Abstract
This study aimed to evaluate the metallo-beta-lactamase (MBL) production in Pseudomonas spp. and Acinetobacter baumannii using phenotypic and genotypic methods and to determine the most appropriate phenotypic method. The study included 55 Pseudomonas spp. (53 Pseudomonas aeruginosa, 1 P. fluorescens and 1 P. putida) and 33 A. baumannii isolates which were resistant to imipenem (IMP) and/or meropenem (MEM). Six phenotypic and one genotypic (real-time polymerase chain reaction [RT-PCR]) methods were used. According to the phenotypic tests, the rates of MBL-positive Pseudomonas spp. and A. baumannii were, respectively: 25.5% and 39.4% by the gradient test; 21.8% and 21.2% by the Rosco rapid CARB screen test; 9.1% and 21.2% by the modified Hodge test (MHT); 32.7% and 66.7% by the combined EDTA disk diffusion test; 56.4% and 100% by IMP + EDTA and 49.0% and 72.7% by MEM + EDTA and 9.1% and 3.0% by IMP + dipicolinic acid (DPA) for the Rosco MBL confirm test; 36.4% and 6.1% by IMP + DPA and 54.5% and 6.1% MEM + DPA for the double disk synergy test. MBL genes were detected only in three Pseudomonas spp. (blaIMP in two P. aeruginosa isolates and blaVIM in a P. fluorescens isolate). For Pseudomonas spp., the MBL positivity rate did not significantly differ between the RT-PCR and MHT and between the RT-PCR and Rosco MBL confirm test (with IMP + DPA) (p > 0.10). In conclusion, the Rosco MBL confirm test (with IMP + DPA) phenotypically predicted the MBL positivity most closely to the RT-PCR method for both Pseudomonas spp. and A. baumannii isolates.
Introduction
Pseudomonas aeruginosa and Acinetobacter baumannii are among the most important causes of healthcare-associated infections. In these bacteria, production of carbapenemases plays an important role in antimicrobial resistance. Among the carbapenemases, metallo-beta-lactamases (MBLs) are becoming increasingly important [Citation1].
In infections for which carbapenems are used, detecting the likely production of carbapenemases using a simple and reliable method has become equally important as the isolation of the agent itself [Citation2]. Although molecular methods are the most reliable methods for detecting carbapenemases, their use in routine applications is impractical; thus, a phenotypic test with a high sensitivity and specificity is needed for daily use [Citation3].
The present study aimed to investigate the presence of MBLs in Pseudomonas spp. and A. baumannii isolates using several phenotypic methods and real-time polymerase chain reaction (RT-PCR), as the molecular method, and to determine the most appropriate phenotypic method yielding the closest result with that of the molecular method.
Materials and methods
The present study included Pseudomonas spp. and A. baumannii isolates, which were isolated from various clinical samples sent to the Laboratory of Medical Microbiology Department in Ankara Dışkapı Yıldırım Beyazıt Training and Research Hospital between January and December 2014 and were found to be resistant to imipenem (IMP) and/or meropenem (MEM) according to the Clinical & Laboratory Standards Institute (CLSI) guidelines [Citation4].
Identification of isolates and antibiotic susceptibility testing
The study included 88 non-duplicate isolates, of which 55 were Pseudomonas spp. and 33 were A. baumannii isolates. The identification and antimicrobial susceptibility testing of the isolates were performed using the BD Phoenix 100 automated system (Becton Dickinson, USA). The minimum inhibitory concentrations (MICs) of IMP and MEM were determined using the gradient test (ETEST®, bioMérieux, France). Escherichia coli ATCC® 25922™, P. aeruginosa ATCC® 27853TM and IMP-, VIM-, and SPM-positive isolates from previous studies were used as controls.
Evaluation of metallo-beta-lactamase production by phenotypic methods
In the present study, six different phenotypic tests were used to evaluate MBL production in the isolates:
Gradient test: MBL gradient strips (ETEST®, bioMérieux, France) with IMP on one end and IMP-EDTA on the other end were used. MBL positivity was evaluated as ≥ eightfold reduction in IMP MIC values detected in the presence of EDTA.
Rosco Rapid CARB screen test: This test was performed using the Rapid CARB Screen (RCS) kit (Rosco Diagnostica A/S, Taastrup, Denmark) and the results were interpreted in accordance with the manufacturer’s instructions. Although the test was for Enterobacteriaceae and P. aeruginosa strains, it was also performed for A. baumannii in the present study.
Modified Hodge Test (MHT): This test is based on the principle of showing the degradation that occurs in the inhibition zone of susceptible bacteria in the presence of carbapenemase-producing bacteria. MHT was performed and interpreted as described previously by Lee et al. [Citation5].
Combined EDTA disk diffusion test: This test is based on the demonstration of ≥7 mm enlargement of the zone diameter of IMP in the presence of 750 μg/disk EDTA due to inhibition of carbapenemase enzyme in the presence of the metal-chelating agent EDTA [Citation6].
Rosco MBL confirm test: This test was performed using the Total MBL Confirm kit (98016; Rosco Diagnostica A/S, Taastrup, Denmark), which uses tablets developed to detect MBL-producing bacteria. The test is based on the inhibition of MBL by EDTA and DPA. The kit contains 10 μg IMP, 10 μg MEM, IMP + EDTA, IMP + DPA and DPA disks. The MBL test was considered positive if the inhibition zone diameters in the presence of EDTA were ≥7 mm larger than the inhibition zone diameters and if the inhibition zone diameters in the presence of DPA were ≥5 mm larger than the inhibition zone diameters.
Double disk synergy test with DPA: The synergy between DPA and IMP and MEM disks was investigated, as the indicator of presence of MBL, as described by Lee et al. [Citation7].
Evaluation of metallo-beta-lactamase production by RT-PCR
The RT-PCR method was used to evaluate the MBL production using the following primers: IMP, VIM, GIM, SPM and SIM. The nucleotide sequences of the primers are presented in . DNA isolation for the isolates was performed according to the manufacturer’s instructions (Qiagen GmbH, Germany).
Table 1. Nucleotide sequences of primers used in polymerase chain reactions.
Statistical analysis
Data analysis was performed using the DOS Microstat statistics package. The results of the descriptive statistics were expressed as numbers and percentages. Comparison of the results from the RT-PCR method with those from the phenotypic methods for each species was performed using the dependent samples t-test. A p-value of <0.05 was considered statistically significant.
Results and discussion
Among the 88 isolates included in this study, 53 were identified as P. aeruginosa, one was identified as P. fluorescens, one was identified as P. putida, and the remaining 33 ones were identified as A. baumannii. The rates of the isolates that were positive for MBLs according to the phenotypic methods and RT-PCR are summarized in . A representative result from each phenotypic test is presented in .
Figure 1. Representative test results. (a) A positive MBL gradient test (Etest); (b) positive and negative results of Rosco rapid CARB screen test; (c) a positive result of a modified Hodge test result; (d) a positive result of a combined EDTA disk diffusion test; (e) a Rosco MBL confirm test (positive result for imipenem EDTA and imipenem DPA test and negative result for meropenem DPA synergy test); and (f) a positive result of a double disk synergy test with DPA.
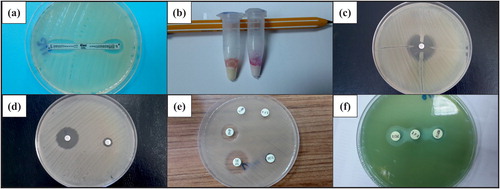
Table 2. Rates of Pseudomonas spp. and A. baumannii isolates that were positive for metallo-beta-lactamase production by phenotypic and genotypic methods.
According to the RT-PCR results, the presence of MBL genes was detected only in three Pseudomonas spp. (): blaIMP was detected in two P. aeruginosa isolates, while blaVIM was found in the P. fluorescens isolate.
Comparison of RT-PCR with phenotypic methods for metallo-beta-lactamase detection
For the Pseudomonas spp. isolates, there was a significant difference between the rates of MBL positive isolates detected by RT-PCR and those detected by all phenotypic methods, except for the Rosco MBL confirm test (IMP + DPA) and MHT (p > 0.10). Although no MBL gene was detected in A. baumannii by RT-PCR, there was no significant difference between the results obtained using RT-PCR with the Rosco MBL confirm test (IMP + DPA) and the double disc synergy test (IMP/DPA) (p > 0.10 for both). There were no significant differences between the rates of MBL positive isolates detected by other phenotypic methods and by RT-PCR. Both for the Pseudomonas spp. and the A. baumannii isolates, false positivity was highest with Rosco MBL confirm (IMP + EDTA; MEM + EDTA) and combined EDTA disk diffusion tests.
P. aeruginosa and A. baumannii are very resistant bacteria, particularly causing infections in the intensive care units, and their antibiotic resistance tends to increase in recent years [Citation8–10]. As the carbapenem group of antibiotics are the last available option, carbapenem resistance has a particular importance. In the 2014 report of the European Antimicrobial Resistance Surveillance Network (EARS-Net), carbapenem resistance was reported as 4%–43% in Pseudomonas spp. and as 0%–93% in Acinetobacter spp. which were isolated from invasive specimens [Citation11]. In the 2016 report, of the EARS-Net, carbapenem resistance was reported as 2.4%–51.6% in Pseudomonas spp. and as 0%–95.4% in Acinetobacter spp. isolated from invasive specimens [Citation12]. In the 2014 report of the Central Asian and Eastern European Surveillance of Antimicrobial Resistance (CAESAR), 10%–87% of P. aeruginosa isolates and 11%–93% of A. baumannii isolates from invasive specimens were reported to be carbapenem resistant [Citation13]. In the more recent 2016 report of the CAESAR, 12%–86% of P. aeruginosa isolates and 11%–95% of A. baumannii isolates from invasive specimens were reported to be carbapenem resistant in 2015 [Citation14]. In the 2013 annual report of the National Antimicrobial Resistance Surveillance System of Turkey (NARSS), the carbapenem resistance rates of P. aeruginosa isolated from blood and cerebrospinal fluid samples were 33.4% and 26.1% for IMP and MEM, respectively [Citation15]. Although the total carbapenem resistance rate was unknown during the collection of the isolates, the IMP and MEM resistance rates were 95.5% and 35% respectively, in blood isolates of A. baumannii and 96.1% and 27.5% respectively, in blood isolates of P. aeruginosa which were collected in 2017 in our hospital (data not shown). In the present study, isolates resistant to either IMP or MEM were included among all the clinical isolates collected during the study period.
Since MBLs play an important role in the resistance against carbapenem group antibiotics, detecting MBL production in bacterial isolates by a simple and reliable laboratory method is of great importance. Although the most reliable methods are molecular methods, such as PCR and sequence analysis, they are not practical options for routine use in laboratories, since they are expensive, require special equipment, and are labor intensive. Thus, phenotypic tests with high sensitivity and specificity to be used in routine laboratory practice are crucial [Citation16,Citation17].
Although Walsh et al. [Citation18] detected a sensitivity of 94% and a specificity of 95% for the Etest MBL strip (gradient test) in P. aeruginosa strains, false positivity has been reported to be still high both in Pseudomonas spp. and A. baumannii strains [Citation18–21], as was also determined in our study. For the Rosco rapid CARB screen test, while AbdelGhani et al. [Citation22] reported a sensitivity of 99% and a specificity of 100%, Dortet et al. [Citation23] reported a sensitivity of 89.5% and a specificity of 71%. In the present study, 12 Pseudomonas spp. were detected as carbapenemase positive by the Rosco rapid CARB screen test; however, only three isolates were found to be MBL positive by RT-PCR. Nine isolates were positively identified by the Rosco rapid CARB screen test but negatively by the molecular method. Therefore, in the present study, it can be assumed that the Rosco rapid CARB screen test was positive because of the presence of other carbapenemases which were not investigated. MHT is a simple method; currently, however, it is not widely used due to its subjectivity and high false positivity [Citation5]. In the present study, MHT was positive in 9.1% of Pseudomonas spp. and 21.2% of A. baumannii isolates. Another test used for the phenotypic determination of MBLs is the combined EDTA disk diffusion test [Citation14]. The sensitivity of this test is between 76% and 96% in different studies [Citation6, Citation21, Citation24]. In the present study, the rate of MBL positivity by the combined EDTA disk diffusion test was 32.7% in Pseudomonas spp. isolates and 66.7% in A. baumannii isolates. As compared with the RT-PCR results, the combined EDTA disk diffusion test had a high rate of false positivity both in Pseudomonas spp. and in A. baumannii isolates.
The Rosco MBL confirm test is based on inhibition of MBLs with EDTA and DPA. Bartolini et al. [Citation25] found that the Rosco MBL confirm test had a sensitivity of 95% and a specificity of 99% and emphasized that it was the most appropriate phenotypic test for routine use.
In their study investigating the presence of MBLs in P. aeruginosa and A. baumannii isolates, Hansen et al. [Citation26] reported that the sensitivities of all carbapenem inhibitor combinations of the Rosco total MBL confirm kit were >80%, whereas the specificities and positive predictive values were low except for the combination of IMP-DPA in A. baumannii isolates. In another study, the sensitivity and specificity of the Rosco MBL confirm test were 89% and 49% respectively [Citation27]. In the present study, the rate of MBL positivity with the Rosco MBL confirm test varied for Pseudomonas isolates. The highest rate was 56.4% with the combination of IMP + EDTA, followed by 49% with the combination of MEM + EDTA. In the A. baumannii isolates, the MBL positivity (100%) was also highest with the combination of IMP + EDTA. Due to its inhibitory property, EDTA has a high false positivity rate when combined either with IMP or with MEM.
In their study, Yong et al. [Citation28] used the double disk synergy method for phenotypically demonstrating the presence of MBLs in Pseudomonas and Acinetobacter isolates, which were previously detected to be MBL positive. They reported that the combinations of IMP + DPA and MEM + DPA showed positive results in 93.2% and 86.4% of the isolates respectively. Picão et al. [Citation29] suggested that the double-disk synergy test was suitable for routine use but emphasized that the best screening method was dependent on the isolate type and common carbapenemases in that area. In the present study, for the double disk synergy test, the rate of MBL positivity in Pseudomonas isolates was 36.4% with the combination of IMP + DPA and 54.5% with the combination of MEM + DPA. The rate of MBL positivity in A. baumannii isolates was 6% both for these two combinations.
Küçükbasmaci et al. [Citation30] did not detect any MBL resistance gene by multiplex PCR in any one of the 51 isolates of P. aeruginosa. Aksoy et al. [Citation31] did not detect any MBL gene in 52 IMP resistant A. baumannii isolates but reported OXA-23-like and OXA-51-like resistance genes. In a study from northern Turkey, among 100 P. aeruginosa isolates studied by Ozgumus et al. [Citation32], VIM-type MBL was detected in one strain, IMP-1 type MBL was detected in nine strains, and clonal analysis of the IMP-1 type positive isolates revealed that all the strains were clonally related. The results from our study were similar to those reported in the study by Ozgumus et al. [Citation32]. In the present study, the Rosco MBL confirm test (combination of IMP + DPA) phenotypically predicted the MBL positivity most closely to the RT-PCR method for both Pseudomonas spp. and A. baumannii isolates.
In our study, the presence of IMP-, VIM-, SPM-, SIM- and GIM-type MBL encoding genes in the isolates of A. baumannii and P. aeruginosa was also investigated and, similar to the results of a recent multiyear multinational study, the IMP- and VIM-type MBL encoding genes were found to be the most frequent [Citation33]. The present study did not examine the presence of OXA-type carbapenemases. It is known that carbapenem resistance can occur due to different carbapenemases other than MBLs, which might affect the positivity of the results of some of the tests used in the present study.
Conclusions
Although molecular tests are gold standard tests for detection of MBLs, they are very labor intensive, expensive, and unavailable for all routine laboratory applications. Identification of Pseudomonas spp. and A. baumannii strains with the most accurate and reliable methods and implementing early isolation measures are very important. In the present study, among the examined phenotypic tests, the Rosco MBL confirm test (combination of IMP + DPA) predicted the MBL positivity most closely to the RT-PCR method for both Pseudomonas spp. and A. baumannii isolates. In order to determine the sensitivities and specificities of phenotypic tests on different species with different carbapenem resistance mechanisms, further studies should be performed on previously detected isolates with already known resistance mechanisms and genes.
Acknowledgments
We are cordially grateful to Prof. Zerrin Aktaş from the Department of Medical Microbiology of Medical Faculty in Istanbul University for supplying the positive control strains used in our molecular studies.
Disclosure statement
The authors report no conflicts of interest.
References
- Pournaras S, Maniati M, Petinaki E, et al. Hospital outbreak of multiple clones of Pseudomonas aeruginosa carrying the unrelated metallo-beta-lactamase gene variants blaVIM-2 and blaVIM-4. J Antimicrob Chemother. 2003;51:1409–1414.
- Khosravi Y, Loke MF, Chua EG, et al. Phenotypic detection of metallo-β-lactamase in imipenem-resistant Pseudomonas aeruginosa. Sci World J. 2012;2012:654939. DOI: 10.1100/2012/654939
- Çakar A. Hacettepe Üniversitesi Hastaneleri’nde ayrıştırılan Pseudomonas aeruginosa izolatlarında metallo-beta-laktamaz enziminin fenotipik ve genotipik yöntemlerle araştırılması [dissertation]. Ankara (Turkey): Hacettepe Üniversitesi Sağlık Bilimleri Enstitüsü; 2005.
- CLSI. Performance standards for antimicrobial susceptibility testing. 24th ed. CLSI document M100. Wayne, PA: Clinical and Laboratory Standards Institute; 2014.
- Lee K, Chong Y, Shin HB, et al. Modified Hodge and EDTA-disk synergy tests to screen metallo-beta-lactamase-producing strains of Pseudomonas and Acinetobacter species. Clin Microbiol Infect. 2001;7:88–91.
- Yong D, Lee K, Yum HJ, et al. Imipenem-EDTA disk method for differentiation of metallo-sz-lactamase-producing clinical isolates of Pseudomonas spp. Acinetobacter spp. J Clin Microbiol. 2002;40:3798–3801.
- Lee K, Lim YS, Yum JHY, et al. Evaluation of the Hodge test and the imipenem-EDTA double-disk synergy test for differentiating metallo-sz-lactamase-producing isolates of Pseudomonas spp. and Acinetobacter spp. J Clin Microbiol. 2003;41:4623–4629.
- Behnia M, Logan SC, Fallen F, et al. Nosocomial and ventilator-associated pneumonia in a community hospital intensive care unit: a retrospective review and analysis. BMC Res Notes. 2014;7:232. DOI: 10.1186/1756-0500-7-232
- Lee CR, Lee JH, Kang LW, et al. Educational effectiveness, target, and content for prudent antibiotic use. Biomed Res Int. 2015;2015:214021. DOI:10.1155/2015/214021
- Tang Q, Song P, Li J, et al. Control of antibiotic resistance in China must not be delayed: the current state of resistance and policy suggestions for the government, medical facilities, and patients. Biosci Trends. 2016;10:1–6.
- Surveillance Report – European Antimicrobial Resistance Surveillance System (EARSS); 2014.
- Surveillance Report – European Antimicrobial Resistance Surveillance System (EARSS); 2016.
- World Health Organization. Central Asian and Eastern European Surveillance of Antimicrobial Resistance. Annual report; 2014.
- World Health Organization. Central Asian and Eastern European Surveillance of Antimicrobial Resistance. Annual report; 2016.
- Republic of Turkey Ministry of Health. Ulusal antimikrobiyal direnç sürveyans sistemi 2013 yillik raporu [National antimicrobial resistance surveillance system 2013 annual report]. Ankara: Turkish Public Health Institution; 2015 (Turkish).
- Oh EJ, Lee S, Park YJ, et al. Prevalence of metallo-beta-lactamase among Pseudomonas aeruginosa and Acinetobacter baumannii in a Korean university hospital and comparison of screening methods for detecting metallo-beta-lactamase. J Microbiol Methods. 2003;54:411–418.
- Shin KS, Son BR, Hong SB, et al. Dipicolinic acid-based disk methods for detection of metallo-beta-lactamase-producing Pseudomonas spp. and Acinetobacter spp. Diagn Microbiol Infect Dis. 2008;62:102–105.
- Walsh TR, Bolmström A, Qwärnström A, et al. Evaluation of a new Etest for detecting metallo-beta-lactamases in routine clinical testing. J Clin Microbiol. 2002;40:2755–2759.
- Ozturk CE, Caliskan E, Sahin I. Antibiotic resistance of Pseudomonas aeruginosa strains and frequency of metallo-beta-lactamases. ANKEM Derg. 2011;25:42–47.
- Rossolini GM, Luzzaro F, Migliavacca R, et al. First countrywide survey of acquired metallo-beta-lactamases in gram-negative pathogens in Italy. Antimicrob Agents Chemother. 2008;52:4023–4029.
- Shivaprasad A, Antony B, Shenoy P. Comparative evaluation of four phenotypic tests for detection of metallo-β-lactamase and carbapenemase production in Acinetobacter baumannii. J Clin Diagn Res. 2014;8:DC05–DC08.
- Abdel Ghani S, Thomson GK, Snyder JW, et al. Comparison of the Carba NP, modified Carba NP, and updated Rosco Neo-Rapid Carb Kit tests for carbapenemase detection. J Clin Microbiol. 2015;53:3539–3542.
- Dortet L, Agathine A, Naas T, et al. Evaluation of the RAPIDEC® CARBA NP, the Rapid CARB Screen® and the Carba NP test for biochemical detection of carbapenemase-producing Enterobacteriaceae. J Antimicrob Chemother. 2015;70:3014–3022.
- Amudhan SM, Sekar U, Arunagiri K, et al. OXA beta-lactamase-mediated carbapenem resistance in Acinetobacter baumannii. Indian J Med Microbiol. 2011;29:269–274.
- Bartolini A, Frasson I, Cavallaro A, et al. Comparison of phenotypic methods for the detection of carbapenem non-susceptible Enterobacteriaceae. Gut Pathog. 2014;6:13.
- Hansen F, Hammerum AM, Skov R, et al. Evaluation of the total MBL confirm kit (ROSCO) for detection of metallo-β-lactamases in Pseudomonas aeruginosa and Acinetobacter baumannii. Diagn Microbiol Infect Dis. 2014;79:486–488.
- Peter S, Lacher A, Marschal M, et al. Evaluation of phenotypic detection methods for metallo-β-lactamases (MBLs) in clinical isolates of Pseudomonas aeruginosa. Eur J Clin Microbiol Infect Dis. 2014;33:1133–1141.
- Yong D, Lee Y, Jeong SH, et al. Evaluation of double-disk potentiation and disk potentiation tests using dipicolinic acid for detection of metallo-β-lactamase-producing Pseudomonas spp. and Acinetobacter spp. J Clin Microbiol. 2012;50:3227–3232.
- Picão RC, Andrade SS, Nicoletti AG, et al. Metallo-beta-lactamase detection: comparative evaluation of double-disk synergy versus combined disk tests for IMP-, GIM-, SIM-, SPM-, or VIM-producing isolates. J Clin Microbiol. 2008;46:2028–2037.
- Küçükbasmaci Ö, Midilli K, Issa G, et al. A new multiplex PCR method for rapid detection of genes encoding VIM and IMP types of metallo-beta-lactamases. Turkiye Klinikleri J Med Sci. 2010;30:1312–1316.
- Aksoy MD, Çavuşlu Ş, Tuğrul HM. Investigation of metallo-beta-lactamases and oxacilinases in carbapenem resistant Acinetobacter baumannii strains isolated from inpatients. Balkan Med J. 2015;32:79–83.
- Ozgumus OB, Caylan R, Tosun I, et al. Molecular epidemiology of clinical Pseudomonas aeruginosa isolates carrying IMP-1 metallo-beta-lactamase gene in a University Hospital in Turkey. Microb Drug Resist. 2007;13:191–198.
- Kazmierczak KM, Rabine S, Hackel M, et al. Multiyear, multinational survey of the incidence and global distribution of metallo-sz-lactamase-producing Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2016;60:1067–1078.
