Abstract
Ricinoleic acid, one of the biggest components in castor oil, is an unusual fatty acid with numerous industrial applications. Despite much research on the oil content of Ricinus communis L., the genes encoding the oil biosynthesis function in the seed developmental stages are still poorly known. Here, we analyzed differentially expressed transcripts during seed development using cDNA-amplified fragment length polymorphism. Almost all ingredients significantly decreased along with seed maturation, except for ricinoleic acid, which increased greatly from 6.7% to 86.8%. A total of 534 distinguished fragments were differentially expressed in six development stages. Among these, 67 fragments showed high homology with known-function genes, and 26 sequences were successfully annotated with fatty acid synthesis or storage proteins in castor and other species. The results showed that fatty acid synthesis and accumulation in castor seeds displayed various temporal patterns. Different cDNA-fragment patterns were mainly found in later development stages, coinciding with the onset of oil synthesis.
Introduction
Castor bean (Ricinus communis L.), from the family Euphorbiaceae, is an important non-edible oilseed crop which being widely cultivated throughout tropical and sub-tropical regions with drought- and saline-tolerance properties. It has high commercial value for the chemical industry and is a feedstock for biodiesel production, as it is the only commercial source of ricinoleic acid, a hydroxylated fatty acid [Citation1,Citation2]. Based on its unique fatty acid composition and high seed oil content, castor has tremendous potential industrial applications [Citation3]. Now, more than 3000 kinds of chemical products could be derived from castor oil using different chemical methods.
With the continuous development and innovation of modern science and technology, castor oil is regarded as a strategic material and is used in the production of sophisticated products. So far, the researches on the measurement of the oil content in castor seeds have reported high values from 40% to 60% [Citation4–6]. Although researches about the biosynthesis process of castor oil started early, its related enzymes and metabolic pathways have been less extensively explored [Citation7]. However, there are few studies on castor oil biosynthesis reported at the molecular level.
Generally, a direct approach to reveal the molecular basis of a biological system is to screen for differentially expressed genes and transcript derived fragments (TDFs) [Citation8]. cDNA-amplified fragment length polymorphism (cDNA-AFLP) analysis is an AFLP-based transcript profiling method for identification of differentially expressed genes by purifying fragments from sequence gels and sequencing [Citation9,Citation10]. This method is an attractive technique for gene discovery on the basis of fragment detection and for temporal quantitative gene expression analysis [Citation9]. From its establishment to the present time, this technology has been used in many plant species, including Arabidopsis thaliana [Citation11], Vitis vinifera [Citation12], Cicer arietinum L. [Citation13] and Robinia pseudoacacia L. [Citation14].
Previous researches showed variations in oil content and fatty acid composition of castor due to seed developmental stages [Citation15]. However, the genes encoding oil biosynthesis have not been tested and identified yet. Here, we analyzed differentially expressed transcripts in castor during seed development using cDNA-AFLP analysis. Our findings provide an insight into genes involved in castor oil biosynthesis and serve as a basis for further and more detailed investigations for better understanding of the genetic and transcriptomic information of castor oil biosynthesis in plant cells.
Materials and methods
Plant materials
Castor seeds (accession 2129) were collected from the Academy of Agricultural Science in Tongliao, China. Then, mature and healthy seeds were cultivated in the farmland of Inner Mongolia University for the Nationalities, Tongliao. The fruits were harvested at 10, 20, 30, 40, 50 and 60 days by bagging after pollination. The morphological characteristics of castor in each development stage have been described in our previous paper [Citation15]. The seeds were collected and stored at −80°C for subsequent experiments.
Measurement of fatty acid composition
The composition and relative percentage of fatty acids was calculated according to the corresponding chromatographic peaks. By applying the computer automatic and manual retrieval with NIST98 and Wiley of Mass Spectral Data, the fatty acid composition of castor oil was measured and analyzed [Citation4]. The fatty acid composition and absolute content were measured as described in our previous reports [Citation4,Citation15].
cDNA-AFLP assay
Total RNA was extracted from seeds using TRNzol reagent according to the manufacturer’s protocol (TIANGEN, Beijing, China). RNA purity and integrity was checked via Nanophotometer (IMPLEN, CA, USA). The quantified RNA samples (1 μg) were used for double-strand cDNA synthesis using M-MLV RTase cDNA Synthesis Kit (TakaRa, Kyoto, Japan). The cDNA-AFLP protocol was adapted according to previous reports with minor modifications [Citation16,Citation17]. First, 150 ng of cDNA was double-digested by MseI (TakaRa, Kyoto, Japan) and EcoRI (TakaRa, Kyoto, Japan) at 37°C for 5 h, then ligated with oligonucleotide adaptors using T4 DNA ligase at 16°C for overnight. The DNA amplification was conducted in a Thermal Cycler 480 (Perkin-Elmer, Norwalk, CA, USA). The ligated products were diluted in a ratio of 1:30 with ddH2O for pre-selective amplification. The pre-amplification reaction was carried out for initial denaturation at 94°C 30 s, followed by 30 cycles of amplification (94°C 30 s, 56°C 30 s and 72°C 1 min), then final extension at 72°C for 10 min. Next, the products were implemented in a 1:10 dilution for the selective amplification with 256 primer combinations. The selective amplification included a touch-down program: a scale-down of 0.7°C per cycle from 65°C. The final selective amplification products were loaded onto a 6% polyacrylamide gel containing urea and electrophoresed at 1500 V for 2.5 h, and then visualized using the silver-nitrate staining protocol according to Zhou et al. [Citation17].
Isolation and sequencing of TDFs
The polymorphic TDFs were excised from the gel and soaked in distilled water. TDFs were re-amplified using the same primers as described for the selective amplification and cloned into a pMD18-T Vector (TakaRa, Kyoto, Japan). The sequencing of TDFs was performed by Sangon Biotech (Shanghai, China). Sequences of TDFs were analyzed for homology searching by Basic Local Alignment Search Tool in the National Center for Biotechnology Information (NCBI) databases.
Data analysis
Data are mean values with standard deviation (±SD) from three independent experiments. Statistical analysis was performed using SPSS 17.0 software package (SPSS Inc., Chicago, IL, USA). Differences were considered statistically significant at p < .05.
Results and discussion
Variations in fatty acid composition of castor bean in seed development stages
During the development stages of the castor bean plants, the 100-seed weight reached the highest value in 40 days, varying from 36.84 to 48.62 g. By gas chromatographic analyses, 11 major ingredients were detected in the fatty acid composition of the castor seeds, including mytistic, palmitic, stearic, oleic, linoleic, linolenic, arachidic, arachidonic, behenic, lignoceric and ricinoleic acids (). Almost all ingredients significantly decreased along with seed maturation, except for ricinoleic acid, which increased greatly from 6.7% to 86.8%. Interestingly, mytistic, behenic and lignoceric acids were just detectable in the initial phase of seed development (10–20 days old seeds).
Table 1. Variations in fatty acid composition of castor bean at different developmental stages.
Ricinoleic acid, one of the biggest components in castor oil, is an unusual fatty acid with numerous industrial applications. Chen et al. [Citation18] summarized that the maturation of castor seeds needs about 60 days after pollination; and the endosperm tissue expansion occurs during the mid-phase (26–40 days). Importantly, they detected ricinoleate after 26 days of seed development. Therefore, they thought that the efficient accumulation of ricinoeate was related to transcriptional activation of genes associated with the ricinoeate and oil synthesis. We obtained similar findings: the content of ricinoleic acid reached the highest value at about 30 days after pollination, and differentially expressed transcripts were significantly induced at later seed development stages, coinciding with the onset of oil synthesis.
cDNA-AFLP analysis
Out of 256 primer combinations, 24 primer pairs successfully produced high intensity, reproducible and polymorphic fragments. A total of 534 clear bands were differentially expressed in six seed development stages, with an average of 22.6 fragments per primer. Based on the presence, absence or differential intensity in the gels, 266 bands were identified as TDFs with unequivocally differential expression, and their sizes ranged from 50 to 2000 bp (). These results indicated that TDFs could modify and change along with seed development.
Figure 1. Polyacrylamide gel of differentially expressed fragments in castor seed developmental stages. cDNA-AFLP analysis. M, DL2000 marker; Lanes 1–6, amplified bands at 10/20/30/40/50/60 days by the primer combinations of E01/M03; Lanes 7–12: amplified bands at 10/20/30/40/50/60 days by the primer combinations of E01/M05 and Lanes 13–15: amplified bands at 10/20/30 days by the primer combinations of E01/M06.
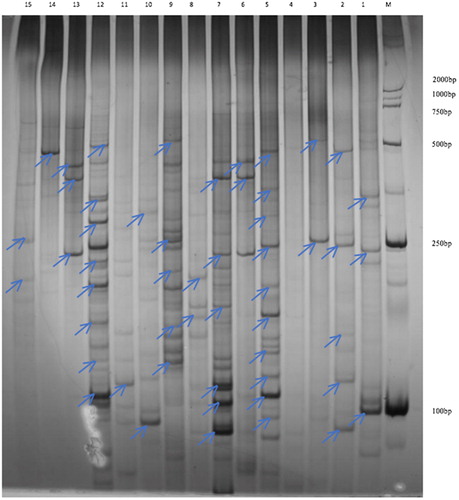
TDFs identification and homology analysis
Among 266 TDFs, 143 fragments were successfully cloned and sequenced. Among these, 67 sequences showed high homology with known-function genes, and 26 sequences were successfully annotated to fatty acid synthesis or storage protein pathways in castor and other species (). Nucleotide sequence homology demonstrated similarity to genes with the following function categories: cysteine protease, ATP synthase, serine/threonine protein kinase, RNA-binding protein, transaldolase, lipid-transfer protein, aspartic proteinase, UDP-glucuronic acid decarboxylase, pyruvate dehydrogenase and histidyl-tRNA synthetase. Additionally, we statistically analyzed the number of TDFs in each seed development phase (). One TDF was detected in the initial three stages, whereas six, five and twelve fragments were found in the 40, 50 and 60-day-old developing seeds, respectively. This indicated that 40–60 days was the vital phase of fatty acid synthesis and accumulation in castor seeds, displaying various temporal patterns; therefore, those TDFs could be involved in the process of fatty acid synthesis.cDNA-AFLP analysis as a differential display technique could tremendously reduce the number of false positives using restriction enzymes to generate specific tags; thereby, it is a reliable, suitable and economical system to detect differentially expressed transcripts without requiring prior sequence knowledge [Citation8,Citation14]. Rantong et al. [Citation19] identified differentially expressed transcripts in lace plant leaf shape during development using cDNA-AFLP, and annotated 79 TDFs related to photosynthesis, biosynthesis pathways, gene regulation and stress responses. Leymarie and Corbineau [Citation20] reported differentially expressed transcripts involved in barley seed germination and dormancy using cDNA-AFLP, and sequenced 25 TDFs. In our study, 143 TDFs were isolated and sequenced, and almost half of these TDFs were homologous to unknown-function genes, suggesting that those TDFs could be directly or indirectly implicated in novel fatty acid synthesis or storage pathways.
Table 2. Sequencing results of transcript derived fragments at different seed development stages.
Table 3. Statistical results of transcript derived fragments at different development stages.
Fatty acid biosynthesis in the cell uses acetyl-CoA produced by glycolysis, as a raw material for initial synthesis, along with a series of other biochemical reactions and enzymes. Acetyl-CoA carboxylase (ACC) is a rate-limiting biotinidase involved in the first committed step of fatty acid synthetic pathway. Davis et al. [Citation21] firstly tested the role of ACC in vivo and identified that its overproduction would result in a high increase in the synthesis of fatty acids. In addition, ACC catalyzes the conversion of acetyl-CoA to malnoyl-CoA, which is a key metabolite in regulating fatty acid synthesis. Turnham and Northcote [Citation22] investigated the role of ACC in regulating fatty acid oxidation and showed that ACC was activated with an increase in malonyl-CoA inhibition of fatty acid oxidation. Intriguingly, in our study, TDFs No. 177 was differentially expressed in different seed development stages, which was consistent with other findings [Citation22–24]. The accumulation of lipids along with a marked rise in ACC activity started from 16 days after pollination during the formation of rape-seeds [Citation22]. Also, researchers detected and purified ACC from developing soybean seeds using chromatography analysis. In the developing castor oil seed, Simcox et al. [Citation24] found that the rate of fatty acid synthesis decreased during seed maturation, and demonstrated a corresponding diminution in the activity of ACC.
In most organisms, the pyruvate dehydrogenase complex (PDC) catalyzes the pivotal irreversible reaction that oxidizes pyruvate into acetyl-CoA [Citation25]. Also, Sutendra et al. [Citation26] found that knockdown of PDC would decrease the de novo synthesis of acetyl-CoA. The properties and subcellular localization of the pea chloroplast PDC demonstrated its role in providing acetyl-CoA and NADH for fatty acid synthesis [Citation27]. Smith et al. [Citation28] suggested that malate and pyruvate were used as carbon sources for fatty acid synthesis, and that the activity of ACC might limit the fatty acid synthesis. Although PDC was required in fatty acid biosynthesis in castor bean, it was not necessary in the proplastid to provide acetyle-CoA during seed germination [Citation29,Citation30]. In addition, researches have also studied the relationship among pyruvate dehydrogenase, acetyl-CoA and fatty acid synthesis in developing seeds, suggesting a predominant role for plastidic pyruvate dehydrogenase in acetyl-CoA formation during lipid synthesis of seeds [Citation31,Citation32]. Here, TDF No. 48 was identified as PDC and was isolated 60 days after pollination, which was consistent with the measurements of the fatty acid content (), suggesting that fatty acid synthesis in castor bean was mainly a process in the later seed developmental stages.
The results obtained in this study could facilitate the efforts to efficiently improve the content of castor oil in breeding programmes. Given the amount of attention that castor oil with its numerous industrial applications has attracted around the world, there are hopes that molecular methods could greatly shorten the selective breeding time as compared to traditional breeding, which need several years.
Conclusions
In this study, we identified and isolated TDFs in the seed development of R. communis using cDNA-AFLP analysis, which detected many genes involved in different seed developmental phases. One TDF, identified as PDC, was isolated 60 days after pollination, and the fatty acid content significantly increased at later developmental stages. These results provided evidence that fatty acid synthesis in castor bean mainly occurred in the later seed developmental stages.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Zou Z, Gong J, Huang Q, et al. Gene structures, evolution, classification and expression profiles of the aquaporin gene family in castor bean (Ricinus communis L.). Plos One. 2015;10:e0141022; DOI: 10.1371/journal.pone.0141022
- Campbell DN, Na CI, Rowland DL, et al. Development of a regional specific crop coefficient (Kc) for castor (Ricinus communis L.) in Florida, USA by using the sap flow method. Industr Crop Product. 2015;74:465–471.
- Severino LS, Auld DL, Baldanzi M, et al. A review on the challenges for increased production of castor. Agron J. 2012;104:931–938.
- Huang FL, Zhu GL, Chen YS, et al. Seed characteristics and fatty acid composition of castor (Ricinus communis L.) varieties in Northeast China. Phyton. 2015;84:26–33.
- Anjani K. Castor genetic resources: a primary gene pool for exploitation. Industr Crop Product. 2012;35:1–14.
- Huang F, Bao C, Peng M, et al. Chromatographic analysis of fatty acid composition in differently sized seeds of castor accessions. Biotechnol Biotechnol Equip. 2015;29:892–900.
- Lin JT, Arcinasc A. Regiospecific analysis of diricinoleoylacylglycerols in castor (Ricinus communis L.) oil by electrospray ionization-mass spectrometry. J Agric Food Chem. 2007;55:2209–2216.
- Xiao D, Liu ST, Wei YP, et al. cDNA-AFLP analysis reveals differential gene expression in incompatible interaction between infected non-heading Chinese cabbage and Hyaloperonospora parasitica. Hortic Res. 2016 [cited 2018 Jan 16];3:16034; DOI:10.1038/hortres.2016.34
- Vuylsteke M, Peleman JD, Eijk MJV. AFLP-based transcript profiling (cDNA-AFLP) for genome-wide expression analysis. Nat Protoc. 2007;2:1399–1413.
- Bachem CWB, Oomen RJFJ, Visser RGF. Transcript Imaging with cDNA-AFLP: a Step-by-Step Protocol. Plant Mol Biol Rep. 1998;16:157–157.
- Diego JGD, Rodríguez FD, Lorenzo JLR, et al. cDNA-AFLP analysis of seed germination in Arabidopsis thaliana identifies transposons and new genomic sequences. J Plant Physiol. 2006;163:452–462.
- Botha FC, Burger AL, Venter M. Molecular analysis of fruit ripening: the identification of differentially expressed sequences in Vitis vinifera using cDNA-AFLP technology. Vitis -Geilweilerhof. 2001;40:191–196.
- Amini S, Maali-Amiri R, Mohammadi R. cDNA-AFLP analysis of transcripts induced in chickpea plants by TiO 2 nanoparticles during cold stress. Plant Physiol Biochem. 2016;111:39–49.
- Xu F, Peng M, Luo Q, et al. Isolation and detection of transcript-derived fragments (TDFs) in NaCl-stressed black locust (Robinia pseudoacacia L.) using cDNA-AFLP analysis. Acta Physiol Plant. 2015;37:1–8.
- Chen X, Peng M, Huang F, et al. A quantitative assay for fatty acid composition of castor seed in different developmental stages. Mol Plant Breed. 2016;7:1–8.
- Vos P, Hogers R, Bleeker M, et al. AFLP: a new technique for DNA fingerprinting. Nucl Acids Res. 1995;23:4407–4414.
- Zhou C, Guo Y, Xie L, et al. Oprimization of AFLP system for hot pepper induced by space. North Horticult. 2011;21:103–105.
- Chen GQ, Ahn Y, He X, et al. Quantitative transcript profiling of lipid biosynthesis genes in developing castor seeds [Abstract]. National Plant Lipid Cooperative Meeting, USA; 2005. Available from: https://www.ars.usda.gov/research/publications/publication/?seqNo115=180014
- Rantong G, Kelen KVD, Breusegem FV, et al. Identification of differentially expressed genes during lace plant leaf development. Int J Plant Sci. 2016;177:419–431.
- Leymarie J, Corbineau F. Identification of transcripts potentially involved in barley seed germination and dormancy using cDNA-AFLP. J Exp Bot. 2007;58:425–437.
- Davis MS, Solbiati J, Cronan JE Jr. Overproduction of acetyl-CoA carboxylase activity increases the rate of fatty acid biosynthesis in Escherichia coli. J Biol Chem. 2000;275:28593–28598.
- Turnham E, Northcote DH. Changes in the activity of acetyl-CoA carboxylase during rape-seed formation. Biochem J. 1983;212:223–229.
- Charles DJ, Cherry JH. Purification and characterization of acetyl-CoA carboxylase from developing soybean seeds. Phytochemistry. 1986;25:1067–1071.
- Simcox PD, Garland W, Deluca V, et al. Respiratory pathways and fat synthesis in the developing castor oil se. Can J Bot. 2011;57:1008–1014.
- Patel MS, Roche TE. Molecular biology and biochemistry of pyruvate dehydrogenase complexes. FASEB J. 1990;4:3224–3233.
- Sutendra G, Kinnaird A, Dromparis P, et al. A nuclear pyruvate dehydrogenase complex is important for the generation of acetyl-CoA and histone acetylation. Cell. 2014;158:84–97.
- Randall DD. Purification and characterization of the pea chloroplast pyruvate dehydrogenase complex : a source of acetyl-CoA and NADH for fatty acid biosynthesis. Plant Physiol. 1985;77:571–577.
- Smith RG, Gauthier DA, Dennis DT, et al. Malate- and pyruvate-dependent fatty acid synthesis in leucoplasts from developing castor endosperm. Plant Physiol. 1992;98:1233–1238.
- Rapp BJ, Randall DD. Pyruvate dehydrogenase complex from germinating castor bean endosperm. Plant Physiol. 1980;65:314–318.
- Parra O, Gallego AM, Urrea A, et al. Biochemical precursor effects on the fatty acid production in cell suspension cultures of Theobroma cocao L. Plant Physiol Biochem. 2017;111:59–66.
- Ke J, Behal RH, Back SL, et al. The role of pyruvate dehydrogenase and acetyl-coenzyme A synthetase in fatty acid synthesis in developing Arabidopsis seeds. Plant Physiol. 2000;123:497–508.
- Yao L, Hui S, Nan W, et al. Elevated acetyl-CoA by amino acid recycling fuels microalgal neutral lipid accumulation in exponential growth phase for biofuel production. Plant Biotechnol J. 2017;15:497–509.
