Abstract
Phytoene synthase (PSY) plays a key role in the biosynthesis and accumulation of carotenoids as the first rate-limiting enzyme in the plant carotenoid pathway. In the present study, the ‘Granny Smith’ apple PSY gene, MdPSY, was cloned using in silico cloning. The length of the MdPSY was 1500 bp, which included a complete open reading frame of 1194 bp, encoding a predicted protein of 397 amino acids. Sequence analysis revealed that the ‘Granny Smith’ apple PSY protein shared high homology with MdPSY2 (99%), MdPSY1 (93.9%), FaPSY (71.8%), CuPSY (70.7%) and NtPSY (69.7%). Phylogenetic analysis showed that the MdPSY protein was closely related to the PSY protein of Rosaceae. An expression profile analysis of different tissues indicated that MdPSY expression in the roots was higher than that in other tissues. MdPSY expression was tested throughout the fruit development period. The results showed that MdPSY plays a role in carotenoid biosynthesis in ‘Granny Smith’ apple. In addition, bagging treatment inhibits PSY gene expression, which in turn affects the synthesis and accumulation of carotenoids.
Introduction
Fruit colour is a significant agronomic character of apples that directly affects their commodity value [Citation1]. Carotenoids are a key component of apple fruit colour and are important pigments in many flowers and fruits, being responsible for bright colours that attract insects, birds and feeding, contributing to the spread of pollen and seeds [Citation2–4]. In addition, as auxiliary pigment for photosynthesis, carotenoids play an important role in light absorption, light protection and free-radical scavenging in plants [Citation4]. As the biosynthetic precursor of abscisic acid (ABA), strigolactone, vitamin A and other physiologically active substances in humans and animals, carotenoids are closely related to plant growth and development and human health [Citation3].
In view of the importance of carotenoids for plants and human health, research on their metabolic mechanisms has become a hot topic in the study of plant secondary metabolism over the past two decades [Citation4,Citation5]. With the advent of molecular biology-related technologies, the biochemical pathway of plant carotenoid biosynthesis has been elucidated, and carotenogenic genes have been isolated from many plants [Citation6,Citation7]. Phytoene synthase (PSY) has been confirmed to act as the rate-limiting enzyme in the accumulation of carotenoids [Citation7,Citation8]. In a recent study, six paralogous genes in the apple PSY gene family have been separated. Among these genes, PSY1 and PSY2 have been identified as being directly involved in apple carotenoid accumulation [Citation9].
Previous studies have shown that carotenoid biosynthesis and accumulation are closely related to the expression levels of carotenogenic genes [Citation10,Citation11]. However, the expression levels of plant carotenoid biosynthesis genes are related to plant genotypes [Citation8,Citation12,Citation13]. In addition, the levels of these genes are affected by a variety of external environmental factors [Citation14]. Light is reported to be an important environmental factor that regulates carotenoid metabolism in plants [Citation14,Citation15]. In Arabidopsis seedlings, the production of carotenoids is boosted by biosynthetic gene expression and enzyme activity levels during deetiolation after shading [Citation16]. The recent research by Dong et al. [Citation17] showed that the expression levels of PSY2 in bagged peach fruits at maturity were lower than those in non-bagged fruits. Now, bagging is the most common technique used for improving fruit quality. Our previous research has found that bagging treatment significantly promoted the synthesis and accumulation of anthocyanins in ‘Granny Smith’ apples [Citation1]. However, the effect of bagging on the synthesis and accumulation of carotenoids is not yet clear.
In the present study, the PSY gene of ‘Granny Smith’ apples was cloned using in silico cloning, based on which the expression characteristics of PSY in different tissues of ‘Granny Smith’ apples and in fruits at different stages of maturity were analyzed. Then, the correlation between the expression level of PSY and carotenoid biosynthesis was analyzed based on the determination of physiological and biochemical indices. This study could help to clarify the molecular mechanism of carotenoid accumulation in apples and provide a theoretical foundation for the scientific regulation of apple carotenoid biosynthesis.
Materials and methods
Plant material and experimental treatment
The 8-year-old ‘Granny Smith’ apple trees used in this experiment were grafted on M26 rootstock and grown at the orchard of the Northwest A&F University apple experimental station in Baishui, Shaanxi Province. For the bagging treatment, each fruit was bagged with double-layer paper bags (Hong Tai, China) at 40 days after full bloom (DAFB). The bags were removed at 165 DAFB (20 days before harvest), and the fruits in the bagged group were collected at 0, 2, 4, 6, 8, 10 and 15 days after bag removal (DABR). The control group fruits were collected at the same time point. At each time point, 10 individual fruits were collected from different locations in the tree canopy. For measurement of the colour parameter, the peels were carefully removed with a knife, including 1 mm of cortical tissue, and then immediately frozen in liquid nitrogen and stored at −80 °C for carotenoids and RNA extraction. In addition, the leaves (young and old), floral buds, root and fruits (55, 75 and 135 DAFB) of the ‘Granny Smith’ apple were collected for gene expression analysis of the different tissues.
Determination of fruit peel colour
The colour parameter of apple peel was measured using a Minolta chromameter (Model CR-400, Minolta, Osaka, Japan). Five fruit samples were measured at each time point, and peel colour was measured at three locations around the equatorial plane of each fruit. Fruit chromaticity was recorded in terms of the CIE parameters a* and b*, which are colour space coordinates [Citation18]. The hue angle (h°) was calculated according to the following equation: h° = arctan (b*/a*).
Carotenoids and chlorophylls extraction
The method of extracting carotenoids and chlorophylls was described previously by Ampomah-Dwamena et al. [Citation19]. Briefly, a sample of lyophilized fruit peel (1–2 g) was ground to a fine powder with a mortar containing liquid nitrogen then homogenized in 5 mL of cold acetone with 0.1% butylated hydroxytoluene and stored in the dark at 4 °C overnight. The reactants were subsequently centrifuged using a 5810R centrifuge (Eppendorf, Hamburg, Germany) at 12,000 rpm at 4 °C for 20 min. The absorbance of the extracted supernatant was measured using a UV-2550 spectrophotometer at 470, 645 and 663 nm. The chlorophylls and carotenoids contents were calculated using the following equations: chlorophyll a = 12.21 A663 nm–2.81 A645 nm; chlorophyll b = 20.13 A645 nm–5.03 A663 nm; carotenoids = (1000 A470 nm–3.27 Chla–104 Chlb)/229.
Total RNA extraction and cDNA synthesis
Total RNA was extracted from apple root, leaves and floral buds using the TRIzol® reagent (Invitrogen), and total RNA was isolated from the pericarp using the hot borate method [Citation20]. The first strand of cDNA was synthesized using the PrimeScript® RT reagent Kit (TaKaRa, Dalian) for gene cloning and expression analysis.
Gene cloning and bioinformatics analysis
The PSY gene of ‘Granny Smith’ apple was cloned using in silico cloning. The method was described previously by Lu [Citation21]. The expressed sequence tag (EST) sequence (JK750344) was used as a seed sequence to blast in the Malus domestica EST database. Based on the cDNA sequence of the apple PSY gene obtained by splicing, specific primers for cloning the ‘Granny Smith’ apple PSY gene were designed using Primer Premier 5.0 software. The forward and reverse primer sequences were 5′-ACTGCTTTCAAAACTTTGCCTTACCC-3′ and 5′-CACATCTATCAACCACTCCATGGC-3′, respectively. The gene sequence was amplified via reverse transcription polymerase chain reaction (RT-PCR) using Platinum® Pfx DNA Polymerase (Invitrogen) under the following reaction conditions: 2 min at 94 °C; 40 cycles of 15 s at 94 °C, 30 s at 58 °C and 2 min at 68 °C; and a 7 min extension at 68 °C on a Veriti™ 96-Well Thermal Cycler (ABI, CA, USA). The obtained PCR fragments were cloned into the pGEM®-T Easy Vector (Promega) and sequenced by Huada Biotech Co., Ltd (Beijing, China). DNAMAN software was used for amino acid sequence alignment, and the phylogenetic tree was constructed using neighbour-joining in Mega5.1 software.
Quantitative real-time PCR analysis
The expression levels of PSY in ‘Granny Smith’ apple were detected via qRT-PCR using SYBR green master mix (Takara, Japan) and an IQ5 real-time PCR cycler (Bio-Rad Laboratories, Hercules, CA, USA) in a reaction volume of 25 µL. The apple Actin gene (AB638619) was used as the housekeeping gene. The following primer sequences were employed: forward primer 5′-TGTGTTTCCTAGTATTGTTGGTCGC-3′, reverse primer 5′-GCCAGATCTTCTCCATGTCATCC-3′. The specific primer sequences used for PSY expression analysis were as follows: 5′-TGAAGTCTGTGCTGAGTATGCCAAA-3′ and 5′-TGTATGAAGCATTAGGTCCATCCACT-3′. The reaction conditions were as follows: 3 min at 95 °C; 40 cycles of 10 s at 95 °C, 30 s at 58 °C and 30 s at 72 °C; and a final step of 7 min at 72 °C. Each reaction was repeated three times. The relative expression levels of the target genes were calculated using the 2–△△Ct formula [Citation22].
Results and discussion
Molecular cloning and homology analysis of MdPSY
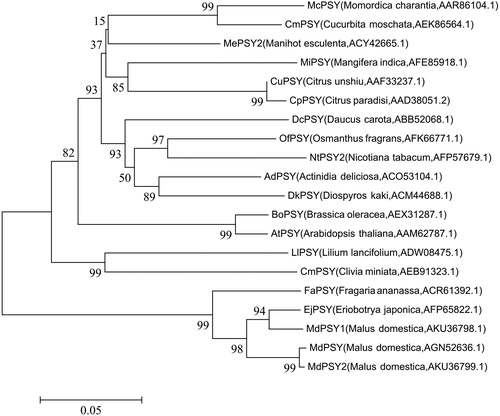
The full length cDNA sequence of ‘Granny Smith’ apple PSY was 1500 bp, including a complete open reading frame (ORF) of 1194 bp, which encoded a predicted protein of 397 amino acid residues (), the gene was designated MdPSY and has been submitted to GenBank (accession no.: KC503839). Homology analysis of the amino acid sequences deduced from the complete ORF of the PSY gene from MdPSY and other PSYs showed that the MdPSY sequence shared similarity of 99% with MdPSY2 from Malus domestica, 93.9% with MdPSY1 from Malus domestica, 89.4% with FaPSY from Fragaria ananassa, 71.8% with CuPSY from Citrus unshiu, 70.7% with NtPSY from Nicotiana tabacum and 69.7% with DcPSY from Daucus carota (). The phylogenetic tree showed that the MdPSY protein was closely related to the PSY protein of Rosaceae and clustered in the same category ().
Changes in colour parameter values in ‘Granny Smith’ fruit peel
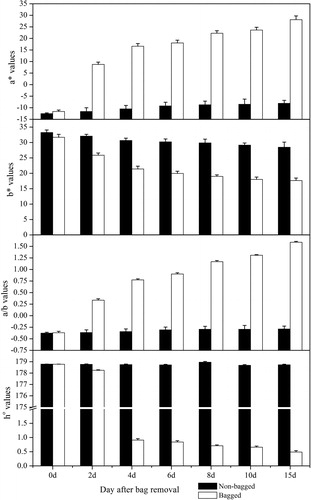
To understand the effect of bagging on the ‘Granny Smith’ fruit peel colour, the colour parameters (a*, b* and ho) were measured and analyzed. As shown in , the values of a*, b*, a/b and ho remained stable in the control group. This finding was consistent with the observed changes in ‘Granny Smith’ apple fruit colour, which remained green at the maturation stage. After bag removal, the fruit colour gradually became red, and the colour parameters changed as well [Citation1]. The b* values decreased gradually, while the ho value sharply decreased, from 178.78 (0 DABR) to 0.49 (15 DABR). The value of a* (negative to positive, from green to red) increased from –11.61 (0 DABR) to 28.13 (15 DABR). The a/b ratio is negative for green, zero for yellow, and positive for orange [Citation19]. In the present study, the obtained a/b ratios were consistent with the visual fruit colour phenotypes ().
Carotenoids and chlorophylls contents
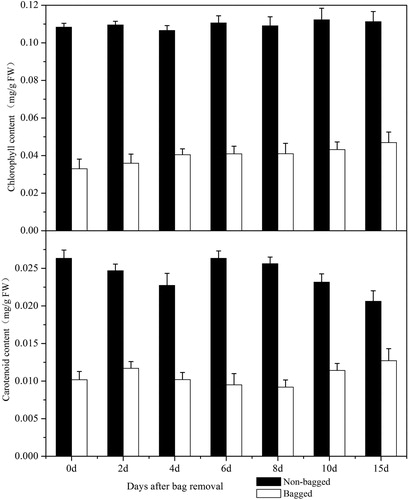
The contents of carotenoids and chlorophylls in ‘Granny Smith’ apple skin in bagged fruits were found to be significantly decreased compared with the non-bagged group after bag removal (), and the observed changes in carotenoids and chlorophylls were consistent with the colour parameters. The contents of carotenoids and chlorophylls in the control group apple skin were relatively stable, being maintained at 0.025 and 0.11 mg/g (FW), respectively. The changes in chlorophyll and carotenoid contents in the treatment group were similar to those observed in the non-bagged fruits and were maintained at 0.01 and 0.04 mg/g (FW), respectively. These results suggested that the light-shading environment created by bagging significantly affected the accumulation of carotenoids and chlorophylls in mature fruits [Citation17].
Expression of the PSY gene in different organs of ‘Granny Smith’ fruit
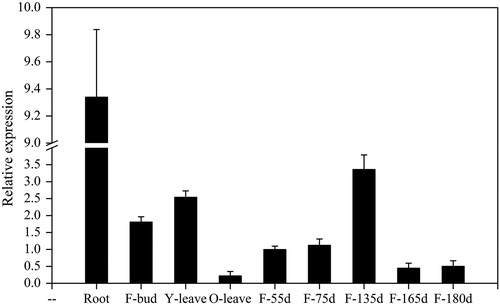
To clarify the expression characteristics of the PSY gene in ‘Granny Smith’ apples, the transcript levels of MdPSY were tested in different tissues and in the fruit skin at different developmental stages (). The expression level of MdPSY was higher in roots (9.34) than in other organs and was lowest in old leaves (0.22). These findings were consistent with previous findings in ‘Royal Gala’ apples [Citation9]. Galpaz et al. [Citation23] suggested that there are two synthetic pathways for carotenoids in plants: the chloroplast pathway and the chromosomal pathway. However, there are differences in the regulatory mechanisms and accumulation patterns of carotenoids in these two pathways. This theory provides a reasonable explanation for the higher expression of PSY found in the roots than that in leaves. In addition, the analysis of MdPSY expression throughout the developmental period in fruits revealed relatively low expression in young fruit, while the highest expression was found at 135 DAFB, after which the expression stabilized and then declined rapidly in the mature phase (). This pattern suggested that MdPSY is involved in ‘Granny Smith’ apples carotenoid biosynthesis and is regulated by fruit developmental stages, which is consistent with findings from bitter melon and ‘Royal Gala’ apples [Citation7,Citation9].
Gene expression of PSY in ‘Granny Smith’ fruit skin after bag removal
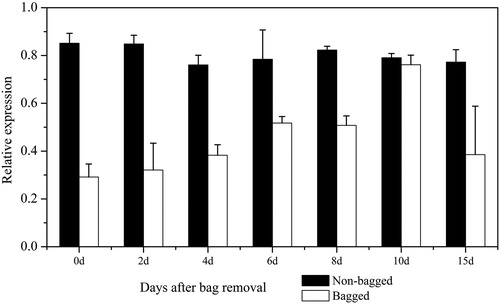
Previous studies have shown that light is one of the key factors regulating plant carotenoid accumulation [Citation3]. In the present study, the expression levels of MdPSY in ‘Granny Smith’ fruit skin after bag removal were assayed to analyze the effects of light on the biosynthesis and accumulation of carotenoids (). The data indicated that the MdPSY expression in the non-bagged fruits tended to be stable and was maintained at 0.8. However, the levels in the bagged fruits were down-regulated. The expression level was relatively low at the early stage after bag removal and then increased with the increase of fruit colour, with maximum expression appearing at 10 DABR. The results further confirmed that light is involved in the regulation of PSY gene expression [Citation17].
Conclusions
Carotenoids are important pigments in apple fruit and play an important role in determining apple fruit colour. In this study, the PSY gene of apple, MdPSY, was successfully cloned from the peel of ‘Granny Smith’ apple, and bioinformatics analysis of MdPSY was performed. In addition, the expression characteristics of ‘Granny Smith’ apple in different tissues and in the pericarp during fruit coloration were analyzed. The results showed that MdPSY plays a role in carotenoid biosynthesis in apples, and lay a theoretical foundation for further research on the molecular mechanism of carotenoid synthesis in apple fruit.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Liu YL, Zhang XJ, Zhao ZY. Effects of fruit bagging on anthocyanins, sugars, organic acids and color properties of ‘Granny Smith’ and ‘Golden Delicious’ during fruit maturation. Eur Food Res Technol. 2013;236:329–339.
- Lancaster JE, Dougall DK. Regulation of skin color in apples. Crit Rev Plant Sci. 1992;10:487–502.
- Cazzonelli CI, Pogson BJ. Source to sink: regulation of carotenoid biosynthesis in plants. Trends Plant Sci. 2010;15:266–274.
- Nisar N, Li L, Lu S, et al. Carotenoid metabolism in plants. Mol Plant. 2015;8:68–82.
- Fraser PD, Bramley PM. The biosynthesis and nutritional uses of carotenoids. Prog Lipid Res. 2004;43:228–265.
- Xu J, Tao NG, Cao HB, et al. Presence of two variants of lycopene β-cyclase gene in genomes of citrus and its relatives. Biotechnol Biotechnol Equip. 2011;25(3):2452–2457.
- Tuan PA, Kim JK, Park NI, et al. Carotenoid content and expression of phytoene synthase and phytoene desaturase genes in bitter melon (Momordica charantia). Food Chem. 2011;126:1686–1692.
- Zhang L, Zhang ZK, Zheng TT, et al. Characterization of carotenoid accumulation and carotenogenic gene expression during fruit development in yellow and white loquat fruit. Hortic Plant J. 2016;2(1):9–15.
- Ampomah-Dwamena C, Driedonks N, Lewis D, et al. The phytoene synthase gene family of apple (Malus domestica) and its role in controlling fruit carotenoid content. BMC Plant Biol. 2015;15:185. DOI:10.1186/s12870-015-0573-7
- Fu XM, Kong WB, Peng G, et al. Plastid structure and carotenogenic gene expression in red- and white-fleshed loquat (Eriobotrya japonica) fruits. J Exp Bot. 2012;63:341–354.
- Wisutiamonkul A, Ampomah-Dwamena C, Allan AC, et al. Carotenoid accumulation and gene expression during durian (Durio zibethinus) fruit growth and ripening. Sci Hortic. 2017;220:233–242.
- Fu CC, Han YC, Fan ZQ, et al. The papaya transcription factor CpNAC1 modulates carotenoid biosynthesis through activating phytoene desaturase genes CpPDS2/4 during fruit ripening. J Agric Food Chem. 2016;64:5454–5463.
- Chan-León AC, Estrella-Maldonado H, Dubé P, et al. The high content of β-carotene present in orange-pulp fruits of Carica papaya L. is not correlated with a high expression of the CpLCY-β2 gene. Food Res Int. 2017;100:45–56.
- Llorente B, Martinez-Garcia JF, Stange C, et al. Illuminating colors: regulation of carotenoid biosynthesis and accumulation by light. Curr Opin Plant Biol. 2017;37:49–55.
- Wu MC, Hou CY, Jiang CM, et al. A novel approach of LED light radiation improves the antioxidant activity of pea seedlings. Food Chem. 2007;101:1753–1758.
- Meier S, Tzfadia O, Vallabhaneni R, et al. A transcriptional analysis of carotenoid, chlorophyll and plastidial isoprenoid biosynthesis genes during development and osmotic stress responses in Arabidopsis thaliana. BMC Syst Biol. 2011;5:77. DOI:10.1186/1752-0509-5-77
- Dong XT, Cao HB, Zhang FY, et al. [Effects of shading fruit with opaque paper bag on carotenogenesis and related gene expression in yellow-flesh peach]. Acta Hortic Sin. 2015;42 (4):633–642. Chinese.
- Mcguire RG. Reporting of objective color measurements. HortScience. 1992;27:1254–1255.
- Ampomah-Dwamena C, McGhie T, Wibisono R, et al. The kiwifruit lycopene beta-cyclase plays a significant role in carotenoid accumulation in fruit. J Exp Bot. 2009;60:3765–3779.
- Yao YX, Zhao LL, Hao YJ, et al. [Effective extraction of total RNA in apple flesh with improved hot borate method]. J Fruit Sci. 2005;22(6):737–740. Chinese.
- Lu LM. [In silico cloning and bioinformatic analysis of TPK1 gene in tobacco]. Sci Agric Sin. 2011;44(1):28–35. Chinese.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25:402–408.
- Galpaz N, Ronen G, Khalfa Z, et al. A chromoplast-specific carotenoid biosynthesis pathway is revealed by cloning of the tomato white-flower locus. Plant Cell. 2006;18:1947–1960.