Abstract
This aim of this study was to investigate the effects of Mycoplasma genitalium lipoproteins on the tumour necrosis factor α (TNF-α)-induced apoptosis in HeLa cells. We separated the M. genitalium lipid-associated membrane proteins (LAMPs) from M. genitalium and used them to treat HeLa cells. Apoptosis was induced by TNF-α treatment. Cell cycle and apoptosis were detected by flow cytometry. Pro-inflammatory cytokine contents were determined by enzyme-linked immunosorbent assay (ELISA). Mitochondrial membrane potential and caspase-3 protease activity were also measured. There was no significant difference in the viable cell percentage in HeLa cells treated with M. genitalium LAMPs at 0–20 μg/mL. However, when treated with 40 μg/mL LAMPs, cytocidal activity was observed during the first 24 h. M. genitalium LAMPs induced cell cycle arrest at the G1 phase in HeLa cells. When apoptosis was induced by TNF-α in HeLa cells, M. genitalium LAMPs significantly reduced the percentage of apoptotic cells. In addition, M. genitalium LAMPs significantly reduced the loss of mitochondrial membrane potential and caspase-3 protease activity in TNF-α-treated HeLa cells. However, the blocking of NF-κB significantly increased the mitochondrial membrane potential and caspase-3 protease activity in TNF-α-induced HeLa cells treated with M. genitalium LAMPs. The obtained results suggest that M. genitalium LAMPs could inhibit TNF-α-induced apoptosis in HeLa cells, which would contribute to the understanding of the pathogenesis of M. genitalium.
Introduction
Mycoplasmas are bacteria with the smallest genome (580–2200 kb), with a relatively high A + T content (67–76%) [Citation1]. With this basic genome, the bacteria have to obtain most nutrients from the host cells. Mycoplasma genitalium is a sexually transmitted pathogen, which was first isolated from males with nongonococcal urethritis (NGU) in 1981 [Citation2]. Researches show that there is an association between the inflammation of female genital tracts and the M. genitalium infection [Citation3]. Even though mycoplasmas are wall-less bacteria, lacking classical modulins (such as lipopolysaccharides, lipoteichoic acid and murein fragments) [Citation4], they are still potent activators for macrophages. Lipoproteins, or lipopeptides, are important pathogenic elements in various biological processes [Citation5,Citation6]. Moreover, the surface adhesin proteins (P140 and P110) are other pathogenic factors [Citation7,Citation8]. However, few host cell models have been established to study the direct invasion and pathogenesis of M. genitalium.
At present, no consensus has been reached about the relationship between mycoplasma infections and apoptosis. Several bacterial lipoproteins could induce apoptosis in HEK293 and THP-1 monocytic cells through toll-like receptor 2 (TLR2) [Citation9]. Apoptosis induced by TLR2 involves the interaction between the death domain of MyD88 and the FADD component from the tumour necrosis factor (TNF) pathway, leading to the activation of caspase-8. However, in another research, cell death could not be observed in the HEK293 cells, indicating that the activation of apoptosis would be sensitive to the culture conditions and treatments [Citation10]. The apoptotic process is known to be regulated by p38 MAPK, MyD88 and FADD. A kinase-inactive mutant of apoptosis signal-regulating kinase (ASK1, an upstream activator for p38 MAPK in the TLR2 signalling pathway) could impair the sustained phosphorylation and activation of p38 MAPK induced by mycoplasma lipoproteins, which attenuates the transcriptional activities of NF-κB and AP-1 [Citation11]. Moreover, mycoplasma lipoprotein-induced cell death, involving DNA fragmentation and caspase-3/7 activation, is also down-regulated by the ASK1 mutant through the inhibition of p38 MAPK [Citation11].
On the other hand, several studies have shown that the mycoplasma infection would inhibit the apoptotic process. For example, M. fermentans could inhibit the TNF-α-induced apoptosis in the human myelomonocytic U937 cells, which is upstream to mitochondria and caspase-8 [Citation12]. Moreover, M. fermentans lipoproteins could inhibit the apoptosis induced by TNF-α in U937 cells [Citation13]. However, the pathogenic mechanisms of M. genitalium have not yet been fully elucidated. In this study, the relationship between M. genitalium lipoproteins and the induction of apoptosis was investigated in the HeLa cells.
Materials and methods
Cell line and cell culture
HeLa cells were purchased from the ATCC (Manassas, VA, USA) and were cultured in Dulbecco’s modified Eagle’s medium (DMEM; HyClone, Logan, UT, USA), containing 10% fetal bovine serum (FBS; HyClone) and 1% penicillin-streptomycin (St. Louis, MO, USA), in a 37 °C, 5% CO2 incubator. These cells were tested every 4 weeks with polymerase chain reaction (PCR) for Mycoplasma contamination.
Mycoplasma culture and lipoproteins preparation
M. genitalium (G-37 strain; ATCC) was grown in the modified SP-4 medium until the stationary phase and the cells were then pelleted by centrifugation. Lipoproteins and aqueous phase proteins (used as control) were prepared according to the previously described method [Citation12,Citation14]. Lipoproteins in the Triton X-114 phase were precipitated by methanol and re-suspended in phosphate buffered saline (PBS) for the stimulation treatment. Protein concentration was determined by a bicinchoninic acid kit (Pierce, Oud-Beijerlands, the Netherlands). In all the experiments, endotoxin-free deionized water was used for reagent preparation and detection tests.
SDS-PAGE and silver staining
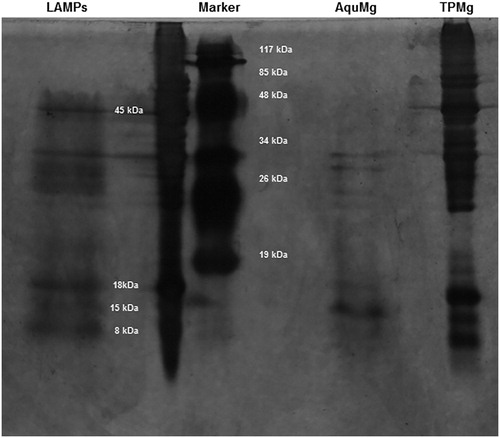
Samples containing equivalent amounts of M. genitalium total proteins (TPMg), lipid-associated membrane proteins (LAMPs) in the TX-114 phase (LPMg) and aqueous phase proteins (hydrophilic proteins, AqMg) were separated by 15% sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE;20 μg each lane) in a mini-gel system (Bio-Rad, Hercules, CA, USA). After electrophoresis, the gel was subjected to silver staining, according to the previously described procedure [Citation15,Citation16]. Briefly, the gel was fixed in 50% methanol and 12% (v/v) acetic acid overnight, followed by incubation in 10% glutaraldehyde for 30 min. After washing with distilled water three times (5 min each time), the gel was treated with silver nitrate for 20 min. The reaction was developed in 2.5% sodium carbonate containing 0.5 mL/L 37% paraformaldehyde, and was stopped by adding 2.3 mol/L citric acid when the bands were clearly visible.
M. genitalium LAMPs stimulation and cell treatments
HeLa cells (1.5 × 105 cells/mL) were seeded onto 6-well plates (Corning, Corning, NY, USA) and cultured for 12 h. Before stimulation with M. genitalium LAMPs, the culture supernatant was removed and replaced with fresh medium. For stimulation, 0, 3, 10, 20 and 40 μg/mL M. genitalium LAMPs was added to treat the HeLa cells. To block the NF-κB secretion, pyrrolidine dithiocarbamate (PDTC) (50 μmol/mL) or TLR2-Ab (1 μmol/mL) was used to treat HeLa cells, at 2 h before the stimulation of M. genitalium LAMPs and apoptosis induction.
Measurements of TNF-α and IL-6
After 24 h stimulation as described above, the cells were lysed by two consecutive cycles of freezing/thawing, and therefore the samples represented the total amount of cytokines produced (both intracellular and those released into the supernatant). The contents of TNF-α, IL-6 and IL-8 in the samples were measured with the enzyme-linked immunosorbent assay (ELISA) kits (Jingmei Biotech, Shenzhen, Guangdong, China), according to the manufacturer’s instructions.
Cell cycle analysis
After 24 h stimulation, HeLa cells were collected and washed twice with PBS. Then these cells were fixed with 1 mL 70% cold ethanol at −20 °C for 24 h. After centrifugation, the pellets were washed and then stained with 150 μg/mL propidium iodide (PI). After incubation in darkness for 30 min, the cells were analysed by a FACS flow cytometer (Beckman Coulter, Inc., Brea, CA, USA).
Apoptosis induction and detection
Apoptosis in HeLa cells was induced with 10 ng/mL TNF-α (R&D, Minneapolis, MN, USA), together with 10 μg/mL cycloheximide (CHX; Sigma, St. Louis, MO, USA), which was used to increase the sensitivity of the cells to TNF-α induction. For the detection of apoptosis, annexin-V-FITC-PI staining was performed, which was able to distinguish between the living, early apoptotic and dead cells [Citation18]. At 4 h after induction, cells were washed with PBS, centrifuged and re-suspended in 400 μL binding buffer (10 mmol/L HEPES/NaOH, 140 mmol/L NaCl, 2.5 mmol/L CaCl2, pH 7.4). In total, 5 μL annexin-V-FITC (10 μg/mL) was added into 195 μL cell suspension. After mixing and incubation in darkness at room temperature for 10 min, the cells were washed with PBS and re-suspended in 190 μL binding buffer containing 10 μL PI (1 μg/mL). Within 10 min, the fluorescence was analysed by flow cytometry (104 cells/sample). Double-stained cells represented late apoptotic cells, rather than necrotic cells. The percentage of apoptotic cells was determined by subtracting the spontaneous apoptotic (untreated) cells from the total apoptotic (treated) cells.
Mitochondrial membrane potential measurement
At 2-h induction with TNF-α, the cells were washed and re-suspended in 1 mL PBS containing 10 μg/mL Rhodamine123 (Rh123; Sigma). After incubation at 37 °C for 30 min, the cells were washed twice and re-suspended in 500 μL PBS. The fluorescence was detected using flow cytometry.
Caspase-3 protease activity assessment
Caspase-3 activities were determined using the assay kit (Beyotime, Haimen, Jiangsu, China), which detected the production of the chromophore p-nitroanilide after its cleavage from the peptide substrate DEVD-p-nitroanilide and LEHD-p-nitroanilide.
Statistical analysis
Data are expressed as mean values with standard deviation (±SD). One-way analysis of variance (ANOVA) was used for group comparison with the IBM SPSS Statistics software (version 24). p < .05 was considered statistically significant.
Results and discussion
M. genitalium LAMPs at low concentrations show no cytocidal activity in HeLa cells
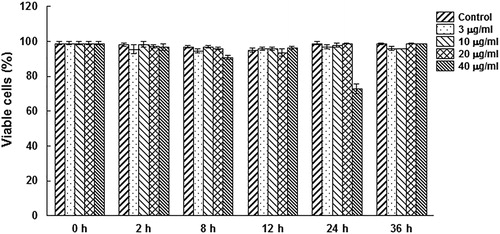
Previous studies have shown that mycoplasma lipoproteins could induce apoptosis in both HEK293 and THP-1 monocytic cells through the TLR2 signalling pathway [Citation9]. In this study, whether M. genitalium lipoproteins could induce cell death in HeLa cells was investigated. The M. genitalium proteins were separated, and then subjected to silver staining. The results showed four protein bands, i.e. P45, P18, P15 and P8 in the LAMPs (), which were different from hydrophilic proteins (AquMg). P45 and P15 were mentioned in previous reports [Citation17,Citation18]. The HeLa cells were treated with 3–40 μg/mL M. genitalium LAMPs, and the cell death was measured with annexin-V-FITC and PI staining by flow cytometry. As shown in , there was no significant difference in the viable cell percentage for the treatment concentrations of 0–20 μg/mL. However, at the highest concentration (40 μg/mL), cytocidal activity was observed during the first 24 h, and the activity gradually declined as the treatment duration increased. These results suggest that M. genitalium LAMPs at low concentrations show no detectable cytocidal activity in HeLa cells.
M. genitalium LAMPs induces cell cycle arrest in HeLa cells
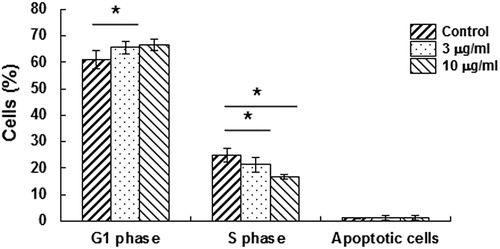
To investigate whether M. genitalium LAMPs affected the proliferation of HeLa cells, flow cytometry was performed at 24 h after treatment. Our results () showed that there was a decrease in the percentage of cells at the S phase, while a corresponding increase was observed in the percentage of cells at the G1 phase (p < .05). This suggests that M. genitalium LAMPs could induce a cell cycle arrest at the G1 phase in HeLa cells.
Effect of M. genitalium LAMPs on pro-inflammatory cytokine production in HeLa cells
It is well known that mycoplasma lipoproteins could activate the macrophages to release IL-6 and further increase the production of other cytokines [Citation19]. To determine the effects of M. genitalium LAMPs on HeLa cells, the production of cytokines (TNF-α, IL-6 and IL-8) was determined in these cells treated with M. genitalium LAMPs. Our results showed that, when the cells were stimulated with 10 μg/mL M. genitalium LAMPs, the content of TNF-α in the culture supernatant was 5.07 ± 2.564 pg/mL, which was negligible compared with the induction dosage. Moreover, the supernatant contents of IL-6 and IL-8 were 23.0644 ± 4.125 pg/mL and 8.126 ± 0.238 pg/mL, respectively.
M. genitalium LAMPs attenuate TNF-α-induced apoptosis in HeLa cells
Next we investigated the effects of M. genitalium LAMPs on TNF-α-induced apoptosis in HeLa cells. To prevent the interference of the stimulated production of TNF-α from the cells, the level of TNF-α in the HeLa cells treated with M. genitalium LAMPs for 24 h was determined. As shown in , TNF-α was secreted at a relatively low level in the HeLa cells treated with M. genitalium LAMPs. Even at the high treatment concentration of 100 μg/mL M. genitalium LAMPs, the content of TNF-α was still very low (18 pg/mL), as compared with the dosage of TNF-α used for the induction of apoptosis (10 ng/mL). Before the induction of apoptosis with TNF-α (10 ng/mL) together with CHX (10 μg/mL), these HeLa cells were first treated with M. genitalium LAMPs (10 μg/mL) for 5 h, and the percentage of apoptotic cells was examined with the annexin-V-FITC and PI staining. Our results () showed a significantly (p < .05) reduced percentage of apoptotic HeLa cells treated with M. genitalium LAMPs (9.36 ± 4.52%), in comparison to the untreated cells (15.51 ± 7.81%) (). This suggests that M. genitalium LAMPs could significantly inhibit the TNF-α-induced apoptosis in HeLa cells.
Figure 4. Effects of M. genitalium LAMPs on the production of pro-inflammatory cytokines in HeLa cells.
Note: HeLa cells were treated with 10 μg/mL M. genitalium LAMPs, and the contents of TNF-α, IL-6 and IL-8 were determined using ELISA. The cytokines of the LP group were increased, to varying degrees, compared with the PBS (control) group. Compared with the control group, *p < .05.
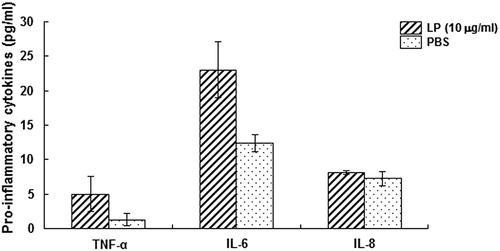
Figure 5. Effects of M. genitalium LAMPs on TNF-α-induced apoptosis in HeLa cells. Lower left quadrant, living cells (annexin V negative/PI negative); lower right quadrant, early/primary apoptotic cells (annexin V positive/PI negative); upper right quadrant, late/secondary apoptotic cells (annexin V positive/PI positive); upper left quadrant, necrotic cells (annexin V negative/PI positive). Representative results from each group.
Note: HeLa cells were first treated with M. genitalium LAMPs (10 μg/mL) for 5 h, and cellular apoptosis was induced with TNF-α (10 ng/mL) together with CHX (10 μg/mL). The apoptotic cells were examined with annexin-V-FITC-PI staining.
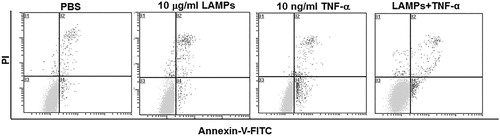
Table 1. Effect of M. genitalium LAMPs on TNF-α-induced cellular apoptosis in HeLa cells.
M. genitalium LAMPs reduce TNF-α-induced mitochondrial membrane potential loss in HeLa cells
Mitochondrial membrane potential (Δψm) collapse has been considered as an important event in the TNF-α-induced apoptotic process [Citation20,Citation21]. The effect of M. genitalium LAMPs on Δψm in the TNF-α-induced apoptosis was then investigated. The HeLa cells were treated with M. genitalium lipoproteins for 4 h, and stimulated with TNF-α (10 ng/mL) together with CHX (10 μg/mL) to induce apoptosis. Then Δψm was measured 2 h later by Rh123 staining and flow cytometry. Our results showed that M. genitalium LAMPs significantly (p < .05) reduced the loss of Δψm in the TNF-α-treated HeLa cells ().
Figure 6. Effect of M. genitalium LAMPs on TNF-α-induced mitochondrial membrane potential loss in HeLa cells.
Note: HeLa cells were treated with M. genitalium lipoproteins for 4 h, and stimulated with TNF-α (10 ng/mL) along with CHX (10 μg/mL) to induce apoptosis. Then Δψm was measured 2 h later, with Rh123 staining by flow cytometry. PDTC or TLR2-Ab was used to block NF-κB in the HeLa cells before M. genitalium lipoproteins treatment and apoptosis induction. Compared with the LAMPs + TNF-α group, *p < .05. MFI: mean fluorescence intensity.
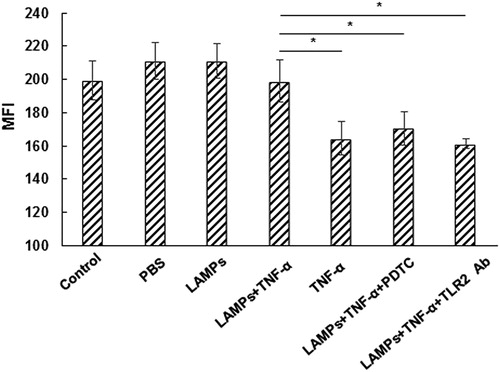
It is well known that PDTC can block the secretion of NF-κB, and that M. genitalium lipoproteins can activate the secretion of NF-κB through TLR2. Therefore, PDTC or TLR2-Ab was used to treat the HeLa cells before treatment of M. genitalium LAMPs and apoptosis induction, which significantly decreased (p < .05) Δψm in the TNF-α-induced HeLa cells treated with M. genitalium LAMPs (). Taken together, these results suggest that M. genitalium LAMPs may inhibit the apoptosis of HeLa cells induced by TNF-α through TLR2 and NF-κB.
M. genitalium LAMPs suppress TNF-α-induced caspase-3 protease activity in HeLa cells
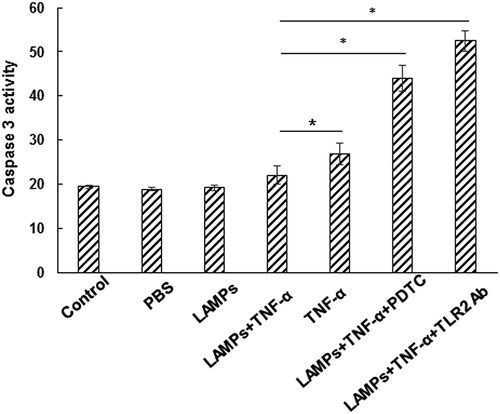
Caspase-3 functions as the initiating factor in the apoptotic signalling pathway, which leads to the morphological manifestation of apoptosis. The effect of M. genitalium LAMPs on the caspase-3 protease activity in the TNF-α-treated HeLa cells was investigated. After treatment with M. genitalium LAMPs for 4 h, the HeLa cells were stimulated with TNF-α (10 ng/mL) together with CHX (10 μg/mL). Then the caspase-3 protease activity was measured 2 h later. Our results showed that, compared with the control group, the caspase-3 activity was significantly reduced in the TNF-α-induced HeLa cells treated with M. genitalium LAMPs () (p < .05). On the other hand, the treatment with PDTC or TLR2-Ab significantly increased the TNF-α-induced caspase-3 protease activity in the HeLa cells treated with M. genitalium LAMPs () (p < .01). Taken together, these results suggest that M. genitalium LAMPs could suppress the TNF-α-induced caspase-3 protease activity in HeLa cells.
Figure 7. Effect of M. genitalium LAMPs on TNF-α-induced caspase-3 protease activity in HeLa cells.
Note: After treatment with M. genitalium LAMPs for 4 h, the HeLa cells were stimulated with TNF-α (10 ng/mL) along with CHX (10 μg/mL). Then the caspase-3 protease activity was measured 2 h later. PDTC or TLR2-Ab was used to block NF-κB in the HeLa cells before M. genitalium lipoproteins treatment and apoptosis induction. Compared with the LAMPs + TNF-α group, *p < .05.
Comparative analysis
In the present study, our results showed that M. genitalium lipoproteins at low concentrations did not directly increase cell death in HeLa cells, while the lipoproteins could inhibit the TNF-α-induced apoptosis. The apoptosis-inhibiting effect of lipoproteins was upstream to mitochondria and downstream to caspase-3. These results shed more light on the mechanism of pathogenesis of M. genitalium, which is the cause of sexually transmitted non-chlamydial NGU in males [Citation22,Citation23] and genital tract inflammation in females [Citation24,Citation25]. Related studies have mainly focused on the adherence-associated proteins and lipoproteins [Citation26]. It has been shown that M. genitalium lipoproteins could induce human monocytic THP-1cells to secrete pro-inflammatory cytokines and lead to apoptosis [Citation27]. Shimizu et al. [Citation17] have found that the lipoproteins P20 and P18 could activate NF-κB through TLR2 and TLR1 in THP-1 cells. Another report also points out that the recombinant C-terminal portion of the immunogenic protein MG309 would activate NF-κB via TLR2 and TLR6 to secrete pro-inflammatory cytokines in the human genital epithelial cells [Citation18]. Herein, the LPMg, TPMg and AqMg were separated on 15% SDS-PAGE, and four intense lanes (i.e. 45, 18, 15 and 9 kDa) were identified.
At present, there are only limited cell models (genital epithelial cells) that could be used to study the pathogenesis of M. genitalium lipoproteins in addition to the THP-1 and HEK293 cell lines. In this study, the cell model was established based on HeLa cells. It has been reported that M. genitalium in women could only induce the lower genital tract inflammation [Citation28], which shows differential capabilities of activating inflammatory cytokines in different genital epithelial cells [Citation18]. Herein, when different concentrations of M. genitalium lipoproteins were used to stimulate the HeLa cells, only very low levels of IL-6, IL-8 and TNF-α could be detected in the HeLa cells after this treatment. Particularly, the stimulated level of TNF-α (8 pg/mL) was negligible compared to the dosage used for apoptosis induction (10 ng/mL). These findings were in consistence with the clinical phenomenon that there are no obvious symptoms in patients with only M. genitalium infection [Citation29]. However, the low levels of these cytokines were sufficient to activate other signalling pathways to amplify the defense function of mononuclear macrophages.
Apoptosis is a process of programmed cell death to maintain balance in the internal environment of the body, as a protective mechanism. Characteristics of apoptosis include membrane blebbing, mitochondrial dysfunction, cellular and cytoplasmic shrinkage, chromosomal fragmentation and condensation, and endonuclease activation, resulting in the characteristic 180-bp nucleosomal DNA ladder [Citation30]. There are two major mediating pathways for apoptosis: the intrinsic pathway, in which signals start from the mitochondria; and the extrinsic pathway, in which the apoptotic signal initiates from the death ligands. Caspase-3 and caspase-7 mediate the downstream events of apoptosis for both of these signalling pathways. In the present study, our results showed that M. genitalium lipoproteins could not induce apoptosis or cell death in HeLa cells, which however were arrested at the G1 phase of the cell cycle. The induced cell cycle arrest delayed the growth of cells, and the harmful factors (other bacterial species, or mixed infection) would invade the cells, which might further lead to cellular apoptosis or inflammation. Therefore, we hypothesize that M. genitalium could not induce apoptosis in HeLa cells, as it requires nutrients from its host cells. Therefore, the M. genitalium infection could induce the apoptotic pathway [Citation34].
As mentioned above, no consensus has been reached about the apoptosis-inducing effects of mycoplasma. In this study, we found that M. genitalium lipoproteins did not directly induce cell death or apoptosis in HeLa cells, and even inhibited the TNF-α-induced apoptosis. NF-κB is a complex transcription factor, which can regulate apoptosis and inflammatory factors [Citation31]. Herein, we blocked the NF-κB function in the TNF-α-induced HeLa cells, and found that the caspase-3 activity was remarkably increased. These results suggest that the NF-κB activation may be related to the anti-apoptotic activity of LAMPs. Some reports have shown that mycoplasma can activate NF-κB through TLRs [Citation18,Citation32]. It could be presumed that M. genitalium lipoproteins could inhibit apoptosis by activating NF-κB through TLR2. It has been reported that M. genitalium can parasitize inside the cells and localize within the nucleus [Citation33], which provides a new way to study the pathogenic mechanisms of M. genitalium. However, further in-depth studies are still needed to investigate the related issues.
Conclusions
Our results showed that M. genitalium LAMPs at low concentrations showed no cytocidal activity in HeLa cells. Moreover, M. genitalium LAMPs induced cell cycle arrest at the G1 phase in HeLa cells. Furthermore, M. genitalium LAMPs significantly attenuated the TNF-α-induced apoptosis in the HeLa cells. In addition, M. genitalium LAMPs could reduce the loss of mitochondrial membrane potential and caspase-3 protease activity in the HeLa cells induced by TNF-α. Our results suggest that M. genitalium LAMPs could significantly inhibit the TNF-α-induced apoptosis in the HeLa cells. These findings would contribute to the understanding of the pathogenesis of M. genitalium.
Disclosure statement
All authors declare no financial competing interests. All authors declare no non-financial competing interests.
Additional information
Funding
References
- You XX, Zeng YH, Wu YM. Interactions between mycoplasma lipid-associated membrane proteins and the host cells. J Zhejiang Univ Sci B. 2006;7:342–350.
- Tully JG, Taylor-Robinson D, Cole RM, et al. A newly discovered mycoplasma in the human urogenital tract. Lancet. 1981;1:1288–1291.
- Horner PJ, Gilroy CB, Thomas BJ, et al. Association of Mycoplasma genitalium with acute non-gonococcal urethritis . Lancet. 1993;342:582–585.
- Henderson B, Poole S, Wilson M. Bacterial modulins: a novel class of virulence factors which cause host tissue pathology by inducing cytokine synthesis. Microbiol Rev. 1996;60:316–341.
- Feng SH, Lo SC. Induced mouse spleen B-cell proliferation and secretion of immunoglobulin by lipid-associated membrane proteins of Mycoplasma fermentans incognitus and Mycoplasma penetrans. Infect Immun. 1994;62:3916–3921.
- da Silva RAG, Churchward CP, Karlyshev AV, et al. The role of apolipoprotein N-acyl transferase, Lnt, in the lipidation of factor H binding protein of Neisseria meningitidis strain MC58 and its potential as a drug target. Br J Pharmacol. 2017;174:2247–2260.
- McGowin CL, Totten PA. The unique microbiology and molecular pathogenesis of Mycoplasma genitalium. J Infect Dis. 2017; 216(Suppl 2):S382–S388.
- Dhandayuthapani S, Rasmussen WG, Baseman JB. Stability of cytadherence-related proteins P140/P110 in Mycoplasma genitalium requires MG218 and unidentified factors. Arch Med Res. 2002;33:1–5.
- Aliprantis AO, Yang RB, Mark MR. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science. 1999;285:736–739.
- Brightbill HD, Libraty DH, Krutzik SR. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science. 1999;285:732–736.
- Into T, Shibata K. Apoptosis signal-regulating kinase 1-mediated sustained p38 mitogen-activated protein kinase activation regulates mycoplasmal lipoprotein- and staphylococcal peptidoglycan-triggered Toll-like receptor 2 signalling pathways. Cell Microbiol. 2005;7:1305–1317.
- Gerlic M, Horowitz J, Farkash S, et al. The inhibitory effect of Mycoplasma fermentans on tumor necrosis factor (TNF)-alpha-induced apoptosis resides in the membrane lipoproteins. Cell Microbiol. 2007;9:142–153.
- Gerlic M, Horowitz J, Horowitz S. Mycoplasma fermentans inhibits tumor necrosis factor alpha-induced apoptosis in the human myelomonocytic U937 cell line. Cell Death Differ. 2004;11:1204–1212.
- Rawadi G, Roman-Roman S. Mycoplasma membrane lipoproteins induced proinflammatory cytokines by a mechanism distinct from that of lipopolysaccharide. Infect Immun. 1996;64:637–643.
- Merril CR, Goldman D, Sedman SA. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981;211:1437–1438.
- Sasse J, Gallagher SR. Staining proteins in gels. Curr Protoc Immunol. 2004;8:Unit 8.9
- Shimizu T, Kida Y, Kuwano K. A triacylated lipoprotein from Mycoplasma genitalium activates NF-kappaB through Toll-like receptor 1 (TLR1) and TLR2. Infect Immun. 2008;76:3672–3678.
- McGowin CL, Ma L, Martin DH. Mycoplasma genitalium-encoded MG309 activates NF-kappaB via Toll-like receptors 2 and 6 to elicit proinflammatory cytokine secretion from human genital epithelial cells. Infect Immun. 2009;77:1175–1181.
- Mitsunari M, Yoshida S, Shoji T. Macrophage-activating lipopeptide-2 induces cyclooxygenase-2 and prostaglandin E(2) via toll-like receptor 2 in human placental trophoblast cells. J Reprod Immunol. 2006;72:46–59.
- Bradham CA, Qian T, Streetz K, et al. The mitochondrial permeability transition is required for tumor necrosis factor alpha-mediated apoptosis and cytochrome c release. Mol Cell Biol. 1998;18:6353–6364.
- Ly JD, Grubb DR, Lawen A. The mitochondrial membrane potential (delta psi(m)) in apoptosis; an update. Apoptosis. 2003;8:115–128.
- Unemo M, Jensen JS. Antimicrobial-resistant sexually transmitted infections: gonorrhoea and Mycoplasma genitalium. Nat Rev Urol 2017;14:139–152.
- Jensen JS. Mycoplasma genitalium: the aetiological agent of urethritis and other sexually transmitted diseases. J Eur Acad Dermatol Venereol. 2004;18:1–11.
- Anagrius C, Loré B, Jensen JS. Mycoplasma genitalium: prevalence, clinical significance, and transmission. Sex Transm Infect. 2005;81:458–462.
- Falk L, Fredlund H, Jensen JS. Signs and symptoms of urethritis and cervicitis among women with or without Mycoplasma genitalium or Chlamydia trachomatis infection. Sex Transm Infect. 2005;81:73–78.
- Burgos R, Pich OQ, Ferrer-Navarro M, et al. Mycoplasma genitalium P140 and P110 cytadhesins are reciprocally stabilized and required for cell adhesion and terminal-organelle development. J Bacteriol. 2006;188:8627–8637.
- Wu Y, Qiu H, Zeng Y, et al. Mycoplasma genitalium lipoproteins induce human monocytic cell expression of proinflammatory cytokines and apoptosis by activating nuclear factor kappaB. Mediators Inflamm. 2008 [cited 2018 Mar 14];2008:195427. DOI: 10.1155/2008/195427
- Moi H, Reinton N, Moghaddam A. Mycoplasma genitalium in women with lower genital tract inflammation. Sex Transm Infect. 2009;85:10–14.
- Seña AC, Lee JY, Schwebke J, et al. A silent epidemic: the prevalence, incidence and persistence of Mycoplasma genitalium among young, asymptomatic high-risk women in the United States. Clin Infect Dis. 2018;67(1):73–79.
- Perlman H, Pagliari LJ, Volin MV. Regulation of apoptosis and cell cycle activity in rheumatoid arthritis. Curr Mol Med. 2001;1:597–608.
- Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733.
- Widjaja M, Harvey KL, Hagemann L, et al. Elongation factor Tu is a multifunctional and processed moonlighting protein. Sci Rep. 2017;7(1):11227. DOI: 10.1038/s41598-017-10644-z
- Ueno PM, Timenetsky J, Centonze VE, et al. Interaction of Mycoplasma genitalium with host cells: evidence for nuclear localization. Microbiology (Reading, Engl.). 2008;154:3033–3041.
- Chernakova GM, Maychuk DY, Klescheva EA, et al. Mixed infections and inflammatory ophthalmic diseases: clinical and laboratory observations. Vestn Oftalmol. 2017;133:74–82.