?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
A yeast that produces high levels of ethyl acetate was isolated and purified from Laobaigan Daqu by traditional microbial methods and was identified as Wickerhamomyces anomalus by its morphology and 26S rDNA D1/D2 sequence. This strain, YF1503, greatly tolerated ethanol and ethyl acetate. The optimal conditions for producing high levels of ethyl acetate were identified using single factor experiments and response surface methodology. Under these fermentation conditions, strain YF1503 produced 17.35 g/L ethyl acetate, the highest level that has been reported. The presence of alcohol acyltransferase (AAT) activity (8.63 U/L) and changes in the concentrations of ethanol and ethyl acetate in the fermentation broth indicate that strain YF1503 synthesises ethyl acetate using the acyltransferase pathway.
Introduction
Hengshui Laobaigan Baijiu (liquor), popular for its transparency, mellow and luscious taste, and persistent aftertaste is an atypical representative of Laobaigan flavour, namely, the eleventh aromatic flavour type in China. This specific distilled Baijiu is produced in multiple stages using solid fermentation conditions [Citation1, Citation2].
The quality and style of Laobaigan Baijiu is reflective of the type, amount and proportion of its flavour components. The balance and stability of the microbial population and the quantities and ratios of the main flavoured substances create unique styles of Baijiu [Citation2]. Therefore, Laobaigan Baijiu most essentially includes a considerable number of flavour substances whose proportions are balanced to enhance the quality of Baijiu [Citation3]. Recent improvements in sample pre-treatment, detection methods and the overall understanding of flavour chemistry has resulted in the identification and characterization of the fragrant compounds contributing to the overall aroma of Laobaigan Baijiu. Gas chromatography-olfactometry (GC-O) and gas chromatography-mass spectrometry (GC-MS) showed that an abundance of esters (up to 22 different types) is present in Laobaigan Baijiu [Citation1, Citation4]. Of the esters, ethyl acetate was found to impart the strongest aroma and contribute the most to the overall aroma in Laobaigan Baijiu.
Ethyl acetate is also important to other kinds of Baijiu. It imparts the primary flavour of Fen Baijiu, which is associated with a fruit aroma [Citation5, Citation6]. Ethyl acetate also has important effects on the Baijiu body styles of rice-flavoured Baijiu and Feng-flavoured Baijiu.
However, due to the limitations and variations in the processing conditions of the fermentations that produce Baijiu, the ethyl acetate content differs from batch-to-batch, and the levels are sometimes lower than those required by the Chinese National Standard. This problem is more common in small- and medium-sized distilleries and seriously impacts the economics of their operations [Citation7]. Previous studies [Citation8–10] have shown that the use of a high ethyl acetate-producing yeast in Baijiu fermentations can effectively increase the content of esters in Baijiu, thereby improving the quality of Baijiu and complying with the Chinese National Standard. The identification of high ethyl acetate-producing yeasts for Baijiu production is consequently very important and receives active interest.
The aim of this investigation was to separate and identify high ethyl acetate-producing strains from a Daqu starter culture used to brew Baijiu. In addition, the ester-producing properties were studied so that the functional strains selected for Baijiu production are better understood and their fermentation conditions can be optimized to give consistent results for all batches, thereby attaining a consistent, high-quality Baijiu.
Material and methods
Materials
Daqu of Laobaigan was provided by Hengshui Laobaigan Co., Ltd. (prepared in December 2015); ethyl acetate standards were acquired from Sigma Co., Ltd.
Culture medium preparation
The preparation of sorghum juice involved crushing of 500 g sorghum and mixing the resulting powder 1:4 (w/w) with distilled water. The suspension was heated to boiling, α-amylase (200 μL, Aladdin Industrial Co., Ltd) was added and the suspension was liquefied at 90 °C for 1 h. The mixture was cooled to 60 °C and α-1,4-glucan glucohydrolase (0.125 g, Beijing Biotopped Science & Technology Co., Ltd) was added. Saccharification proceeded at 60 °C for 2 h. The suspension was centrifuged at 8000 rpm (CR22N, High-Speed Refrigerated Centrifuge, Hitachi Koki Co., Ltd.) for 7 min and the supernatant was adjusted to 10°Bx (LYT-390, Portable Refractometer, Shanghai Linyu Co., Ltd) and pH 6.0. This mixture was sterilized at 121 °C for 20 min [Citation11].
Wallerstein Laboratory Nutrient Agar (WL) medium was prepared with 0.5% yeast extract, 0.5% tryptone, 5% glucose, 2% agar, 0.055% potassium dihydrogen phosphate, 0.0425% potassium chloride, 0.0125% calcium chloride, 0.00025% ferric chloride, 0.0125% magnesium sulphate, 0.00025% manganese sulphate and 0.0022% bromocresol green, adjusted to pH 6.5 and then autoclaved at 121 °C for 20 min [Citation12].
Isolation of yeast strains
A sample of 1 g mixed Daqu of Laobaigan was added to 99 mL of sterilized double distilled (dd) H2O and shaken for 5 min. The culture filtrate (2 mL) was diluted from 10−1 to10−5 and then, 100 μL of each dilution was coated onto the YPD (Yeast extract Peptone Dextrose) plates and cultured by inverting the plates in a 30 °C incubator for 2–4 days. Single colonies of different morphologies were selected from the plates and examined under a microscope. Single colonies were streaked onto new YPD plates and cultured in a 30 °C incubator, and the growth of cells was monitored every 6 hours. These steps were repeated until isolated colonies appeared. Each isolated colony was then used to inoculate a separate YPD slant tube. The above method is referred to as the dilution plate method [Citation13].
Screening of high ethyl acetate-producing yeasts
Yeasts isolated from Daqu were activated in YPD liquid medium in an incubator at 30 °C shaking at 180 rpm for 12 h. Then, 1 mL of this seed culture was inoculated into 50 mL of sterilized sorghum juice supplemented with ethanol (4%, v/v) and acetic acid (0.01%, v/v). The medium was cultured in a 30 °C incubator at 180 rpm for 3 days. The broth was centrifuged at 10,000 rpm (Microfuge 20R, Beckman Coulter, Inc.) for 10 min at the end of the fermentation. The supernatant was diluted with 60% ethanol to the appropriate concentration and its ethyl acetate content was quantified using high-performance liquid chromatography (HPLC-1260 Agilent Technology Co., Ltd.). The yeasts that produced the highest level of ethyl acetate were selected for further study.
HPLC conditions
The analyses were conducted with an HPLC (HPLC-1260 Agilent Technology Co., Ltd.) using a ZORBAX Eclipse Plus C-18 column with dimensions 4.6 mm ×150 mm ×5 μm (Agilent Technology Co., Ltd.). The HPLC conditions were as follows: mobile phase—methanol:0.1 mol/L KH2PO4 (50:50, v:v); flow rate—1 mL/min; UV detection wavelength—210 nm; column temperature—35 °C; injection volume—5 μL and detection time for ethyl acetate—5.0 min [Citation14].
Identification of yeast strain
Morphological observation
The cell morphology and the colony morphology of the candidate yeast strain were examined using a microscope (Nikon, Japan) and on a WL plate, respectively.
DNA sequencing
The total DNA of the yeast was extracted using a EZNA Fungi Genomic DNA Extraction Kit (Sigma). Sequencing of the D1/D2 domain of 26S rDNA was performed as described by Lopandic et al. [Citation15]. A fragment of approximately 600 bp was amplified with primers NL1 (5´-GCATATCAATAAGCGGAGGAAAAG-3´) and NL4 (5´-GGTCCGTGTTTCAAGACGG-3´). The polymerase chain reaction (PCR) working mixture consisted of 1 μL of DNA sample, 1 μL of primer NL1 (20 μmol/L), 1 μL of primer NL4 (20 μmol/L), 4 μL of deoxynucleoside triphosphates (dNTPs), 4.5 μL of 10 × Taq Reaction Buffer, 0.5 μL of Taq polymerase (5 U/μL) and 37.5 μL of ddH2O. PCR was performed (T100-Thermal Cycler, Bio-Rad Laboratories, Inc.) with the following temperature program: 95 °C for 5 min, then 30 cycles of 95 °C for 30 s, 55 °C for 30 s and 72 °C for 1 min, and a final extension of 72 °C for 10 min. Partial sequences of the 26S rDNA gene were used in a similarity search by the BLAST program from NCBI (http://www.ncbi.nlm.nih.gov/). Strains showing identity scores higher than 99% were selected as type strains. MEGA 6.0 biology software was used to analyse the multiple sequences of the tested and the type strains based on the homology search results, and the phylogenetic tree was then constructed using the neighbour-joining method.
Strain characterization
Strain tolerance
The tolerance of the yeast strain to glucose (20–70%, w/v), sodium chloride (5–25%, w/v) and acetic acid (0.1–0.3%, v/v) were determined by the streaking method [Citation16]. The strains’ tolerances to ethanol (8–20%, v/v) and ethyl acetate (16–30 g/L) were determined by monitoring the optical density (OD560) of liquid cultures.
Optimum growth of initial pH, and temperature
The isolated yeast was inoculated into YPD liquid medium at 30 °C and was then shaken at 180 rpm for 24 h. The activated strain was inoculated into YPD liquid medium with different pH values (pH 3–7) and cultured statically in a 30 °C incubator for 2 days. Cell density was measured by monitoring the absorbance at 560 nm and the initial pH value of the medium, which resulted in the highest absorbance value, was identified as the optimum pH.
The activated strain was also inoculated into YPD liquid medium and then cultured at different temperatures (20–36 °C) and pH 6 for 2 days. The optimum temperature was determined by the absorbance of the culture (as stated above for determining optimum pH).
Effect of different conditions on the level of ethyl acetate
The effects of degrees Brix (6–14°Bx), ethanol concentration (0–10%, v/v), inoculum volume (2–10%, v/v), temperature (18–30 °C), mixing (rotation speed of 0–240 rpm) and fermentation time on the level of ethyl acetate were studied using a single factor experiment [Citation17].
Optimization by response surface methodology
The ethanol concentration, rotation speed, and degrees Brix were selected as the independent variables for a Box-Behnken center combination design. With ethyl acetate concentration as the response variable, a response surface methodology (RSM) using three factors and three levels was designed, and the experimental combinations of factors and levels were selected ().
Table 1. Factors and levels of Box–Behnken design for RSM.
Ethanol and acetic acid
The levels of ethanol and acetic acid in the broth were quantified by HPLC [Citation18] and biosensor measurements [Citation19], respectively.
Enzyme measurements
Preparation of yeast cell extracts
Yeast cells cultured under the optimal conditions for ethyl acetate were separated by centrifugation at 10,000 rpm for 10 min and collected from the culture broth. The cells were washed twice with distilled water and then with 5 mL of Tris–HCl buffer (pH 8, containing 0.1% Triton X-100, 10% glycerol, 5 mmol/L DTT). The cells were disrupted with ultrasonic crushers at 50% power for 15 min in ice water. Enzyme activity was measured in the supernatant after centrifugation (10,000 rpm for 10 min) [Citation20].
Alcohol acyltransferase
Alcohol acyltransferase (AAT) activity was measured [Citation21] by mixing 300 μL of acetyl-CoA solution (2.5 mmol/L acetyl-CoA in 0.5 mol/L Tris-HCl, pH 8.0), 300 μL of ethanol solution (200 mmol/L ethanol in 0.5 mol/L Tris-HCl, pH 8.0) and 400 μL of enzyme extract. The mixture (1 mL) was incubated at 30 °C for 30 min. Next, 100 μL of 10 mmol/L 5,5-dithiobis(2-nitrobenzoic acid) (DTNB) was added, and the solution stood at room temperature for 10 min. The increase in absorbance at 412 nm was measured as a function of time (TU-19UV-VIS spectrophotometer, Beijing Purkinje General Instrument Co., Ltd). The increase in absorbance was due to the formation of a yellow thiophenol product from the reaction of DTNB with the free CoASH that had been liberated during the catalytic reaction. One activity unit (U) is defined as an increase of one unit of absorbance per minute. The results are expressed as specific activities.
Lipase and esterase
Lipase and esterase activities were quantified in cell extracts according to the description by Winkler and Stuckmann [Citation22] with some modifications and using p-nitrophenyl palmitate (p-NPP) and p-nitrophenyl butyrate (p-NPB) as substrates, respectively. A stock solution of p-NPP/p-NPB (16.5 mmol/L) was freshly prepared in 2-propanol. Next, 100 μL of this substrate stock solution was added to 2.8 mL of 50 mmol/L Tris-HCl (with 0.1% (w/v) gum arabic and 0.4% (v/v) Triton X-100). The solution was mixed and pre-incubated for 5 min at 37 °C before 100 μL of the cell extract sample was added. The sample mixture was then incubated at 37 °C for 10 min and the reaction was terminated by the addition of 2 mL of 0.2 mol/L Na2CO3 solution. Released p-nitrophenol (p-NP) was immediately quantitated by measuring the absorbance at 410 nm in a UV-VIS spectrophotometer. Appropriate controls were used to subtract the absorbance of the reaction mixture from that produced by the specific hydrolysis of substrate (p-NPP/p-NPB). One international unit of lipase/esterase activity is expressed as the amount of enzyme liberating 1 μmol p-NP per minute under the conditions of the assay.
Analytical methods and statistical analysis
Design Expert software (version 8.0) was used for the experimental design, data analysis and quadratic model building. SPSS 8.0 was applied to perform an analysis of variance on experimental results by one-way analysis of variance (ANOVA). Variable effects were regarded as significant if the reported p-values were less than 0.05.
Results and discussion
Screening for high ethyl acetate-producing yeasts
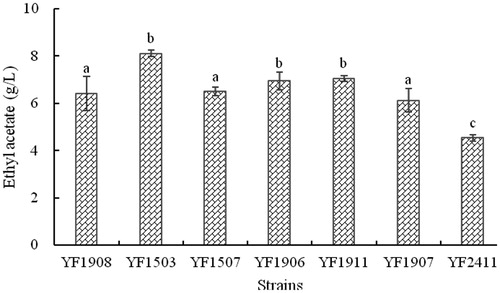
A total of 30 yeast strains were obtained from the dilution plate method and the ability of each strain to produce ethyl acetate in the culture medium was analysed according to the conditions described above (data not shown). Seven yeast strains produced high levels of ethyl acetate (): strains YF1503, YF1906 and YF1911 produced the highest concentration of ethyl acetate (8.10, 6.95 and 7.06 g/L, respectively), while strains YF1908, YF1507 and YF1907 produced less (approximately 6 g/L) and strain YF2411 produced the least (4.54 g/L). Strain YF1503 gave the highest concentration of ethyl acetate under the selected conditions and therefore, was selected for further study.
Identification of yeast strain YF1503
Morphological observation
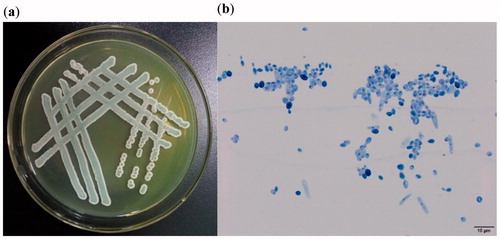
As shown in , the colony of strain YF1503 on WL medium was flat and white to grayish green with a rough surface. Under an optical microscope (10 × 100), the cells of strain YF1503 had the following characteristics: oval, with a diameter of 3–5 μm, with bud propagation and no mycelia (). The observed cell morphology corresponds to the W. anomalus characteristics.
DNA sequencing of strain YF1503
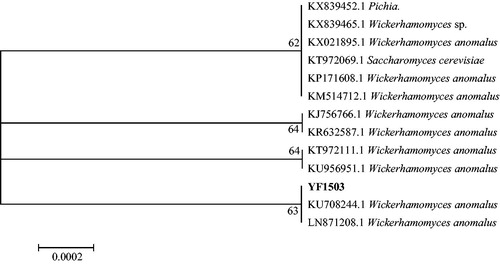
The 26S rDNA D1/D2 region sequence of yeast strain YF1503 was queried against the NCBI database. The highly homologous strains all belonged to W. anomalus. A phylogenetic tree for related strains was constructed according to strain similarities (). Strain YF1503, W. anomalus strains LN871208.1 and KU708244.1 clustered into a branch and were each other's closest relatives, which identified strain YF1503 as W. anomalus. A subset of W. anomalus, Pichia anomala and Hansenula anomala were classified as synonyms in the most recent taxonomy reports [Citation23]. W. anomalus is an important yeast strain in Baijiu brewing and traditional food fermentation [Citation24–26]. Strain YF1503 synthesizes high concentrations of ethyl acetate [Citation27]. The culture media and fermentation conditions needed to maximize the production of ethyl acetate from strain YF1503 were determined in this study.
Characterization of yeast strain YF1503
Strain tolerance
The tolerance of strain YF1503 for ethanol, acetic acid, sodium chloride, glucose and ethyl acetate was studied. shows that this strain tolerated as much as 12% ethanol, making it highly resistant to ethanol. This characteristic is beneficial for the strain’s use in a number of applications. Strain YF1503 also tolerated as much as 22 g/L ethyl acetate, which is one of the highest tolerance levels reported in the literature [Citation27]. In addition, the strain tolerated normal concentrations of acetic acid, sodium chloride and glucose.
Table 2. Tolerance of yeast strain YF1503.
Initial pH and temperature for optimum growth
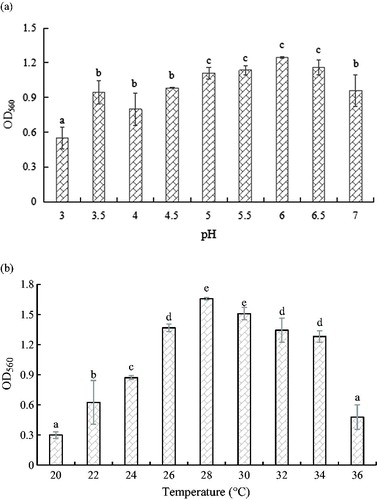
The optimum determined initial pH range for yeast YF1503 growth was between 5.0 and 6.5 (), which is higher than the typical values for yeasts (pH 4.0–5.5) [Citation28]. The optimum growth temperature is from 28 to 30 °C, which is consistent with the behaviour of most yeasts.
Effect of conditions on the level of ethyl acetate
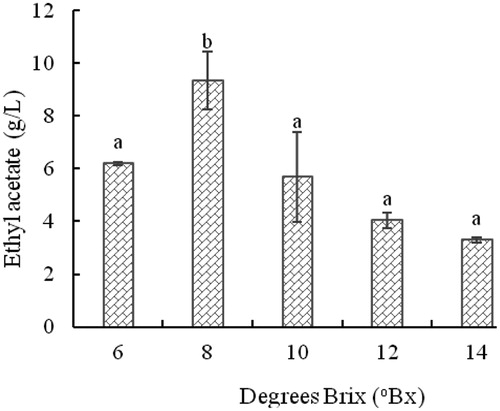
Effect of degrees Brix on the level of ethyl acetate
The effect of degrees Brix, a measure of total sugars, on the level of ethyl acetate is shown in . When the degrees Brix value was low, the nutrients in the medium were not sufficient, affecting the ability of strains for producing esters. High degrees Brix values led to high osmotic pressures, which affected the growth of strains and thereby inhibited their ability to synthesize esters. The strain produced the highest level of ethyl acetate (9.34 g/L) at 8°Bx in sorghum broth. This result is consistent with the previous results of Li et al. [Citation10] and Liu et al. [Citation29].
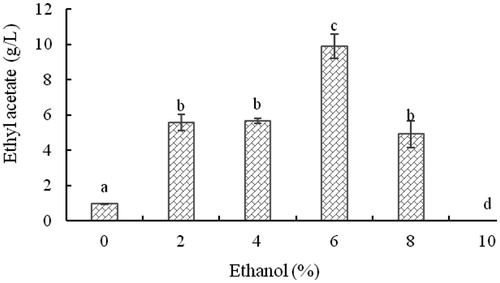
Effect of ethanol concentration on the level of ethyl acetate
The effect of ethanol concentration on the level of ethyl acetate produced by strain YF1503 was determined (). The strain synthesized tiny amounts of ethyl acetate without the precursor ethanol. The strain produced ethyl acetate with a minimal amount of ethanol in the fermentation broth, indicating that ethanol is the necessary precursor for the synthesis of ethyl acetate. However, high concentrations of ethanol inhibited the growth of the strain, resulting in unfavourable production of ethyl acetate. The level of ethyl acetate was highest at 6% ethanol, reaching 9.90 g/L. The ethanol additions (6%) used in this study were higher than those used in previous studies [Citation17, Citation29].
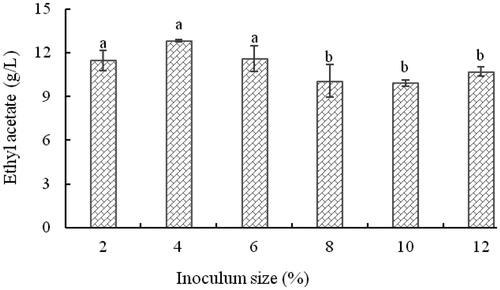
Effect of inoculum size on the level of ethyl acetate
shows that the production of ethyl acetate was highest when the inoculum level was less than 6% (v/v). Excessive amounts of inoculum (>6%) inhibited the ability of the strain to synthesize ethyl acetate. The optimum inoculum level, at which the strain produced the largest quantity of ethyl acetate, was 4% (v/v). At 4% inoculum level, combined with the use of degrees Brix and ethanol concentration at optimal values, the level of ethyl acetate reached its highest value of 12.78 g/L.
Effect of temperature on the level of ethyl acetate
The effect of temperature on the level of ethyl acetate was not significant, but the lower temperatures were more favourable for synthesising ethyl acetate [Citation30]. The yield of ethyl acetate was 12.95 g/L at 20 °C. The optimum temperature for strain is consistent with the one, determined by Li et al. [Citation17], and lower than the one, determined of Zhong et al. [Citation31].
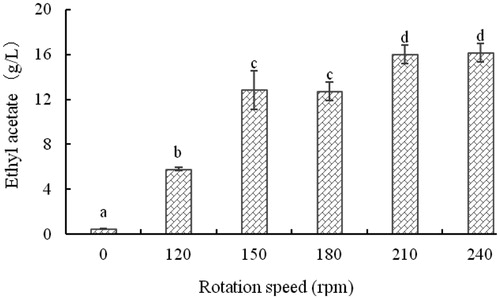
Effect of agitation on the level of ethyl acetate
The effect of mixing at different rotation speeds on the production of ethyl acetate by strain YF1503 is shown in . The strain synthesized little ethyl acetate in static culture. The level of ethyl acetate increased with the increase of the rotation speed. The synthesis of ethyl acetate by this strain is aerobic. The level of ethyl acetate peaked at 16.01 g/L at a rotation speed of 210 rpm.
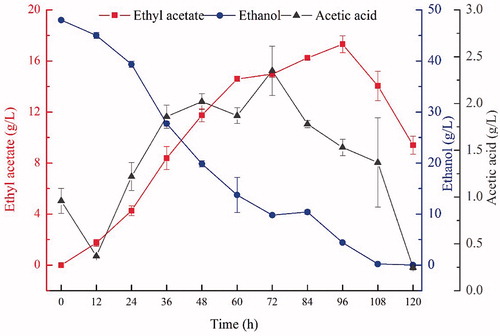
Changes in ethyl acetate, acetic acid and ethanol levels during fermentation
The effect of fermentation time on the level of ethyl acetate is shown in . Ethyl acetate accumulates for the first 96 h and peaks at 17.31 g/L. As time increased beyond 96 h, the concentration of ethyl acetate decreased rapidly. Ethyl acetate seemed to accumulate and decompose simultaneously and may be lost by metabolic transformation [Citation32], hydrolysis by esterases produced by the strain [Citation33, Citation34] or by volatilization [Citation27].
The level of ethanol decreased with time and was completely consumed by 108 h. This behaviour is likely because ethanol is used as the direct substrate in the synthesis of ethyl acetate [Citation35]. In contrast, the acetic acid concentration declined slightly at the beginning of fermentation and then accumulated over time until reaching its peak at 72 h, which was followed by a decline. Acetic acid is produced by the glucose metabolism and the decomposition of ethyl acetate [Citation32]. The decrease in acetic acid concentration might be caused by acetic acid reacting with ammonia, which is generated by the deamination of the amino acids derived from the proteolytic cleavage caused by proteases secreted by the yeast [Citation36].
Optimization by RSM
RSM was used as an efficient and effective statistical technique to optimize the production of ethyl acetate by YF1503. RSM determines the effect of variable factors with a minimum number of experiments. A total of 17 experiments with the dependent variable of ethyl acetate concentration (Y) and independent variables were randomly conducted (). Coefficients and p-values of first-order polynomials (A, B, C), second-order polynomials (AA, BB, CC), cross-polynomials (AB, AC, BC) and constants were determined via regression analysis (). The p-values were also used to check the significance of each of the coefficients, which must be understood. The P-values for A (0.0059), B (0.0126), AA (0.0007) and BB (0.0043) were much less than 0.05, which suggested that the ethanol concentration and rotation speed strongly influence the synthesis of ethyl acetate by YF1503. shows that these results correspond to a lack-of-fit value of 0.084 for the model when the p-value exceeds 0.05. From these data, a second-order model equation derived from this response model was estimated as shown below:
Table 3. Analysis of variance for the quadratic model.
The interactions of the three parameters and the optimal concentrations of ethyl acetate that were produced under these parameters were further analysed by three-dimensional response surface curves and respective contour plots (). The plot was convex, meaning that the optimal conditions for producing ethyl acetate were determined. The optimal concentration of ethyl acetate (approximately 17.26 g/L) was predicted to be obtained when the independent variables are 6.7% (ethanol), 223.62 rpm (rotation speed), and 8.64°Bx (degrees Brix).
Figure 10. Three-dimensional response surface plots of the interaction of (a) concentration of ethanol, (b) rotation speed and (c) degrees Brix on the concentration of ethyl acetate produced by yeast strain YF1503.
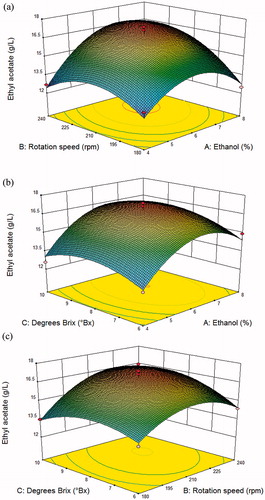
The model was validated by performing three experiments in shake flasks under the predicted conditions. The parameters of the actual experiment were 6.5% (ethanol), 220 rpm (rotation speed) and 8.5°Bx (degrees Brix). The predicted response (ethyl acetate production) was 17.26 g/L, and the actual (experimental) response was 17.35 g/L. Because the experimental values were close to the predicted values, the model was successfully validated. The final concentration of ethyl acetate was higher than most previously reported values [Citation17]. Strain YF1503 thus has a strong capacity for ethyl acetate synthesis and a good potential to be further studied in commercial applications.
Enzyme activity measurement
The activities of AATs, lipases and esterases of YF1503 cultured under the optimal conditions for producing high levels of ethyl acetate were determined. The AAT enzyme activity was 8.63 U/L at the peak ethyl acetate level; however, lipase and esterase activities were not detected. When this result is considered with the ethanol and acetic acid levels, the synthesis of ethyl acetate by strain YF1503 by way of the acyltransferase pathway, i.e. through AAT catalysing the consumption of ethanol and acetyl coenzyme A, is plausible. These results provide further evidence for previously suggested hypotheses [Citation27, Citation34].
Conclusions
This study showed that a strain of W. anomalus, YF1503, which was isolated from Laobaigan Daqu produced high levels of ethyl acetate and tolerated high concentrations of ethanol (12%) and ethyl acetate (22 g/L). These characteristics make this strain suitable for utilization in brewing and improving the consistency and high-quality flavour of Baijiu. The optimum conditions for the yeast to synthesize ethyl acetate, determined by single factor analysis and RSM, are as follows: an initial pH of 6.0, degrees Brix of 8.5, a culture medium containing 0.01% acetic acid and 6.5% ethanol, 4% (v/v) inoculum volume percentage, a fermentation temperature of 20 °C, mixing at 220 rpm and a total batch fermentation time of 4 days. Yeast YF1503 reached its maximum ethyl acetate concentration of 17.35 g/L under these conditions, which is one of the highest reported concentrations. This strain thus has a strong potential for increasing the amount of ethyl acetate in Baijiu, thereby improving its flavour quality and consistency. The levels of ethanol and ethyl acetate found during the course of fermentation and the corresponding AAT activity (8.63 U/L) indicate that strain YF1503 primarily synthesizes ethyl acetate through the acyltransferase pathway.
Acknowledgment
We thank Dr. Sharon Shoemaker for insightful discussions and providing English assistance for this manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ding YL, Fan WL, Xu Y, et al. Analysis of aroma components in Laobaigan-flavor liquor. Liquor Making. 2008;4:109–113.
- Zhu HX, Zhang YH, Cheng ZZ, et al. The change rules of the main flavoring components during the fermentation of Hengshui Laobaigan Liquor. Liquor-Making Sci Technol. 2015;2:36–39.
- Lv H. Sensory evaluation and appreciation of Laobaigan-flavour Liquor. Liquor Making. 2009;1:43–44.
- Ding YL. Studies on characteristic aroma compounds in Fen-liquor [D]. Wuxi: Jiangnan University; 2008.
- Shen YF. Study on the formation mechanism of four main kinds of ethyl esters in the fermentation of Liquor. Liquor-Making Sci Technol. 2003;5:28–31.
- European Committee for Electrotechnical Standardization (CENELEC). Mild aromatic Chinese spirits. China: CENELEC; 2006. Standard No. GB/T 10781.2–2006.
- Song W, Lv LH, Zhao LQ, et al. Optimization of fermentation conditions of Hansenula anomala for ethyl acetate production and study on the metabolic pathway. China Brewing. 2011;30(1):116–119.
- Xu L, Zhang QY. Screening and application of several aroma-producing yeast strains. Liquor-Making Sci Technol. 2010;12:48–49.
- Hu JH, Wang XL, Sheng L, et al. Screening of a yeast strain with high-yield of ethyl acetate and its application in the production of Niulanshan Liquor. Liquor-Making Sci Technol. 2013;1:69–70.
- Li RL. Breeding of high-yield ethyl acetate yeast and research of application in fen-flavour Xiaoqu Liquor [Dissertation]. Wuhan: Hubei University of Technology; 2011.
- Zhao QL. Screening of brewing microorganisms from Maotai-flavor Daqu and studying on fermentation conditions [Dissertation]. Guiyang: Guizhou University; 2016.
- Xue JX, Xu YW, Yang Y, et al. Screening and identification of Saccharomyces cerevisiae with WL medium. China Brewing. 2007;26(9):36–39.
- Zhao C, Yan X, Yang S, et al. Screening of Bacillus, strains from Luzhou-flavor liquor making for high-yield ethyl hexanoate and low-yield propanol. LWT – Food Sci Technol. 2017;77:60–66.
- Zhang FY. Determination of the content of ethyl lactate, ethyl acetate and β-phenylethanol in liquor by RP-HPLC. Liquor-MakingSci Technol. 2008;11:108–111.
- Lopandic, K., Sugita, T., Middelhoven, et al. Frontiers in Basidiomycote mycology: Trichosporon caseorum sp.nov. and Trichosporon lactis sp. nov., two basidiomycetous yeasts isolated from cheeses. Germany: IHW Verlag, Eching; 2004.
- Liu XY, Pan J, Gu YH, et al. Stress tolerances of four wild yeast strains. Sci Technol Food Industry. 2015;36:149–153.
- Li XY, Wang XL, Wang DL, et al. Study on the ester-producing conditions of Pichia anomala strains with high-yield of ethyl acetate. Liquor-Making Sci Technol. 2014;5:11–16.
- Guo Y, Liang J, Li MM, et al. Determination of organic acids in apple fruits by HPLC. Food Sci. 2012;33:227–230.
- Feng D, Wang BL, Liang XH, et al. Rapid determination of ethanol content in liquor by biosensor. Liquor-Making Sci Technol. 2012;2:83–86.
- Rojas V, Gil JV, Manzanares P, et al. Measurement of alcohol acetyltransferase and ester hydrolase activities in yeast extracts. Enzyme Microb Tech. 2002;30:224–230.
- Li D, Xu Y, Xu G, et al. Molecular cloning and expression of a gene encoding alcohol acyltransferase (MdAAT2) from apple (cv. Golden Delicious). Phytochemistry. 2006;67:658–667.
- Winkler U K, Stuckmann M. Glycogen, hyaluronate, and some other polysaccharides greatly enhance the formation of exolipase by Serratia marcescens. J Bacteriol. 1979;138:663–670.
- Kurtzman C P. Phylogeny of the ascomycetous yeasts and the renaming of Pichia anomala to Wickerhamomyces anomalus. Antonie van Leeuwenhoek. 2011;99:13–23.
- Hou JG, Guo FX, Fan JH, et al. Screening and identification of a strain with high yield of ethyl acetate from Taoxiang Pits. Liquor-Making Sci Technol. 2016;11:56–59.
- Dong KF, An N, Li DL, et al. Isolation and identification of yeasts in Daqu for aged vinegar production and their capacity for producing ethyl alcohol and ethyl acetate. Sci Technol Food Ind. 2016;37:213–216.
- Ming HM, Zhou J, Chen ME, et al. Optimization of phenylethanol fermentation conditions of Wickerhamomyces anomalus. Hubei Agric Sci. 2015;14:3492–3496.
- Löser C, Urit T, Bley T. Perspectives for the biotechnological production of ethyl acetate by yeasts. Appl Microbiol Biotechnol. 2014;98:5397–5415.
- Narisu, S, Gao FQ, et al. Screening and identification of yeast for tolerance to high temperature, alcohol and low acid. Pratacult Sci. 2013;10:1625–1632.
- Liu YC, Guo SX, Li RL, et al. Researching the ester production conditions of the high production ethyl acetate yeast. Food Ferment Technol. 2011;47:22–24.
- Huang GJ, Xu XF, Guo MJ, et al. Isolation and identification of a high-yield ethyl acetate yeast from xiaoqu of soybean-flavor liquor and its fermentation characterization. Sci Technol Food Industry. 2015;11:153–158.
- Zhong SX, Wang SL, Bian MH, et al. Identification and ester-producing conditions of a high ethyl acetate-producing yeast. China Brewing. 2017;36:75–79.
- Tabachnick J, Joslyn M A. Formation of esters by yeast. II. Investigations with cellular suspensions of Hansenula anomala. Plant Physiol. 1953;28:681–692.
- Inoue Y, Trevanichi S, Fukuda K, et al. Roles of esterase and alcohol acetyltransferase on production of isoamyl acetate in Hansenula mrakii. J Agr Food Chem. 1997;45:644–649.
- Kurita O. Increase of acetate ester-hydrolysing esterase activity in mixed cultures of Saccharomyces cerevisiae and Pichia anomala. J Appl Microbiol. 2008;104:1051–1058.
- Tabachnick J, Joslyn MA. Formation of esters of yeast. I. The production of ethyl acetate by standing surface cultures of Hansenula anomala. J. Bacteriol. 1953;65(1):1–9.
- Tang J, Wang HY, Xu Y, et al. Effect of mixed culture of Saccharomyces cerevisiae and Pichia anomala on fermentation efficiency and flavor compounds in Chinese liquor. Microbiol Chin. 2012;39:921–930.