilactici strains c 27 and c 59, and Str. thermophilus strain c 21. Interestingly, the only two strains that possessed activity against Listeria monocytogenes ATCC®19115™ were of the species P. acidilactici – c 15 and c 53. As this activity is in agreement with pediocin production in milk by P. acidilactici in co-culture with Lb. bulgaricus [Citation30], putative bacteriocin production by these strains will be subject to further investigation.
Table 5. Antimicrobial activity* of the newly isolated strains of Lactic acid cocci against pathogenic test-microorganisms.
3.6. Production of galactooligosaccharides (GOS)
A valuable and rare property of the Lb. bulgaricus strains in our collection is the ability to produce GOS. In Lb. bulgaricus, GOS are a product of enzymatic trans-glycosylation by β-galactosidases of the GH2 family (LacZ). These GOS comprise 2–8 β-linked galactose residues connected with glucose at the reducing end. GOS are compounds with a strong prebiotic effect, since they are not metabolised by humans, and at the same time stimulate the growth of all probiotics. GOS are able to reorganise the intestinal flora by growth enhancement of Bifidobacterium sp. They have a positive effect on the intestinal immune system, inhibit the adhesion of pathogens; prevent infections and cancer, enhance mineral absorption, and are used in specialised foods for individuals with lactose intolerance [Citation31].
Seven of the novel Lb. bulgaricus strains, cultured either in milk or in defined Lactose medium, formed galactooligosaccharides (GOS) with a degree of polymerization (DP) DP3. In addition, another three strains were detected to form GOS in Lactose medium only. The highest amounts of GOS with DP3 were produced by Lb. bulgaricus 41, 43 and 44 (). GOS quantification showed that Lb. bulgaricus strain 41 reached 3.05 mg/mL in yogurt after 48 h of fermentation, followed by Lb. bulgaricus 43 (2.83 mg/mL). Aiming to prove GOS production and to establish their structure, cultural liquids were subjected to high-resolution accurate mass spectra analysis (HRAMS), with preliminary separation of the metabolites by liquid chromatography. The structural analysis revealed that the produced GOS are trisaccharides and tetrasaccharides, the last galactose residue being connected by either a β(1→4), or a β(1→6) glycosidic bond with the lactose core. The produced quantities of GOS with DP4 were several times less than those with DP3; GOS with higher degrees of polymerization than DP4 were not observed. The results from the LC-MS/MS analysis of Lb. bulgaricus 43 are presented in .
Table 6. Production of GOS by Lb. bulgaricus strains grown for 24–48 h in skimmed milk or Lactose medium.
Figure 4. LC-MS/MS analysis of liquid supernatant of Lb. bulgaricus strain 43 grown for 24 h in Lactose medium: total ion chromatogram (A); extracted ion chromatogram for disaccharides (B), trisaccharides (C) and tetrasaccharides (D); MS/MS spectra (E) of two major trisaccharides with m/z = 503.16.
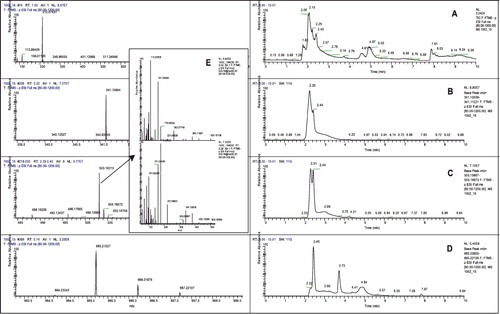
The information about GOS production in yogurt is very scarce. Examining yogurts obtained from the Spanish markets, Martinez-Villaluenga et al. [Citation32] reported that the commercial yogurts contain between 0.2 and 0.5% GOS, depending on the starter culture. Venica et al. [Citation13] reported that the GOS amount of commercial milk fermented by Lb. acidophilus La-5 could be increased by the addition of β-galactosidase of Kluyveromyces lactis. To the best of our knowledge, our study performed the first analysis of GOS formation by particular Lb. bulgaricus strains to date. The results revealed that several strains spontaneously formed tetrasaccharides and relatively high amounts of trisaccharides that are stable after 48 h of fermentation. The structural analysis of GOS formed by Lb. bulgaricus 43 by HRAMS (E) showed that the obtained trisaccharides contain β(1→4) and β(1→6) glycosidic bonds. The bond β(1→4) is quite unusual and is reported in GOS formed by Lb. bulgaricus for the first time. Observing the transgalactosylation activity of LacZ of Lb. bulgaricus DSM 20 081, Nguyen et al. [Citation33] obtained the trisaccharides β-D-Galp-(1→6)-Lac and β-D-Galp-(1→3)-Lac. The β-galactosidase of Lb. bulgaricus strain L3, employed by Lu et al. [Citation34] to produce galactosyl-sucralose, also formed predominantly β(1→6) galactosyl bonds. Only β(1→6) and β(1→3) bonds were reported in GOS, formed in yogurts [Citation32]. Therefore, the unique property of Lb. bulgaricus to form β(1→4) galactosyl bonds may be associated with novel functional properties of GOS produced by these isolates and with particularities in β-galactosidase enzymes that need further investigations.
4. Conclusions
The specific natural and climatic conditions in Bulgaria have contributed to the spontaneous evolution of yogurt starter cultures with unique features. Lb. bulgaricus able to produce prebiotics (GOS) were predominantly isolated from Rhodope villages, and from sheep and buffalo yogurts. LAB strains possessing antimicrobial activity were evenly distributed throughout the country, with predominance in mountainous areas with altitudes above 1500 m. The comprehensive examination of the current status of the authentic Bulgarian yogurt microbial diversity showed that homemade yogurts produced in small farms in Bulgarian mountainous areas are a rich inexhaustible source of lactic acid bacteria with health-promoting effects. The valuable combination of beneficial qualities and good technological properties made the novel isolates very promising for successful engagement in yogurt manufacturing.
5. Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Rul F. Yogurt: microbiology, organoleptic properties, and probiotic potential. In: Ray RC, Montet D, editors. Fermented Foods, Part II: Technological Interventions. Boca Raton: (FL): CRC Press; 2017. p. 418–450. (Food Biology Series).
- Metchnikoff E. The prolongation of life: Optimistic studies. Mitchell PC, translator; London (UK): William Heinemann; 1907.
- Grigoroff S. Étude sur une lait fermentée comestible. Le “Kissélo mléko” de Bulgarie [Study on an edible fermented milk. The “Kisselo mléko” from Bulgaria]. Revue Médicale de la Suisse Romande. 1905;25:714–720.
- Miteva V, Stefanova T, Budakov I, et al. Characterization of bacteriocins produced by strains from traditional Bulgarian dairy products. Syst Appl Microbiol. 1998;21(1):151–161.
- Morelli L. First global summit on the health benefits of yogurt: yogurt, living cultures, and gut health. Am J Clin Nutr. 2014;99(5):1248–1250.
- Viega P, Pons N, Agrawal A, et al. Changes of the human gut microbiome induced by a fermented milk product. Sci Reports. 2014 [cited 2018 Apr 03];4:6328. DOI:10.1038/srep06328
- Makino S, Sato A, Goto A, et al. Enhanced natural killer cell activation by polysaccharides derived from yogurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1. J Dairy Sci. 2016;99(2):915–923.
- Stefanova T, Urshev Z, Dimitrov Z, et al. Species affiliation of dairy lactobacilli with angiotensin converting enzyme inhibitory activity. Biotechnol Biotechnol Equip. 2009;23(2):1250–1254.
- Suzuki Y, Ikeda K, Sakuma K, et al. Association between yogurt consumption and intestinal microbiota in healthy young adults differs by host gender. Front Microbiol. 2017 [cited 2018 Apr 03];8:847. DOI:10.3389/fmicb.2017.00847
- Boyanova L, Gergova G, Markovska R, et al. Bacteriocin like inhibitory activities of seven Lactobacillus delbrueckii subsp. bulgaricus strains against antibiotic susceptible and resistant Helicobacter pylori strains. Lett Appl Microbiol. 2017;65(6):469–474.
- Toyoda Y, Erkut C, Pan-Montojo F, et al. Products of the Parkinson's disease-related glyoxalase DJ-1, D-lactate, and glycolate, support mitochondrial membrane potential and neuronal survival. Biol Open. 2014;3(8):777–784.
- Chandan RC, Gandhi A, Shah NP. Yogurt: Historical background, health benefits, and global trade. In: Shah NP, editor. Yogurt in health and disease prevention. London (UK): Academic Press; 2017. p. 3–29.
- Venica CI, Bergamini CV, Rebechi SR, et al. Galacto-oligosaccharides formation during manufacture of different varieties of yogurt. Stability through storage. LWT Food Sci Technol. 2015;63(1):198–205.
- Dubois M, Gilles KA, Hamilton JK, et al. Colorimetric methods for determination of sugars and related substances. Anal Chem. 1956;28(3):350–356.
- Petrova P, Petrov K. Antimicrobial activity of starch-degrading Lactobacillus strains isolated from Boza. Biotechnol Biotechnol Equip. 2011;25(4):114–116.
- Tserovska L, Stefanova S, Yordanova T. Identification of lactic acid bacteria isolated from katyk, goat's milk and cheese. J Cult Collect. 2002;3:48–52.
- Dimitrov Z, Michaylova M, Mincova S. Characterization of Lactobacillus helveticus strains isolated from Bulgarian yogurt, cheese, plants and human faecal samples by sodium dodecyl sulfate polyacrylamide gel electrophoresis of cell-wall proteins, ribotyping, and pulsed-field gel fingerprinting. Int Dairy J. 2005;15:998–1005.
- Michaylova M, Minkova S, Kimura K, et al. Isolation and characterization of Lactobacillus delbrueckii ssp. bulgaricus and Streptococcus thermophilus from plants in Bulgaria. FEMS Microbiol Lett. 2007;269(1):160–169.
- Petrova P, Emanuilova M, Petrov K. Amylolytic Lactobacillus strains from Bulgarian fermented beverage Boza. Z Naturforschung C. 2010;65(3-4):218–224.
- Velikova P, Stoyanov A, Blagoeva G, et al. Starch utilization routes in lactic acid bacteria: New insight by gene expression assay. Starch/Starke. 2016;68(9–10):953–960.
- Miteva VI, Abadjieva AN, Stefanova TT. M13 DNA fingerprinting, a new tool for classification and identification of Lactobacillus spp. J Appl Bacteriol. 1992;73(4):349–354.
- Zhang X, Kong J, Qu Y. Isolation and characterization of a Lactobacillus fermentum temperate bacteriophage from Chinese yogurt. J Appl Microbiol. 2006;101(4):857–863.
- Lamothe GT, Jolly L, Mollet B, et al. Genetic and biochemical characterization of exopolysaccharide biosynthesis by Lactobacillus delbrueckii subsp. bulgaricus. Arch Microbiol. 2002;178(3):218–228.
- Patel S, Majumder A, Goyal A. Potentials of exopolysaccharides from lactic acid bacteria. Indian J Microbiol. 2012;52(1):3–12.
- Simova E, Beshkova D. Effect of growth phase and growth medium on peptidase activities of starter lactic acid bacteria. Le Lait. 2007;87(6):555–573.
- Kabadjova-Hristova P, Bakalova S, Gocheva B, et al. Evidence for proteolytic activity of lactobacilli isolated from kefir grains. Biotechnol Biotechnol Equip. 2006;20(2):89–94.
- Chang OK, Perrin C, Galia W, et al. Release of the cell-envelope protease PrtS in the growth medium of Streptococcus thermophilus 4F44. Int Dairy J. 2012;23:91–98.
- Cui Y, Xu T, Qu X, et al. New insights into various production characteristics of Streptococcus thermophilus strains. Int J Mol Sci. 2016 [cited 2018 Apr 03];17:1701. DOI: 10.3390/ijms17101701
- Hafeez Z, Cakir-Kiefer C, Girardet J-M, et al. Hydrolysis of milk-derived bioactive peptides by cell-associated extracellular peptidases of Streptococcus thermophilus. Appl Microbiol Biotechnol. 2013;97(22):9787–9799.
- Somkuti GA, Steinberg DH. Pediocin production in milk by Pediococcus acidilactici in co-culture with Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus. J Ind Microbiol Biotechnol. 2010;37(1):65–69.
- Markowiak P, Śliżewska K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients. 2017 [cited 2018 Apr 03];9(9):1021. DOI:10.3390/nu9091021.
- Martinez-Villaluenga C, Cardelle-Cobas A, Corzo N, et al. Study of galactooligosaccharides composition in commercial fermented milks. J Food Compos Anal. 2008;21(7):540–544.
- Nguyen TT, Nguyen HA, Arreola SL, et al. Homodimeric β-galactosidase from Lactobacillus delbrueckii subsp. bulgaricus DSM 20081: Expression in Lactobacillus plantarum and biochemical characterization. J Agric Food Chem. 2012;60(7):1713–1721.
- Lu L, Xu S, Jin L, et al. Synthesis of galactosyl sucralose by β-galactosidase from Lactobacillus bulgaricus L3. Food Chem. 2012;134(1):269–275.